Abstract
The amniotic membrane (Amnio-M) has various applications in regenerative medicine. It acts as a highly biocompatible natural scaffold and as a source of several types of stem cells and potent growth factors. It also serves as an effective nano-reservoir for drug delivery, thanks to its high entrapment properties. Over the past century, the use of the Amnio-M in the clinic has evolved from a simple sheet for topical applications for skin and corneal repair into more advanced forms, such as micronized dehydrated membrane, amniotic cytokine extract, and solubilized powder injections to regenerate muscles, cartilage, and tendons. This review highlights the development of the Amnio-M over the years and the implication of new and emerging nanotechnology to support expanding its use for tissue engineering and clinical applications.
Graphical Abstract

Similar content being viewed by others
Introduction
The most inner part of the placenta in direct contact with the fetus is called the amniotic membrane (Amnio-M) [1]. The Amnio-M comprises three layers, an epithelial layer toward the fetus, a basement membrane, and a stroma. The latter consists of a compact layer, a fibroblast layer, and finally, a spongy outer layer [2, 3]. These layers comprise two types of cells: the amniotic epithelial cells (AECs) and the amniotic mesenchymal stromal cells (AMSCs) [4]. Cells of the Amnio-M are essential for regulating the development of the embryo by providing several cytokines and growth factors and contributing to the extracellular matrix (ECM) production [5, 6].
Historically, the applications of the Amnio-M in medical therapy started in the early 1900, when Davis [7] proposed its application in skin transplantation. In 1940, De Rötth [8] proposed its usage as the ideal material to replace damaged conjunctiva instead of the mouth mucous membrane, based on its thin, smooth, and transparent structure that mimics the conjunctiva native tissue. In the same year, Chao, Humphreys [9] successfully used the Amnio-M to reconstruct the dura in experimental severe head injury with dural perforation. The Amnio-M was dried using in an oven or an autoclave and referred to as “amnioplastin,” and used to prevent meningocerebral adhesions and posttraumatic epilepsy [9].
Later, in the early eighties, the Amnio-M was used as an adjuvant to autografts in chronic skin ulcers and to prepare for subsequent skin autograft applications, resulting in successful skin healing [10]. In 1986, the Amnio-M provided an excellent alternative to split skin graft to reconstruct the vagina in vulvovaginoplasty [11]. In an effort to preserve its biological and physical properties, Kim and Tseng [12] proposed cryopreservation of the Amnio-M at − 80 °C. The use of the cryopreserved Amnio-M to reconstruct ulcerated cornea in 11 patients achieved a more than 90% success rate [13]. In 1997, Güler and Ercan [14] were the first to test lyophilized (freeze-dried and sterile) Amnio-M in mandibular vestibuloplasty in which they reported potent angiogenic effect.
To facilitate its application, commercial products of the Amnio-M in the form of suspension are becoming recently available. In 2005, it was first applied in the form of suspension eye drops (AMEED®) for corneal ulcer treatment to overcome the invasive procedure of suturing the Amnio-M graft [15]. In other clinical trials, micronized dehydrated human amnion/chorion membrane (μ-dHACM, EpiFix®) was also shown to be effective in treating diabetic foot ulcers, plantar fasciitis, and osteoarthritis (OA) with minimal invasiveness [16,17,18,19]. More recently, the Amnio-M was used as an effective dermal filler for facial wrinkles to restore smooth skin appearance in an in vivo rabbit model [20]. Its cosmetic applications showed rapid improvement in midface aging correction cases, including filling the nasolabial folds, malar fat pad, and descent of lid skin below the orbital rim [21]. The addition of cytokines and growth factors enhanced the usage of the Amnio-M in many other clinical applications in regenerative medicine, such as a three-dimensional (3D) scaffold for tissue engineering and in drug delivery.
The Amnio-M applications have contributed to a better understanding of stem cell biology by providing an optimal platform for cell culture. Our laboratory reported that Amnio-M could provide biologically enriched, well optimized, and topographical mechanical 3D scaffold for culturing stem cells at a low cost compared to the commercially available scaffolds [22]. We also were among the first to provide a microfluidic chip coated with decellularized Amnio-M to introduce a continuous fluid flow to mimic the extracellular fluid dynamics. Recently, the fabrication of Amnio-M organ-on-a-chip has provided an innovative platform for studying the AECs and AMSCs transition and migration in the presence of oxidative stress during preterm birth [23].
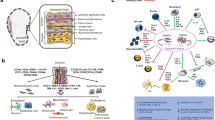
In this review, we highlight the structural and functional properties of the Amnio-M and their role in its diverse applications in regenerative medicine. We also provide an overview of the generation of new forms of the Amnio-M using nanotechnology and their potential applications in tissue engineering. The history of the development of the Amnio-M for research and clinical applications over the past century is summarized in Fig. 1. Figure 2. depicts the main three main components of the Amnio-M and their adaptation to the main pillars of the tissue engineering pyramid that include cells, scaffolds, and growth factors. We will discuss each of these components throughout the review.
Cellular components of the Amnio-M
The human Amnio-M (h-Amio-M) was shown to include two main types of stem cells, the AECs that rest on a thicker basement membrane and the AMSCs that exist in the deeper spongy layer of the membrane [24, 25]. During embryogenesis, both types of cells originate before the delineation of the three primary germ layers in the pre-gastrulation stages and are typical of epithelial origin [26]. The AMSCs are derived from the extraembryonic mesoderm of the primitive streak, while the AECs are derived from the fetal ectoderm on the eighth day of fertilization and preceding organogenesis [27]. The AECs are specialized fetal epithelial cells that live for less than ten months. The AMSCs are similar to adult stem cells in their capability to differentiate into more specialized cells, such as osteocytes, chondrocytes, adipocytes, cardiomyocytes, myocytes, neurocytes, hepatocytes, and vascular endothelial cells [28, 29]. Unlike embryonic stem cells (ESCs), the AMSCs do not form teratomas upon transplantation in vivo, supporting their safe application for clinical transplantation [30,31,32,33,34]. Other types of stem cells are present in the amniotic fluid (AF), and are known to shed during fetal development from both embryonic and extraembryonic tissues [35]. The AF is formed in the amniotic cavity of early gestation two weeks after fertilization [36]. The first progenitor cells derived from the AF were reported in 1993 [37]. The amniotic fluid stem cells (AFCs) include human amniotic fluid epithelial cells (AF-AECs) and amniotic fluid MSCs (AF-MSCs). The former were reported to have the ability to differentiate into neurons, astrocytes, oligodendrocytes and can be used for transplantation therapy in neurodegenerative diseases [38]. The AF-MSCs express the pluripotent marker Oct-4 and have multiple differentiation capacities similar to the AM-MSCs [32, 35]. They can be easily isolated from the AF and have been used in several therapeutic applications [36, 39]. They were also reported to have no tumorigenicity, low immunogenicity, and minimal ethical concerns [36].
The amnio-M cells are also easily accessible, provide high yield, and represent an ethically acceptable source of stem cells for applications in regenerative medicine. Moreover, cells obtained from the hAmnio-M have high plasticity and show multilineage differentiation potential while presenting no risk of tumorigenicity following transplantation [40]. These advantages render the hAmnio-M desirable use in clinical application, including cardiac repair, neurological reconstruction, bone remodeling, and hepatic regeneration [41]. Of particular interest, the lack of histocompatibility antigens on the surface of the Amnio-M cells supports their applications as an excellent biocompatible scaffold that evades the body’s immune reactions upon transplantation [42].
Development of the Amnio-M: epithelial-mesenchymal transition
Epithelial-mesenchymal transition (EMT) is a process in which epithelial cells lose their polarity and acquire mesenchymal phenotype. EMT is not a simple binary decision between the mesenchymal and epithelial phenotype but a chain of forwarding and backward dynamic transitional states between the mesenchymal and epithelial fate [43]. EMT is involved in embryological development, wound healing, and stem cell differentiation. In pathological conditions, it plays a role in tumor generation, progression, metastasis, and organ fibrosis [43, 44].
EMT is regulated by a myriad of transcription factors, including tumor necrosis factor alpha (TNF-\(\alpha )\), Interleukin 6 (IL-6), IL-8, Zinc finger protein of Snail1 (SNAIL1), SNAIL2, prostaglandin, and extracellular signals such as Wnt, transforming growth factor beta (TGF-β), and fibroblast growth factor (FGF) [45, 46]. Zinc finger E-box (ZEB) and Twist are other crucial EMT regulators that modulate epithelial marker loss, such as E-Cadherin and IL-8, along with up-regulation of mesenchymal markers including vimentin, N-Cadherin, and fibronectin [45, 47].
During in vitro amplification, AECs undergo various cellular modifications toward the AMSC phenotype via spontaneous EMT. As reported by Canciello et al., prostaglandin altered this process by inhibiting EMT, suggesting a significant contribution of EMT in the AEC and AMSC transdifferentiation [45]. EMT-associated cellular events include ECM degradation, cytoskeleton disorganization in addition to alteration in the expression level of intracellular adhesion molecules, enabling the cells to acquire migratory behavior [43, 48]. Although these events were studied and reported in cancerous transformations, the same cellular events were also reported to be associated with the ruptured amniotic membrane [48]. Janzen et al. reported that TNF-α, IL6, IL8, prostaglandin, and Matrix metalloproteinase (MMP) are all EMT inducers and biologically active and in substantial concentrations in the fetal-placental unit preterm. They concluded that EMT plays a role in the amniotic membrane rupture via decreasing its tensile strength just before labor (Fig. 3) [48,49,50].
Applications of the AMSCs in regenerative medicine
The rich structure of the Amnio-M supported its use as a natural bio-scaffold for clinical applications. The AMSCs possess unique characteristics that render them useful in diverse applications for tissue repair. These include using as cartilage grafts for tracheal reconstruction of the fetus [51, 52], restoration for the diaphragm muscles [53, 54], bone grafts [55, 56], and heart valve leaflets [57,58,59]. Furthermore, seeding human AMSCs in gelatin microcarriers could successfully generate modular bone-like tissues upon osteogenic differentiation [60].
In mice, the Amnio-M cells were shown to be effective in treating acute tendinopathy [61], and skin repair [59]. They promoted protection against cellular damage in a liver cirrhosis animal model [62, 63] and improved the heart’s function in a cardiac infarction model [64,65,66,67]. Both the AECs and the AMSCs showed promising results when transplanted in diabetic mouse model and effectively brought back glucose to its normal levels [68,69,70]. This promising therapeutic effect in treating type 1 diabetes has been attributed to the cells’ capacity to differentiate into β-cell in vivo. Furthermore, the AECs have been proposed for spinal cord regeneration, as they expressed neural and glial markers [71] and secreted catecholamine neurotransmitters [72]. For example, injection of AECs in combination with umbilical cord MSCs (UC-MSCs) in spinal cord injury showed significant suppression of microglia activity and reduced neuropathic pain [73].
The AFCs on the other hand were used as an effective cell-based therapy for acute or chronic renal failures and acute tubular necrosis in animal models [74]. The AFCs were reported to facilitate neuroprotection during intercellular coupling due to their high expression levels of gap junction protein [75]. Moreover, the AFCs were found to support intercellular communication with astrocytes, highlighting their role in delivering therapeutic factors, such as microRNAs, to damaged tissues [75].
The regenerative utility of stem cells is not mediated only by direct effects but also via paracrine mechanisms, as shown in animal models [76,77,78]. Both the amniotic fluid conditioned media (AF-CM) [79] and AMSCs conditioned media (AMSCs-CM) [80] restored blood flow in a murine hindlimb ischemia model. This effect was attributed to the cytokines and pro-angiogenic growth factors released by the cells into the culture medium, including vascular endothelial growth factor (VEGF), TGF-β, and stromal cell-derived factor-1 (SDF-1). AFCs-CM were shown to stimulate endogenous repair mechanisms, such as dermal fibroblast proliferation at the site of injury in a mouse skin wound model [81]. Recruitment of endothelial progenitor cells to ischemic skin in rat models supported therapeutic angiogenesis by delivering angiogenic growth factors and cytokines [82]. In these studies, the potential of both the Amnio-M-derived cells and the AFCs to stimulate tissue repair was mediated by several paracrine mechanisms, such as the release of trophic factors [83], immunomodulation [84, 85], and the establishment of a supportive environment for renewal [86]. Furthermore, both in vitro and in vivo studies showed that the derivatives and protein extracts of the AMSCs and hAECs display potent anti-tumor effects [87,88,89].
Amnio-M-derived growth factors and cytokines
The anti-inflammatory and antibacterial properties of the Amnio-M are mediated, for the most part, by released growth factors and cytokines. For instance, the angiogenic properties of the Amnio-M were attributed to its capacity to produce VEGF and platelet-derived growth factor (PDGF), both of which mediate wound healing. Moreover, the potent anti-inflammatory and immune-modulatory effects were attributed to the secretion of IL-10 and IL-6 [2, 90]. Hyaluronic acid (HA) in the Amnio-M matrix was reported to inhibit the potent pro-fibrogenic cytokine TGF-β; this could be modulated via increased receptor turnover and decreased endosomal internalization. HA was found to attenuate both SMAD- and non-SMAD-dependent TGF-β1 signaling events [91]. Moreover, Zofia et al. reported that the Amnio-M's secretome contains a wide range of factors that contribute to the regenerative potential and the induction of HUVEC cell migration. These include FGF-6, PDGF-AB, macrophage colony-stimulating factor receptor (M-CSFR), VEGFR3, neurotrophin-4 (NT-4), insulin-like growth factor binding protein 4 (IGFBP-4), and IGFBP-6 [6]. The contribution of the Amnio-M secretome and cytokines in regeneration is summarized in Fig. 4 and Table 1.
Immunomodulatory and anti-inflammatory properties
The Amnio-M plays an essential role in combating inflammation via its potential to suppress the pro-inflammatory cytokines. Secreted elafin (peptidase inhibitor 3) and secretory leukocyte proteinase inhibitors were shown to have an anti-inflammatory effect [6, 92], so was IL-10, which is known to suppress the pro-inflammatory cytokines IL-6 and TNF \(\alpha\). In addition, the Amnio-M was reported to contain various protease inhibitors that play an essential role as anti-inflammatory mediators such as \(\alpha\) 1 anti-trypsin, inter-\(\alpha\) -trypsin inhibitor, and IL-1 inhibitors (IL-1RA) that suppress the IL-1-mediated inflammation [93]. Interestingly, the anti-inflammatory action of the Amnio-M was attributed to its ability to trap the inflammatory cells which undergo apoptosis, making it an excellent candidate for transplantation on the ocular surface [94].
Exosomes are nano-sized extracellular vesicles that contain a wide range of bioactive molecules such as nucleic acids, lipids, and proteins. These vesicles participate in intercellular communication and regulate various intracellular biological functions [95]. Tan et al. reported that AECs-derived exosomes mediate an anti-inflammatory response by augmenting macrophages’ phagocytosis properties along with diminished neutrophil myeloperoxidases and inhibition of T cell proliferation. The same group also reported that administering specific doses of AECs-derived exosomes along with bleomycin, an anti-cancer drug, reduced lung inflammation and fibrosis, in addition to increasing the bronchoalveolar stem cell proliferation [96]. The anti-inflammatory effect of the AEC's exosomes was attributed to their effect on decreasing neutrophil myeloperoxidase (MPO) activity, increasing the phagocytic activity of macrophages, shifting their polarization state toward M2, and repressing proliferation activity of CD3 and CD28-activated T cells [5]. Several studies showed that AECs transplanted into immunocompetent animals were highly tolerated and survived in host tissues. These studies confirmed the clinically relevant value of the low immunogenicity displayed by the Amnio-M. This low immunogenicity was attributed to low expression of HLA IA, besides negative expression of CD86, CD40, CD80, and HLA-DR immunogenic markers [97,98,99]. Furthermore, AEC and AMSCs failed to induce T-cell proliferation in mixed lymphocyte reactions, further confirming their low immunogenicity in vitro [2].
Kupo et al. investigated the immune response generated by xenotransplanted human Amnion-M in the limbus, intracorneal space, and under the kidney capsule of immunocompetent rats. In these experiments, all intracorneal transplanted grafts were tolerated, as well as the grafts under the kidney capsule. However, the latter did not show much host-integrated vascularization when compared to skin graft controls. Interestingly, the skin graft controls showed signs of immune rejection, confirming the superiority of the amnion-M in overcoming graft rejection [100]. In another study by Cargnoni et al., infusion of placenta-derived cells in a lung fibrosis animal model significantly reduced the numbers of infiltrated neutrophil and fibrosis severity [101].
Dual-effect on angiogenesis
The Amnio-M produces several potent anti-angiogenic factors, including endostatin, tissue inhibitors of metalloproteases (TIMP-1, 2, 3, and 4), and thrombospondin -1 [6, 92]. Both the AMSCs and AECs have been shown to express Collagen XVIII, which displays anti-angiogenic properties [102]. AECs, in particular, were reported to secrete IL-1Ra, TIMP4, and 3, which are known for their anti-angiogenic activity in addition to their anti-cancer properties [103]. AECs were able to suppress capillary formation, as evidenced by aortic ring assay in vitro [104]. Interestingly, pro-angiogenic activity was also reported in the Amnio-M and was found to differ from one cell type to another. This could be attributed to the angiogenesis inducers such as angiogenin, PDGF, and VEGF secreted by the AMSCs, proposing them a candidate for skin ulcer treatment and wound healing [5]. In addition to the cellular component, both the integrin and fibronectin protein content in the ECM of Amnio-M have been demonstrated to interact with PDGF, EGF, and b-FGF growth factors for activation of the ERK pathway [105]. A recent study by Tsai et al. demonstrated that the Amnio-M could be considered an excellent matrix for establishing mature vascular constructs. This is due to its potential for enhancing integrin expression, platelet-endothelial cell adhesion molecule-1, and adhesion molecules such as VE-cadherin in the cultured endothelial cells [106].
Anti-fibrotic effect
The Amnio-M showed an anti-fibrotic effect via the downregulation of the expression of TGF-β3 and its receptor, promoting wound healing instead of scar formation. TGF-β3 is an antagonist for TGF- β1 and TGF-β2, which stimulates ECM synthesis, increases collagen deposition in the wound area and promotes scar formation [107]. Tseng et al. reported that the Amnio-M stromal matrix could exert direct and potent anti-scarring action on the ocular surface fibroblasts via suppressing TGF-β transcription and signaling [93]. AEC-derived exosomes showed anti-fibrotic properties by virtue of their protein cargo involved in EFG, FGF, and PDGF signaling pathways [96]. In addition, anti-fibrotic miRNAs were found in AEC’s exosomes that target various aspects of TGFβ signaling [96].
Antibacterial properties
The antibacterial properties of the Amnio-M was shown against both gram-positive and gram-negative bacteria. Zare-Bidaki et al. reported the significant growth inhibitory effect of both the amniotic and the chorionic membranes against eight bacterial strains using disk diffusion assays. These included Escherichia coli, Bacillus cereus, Klebsiella pneumonia, Streptococcus pyogenes, Pseudomonas aeruginosa, Staphylococcus aureus, Shigella flexneri and probiotic Lactobacillus plantarum [108]. In the same direction, Tehrani et al. tested the Amnio-M extract before and after its exposure to IL-1β against Pseudomonas aeruginosa and Staphylococcus aureus, in addition to two clinically isolated sensitive strains of Escherichia coli. The data showed that pre-exposure of the Amnio-M to IL-1β augmented the antibacterial peptide secretion, including elafin, HBD-2, HBD-3, and cathelicidic LL-37, which in turn enhanced the antibacterial properties of the membrane [109].
A clinical study that compared the therapeutic effect of autologous skin graft and Amnio-M dressing in 33 patients suffering from burn showed that the latter was more effective in alleviating the pain, fastening the healing and epithelialization, and protecting the wounds from infection [110]. Moreover, anti-microbial agents in the AF such as beta-lysin, bactericidin, lysozyme, and transferrin could be involved in mounting that effect [92]. The antibacterial potential of the Amnio-M may also be attributed to its sealing capacity. After implantation, the Amnio-M lies in direct and very close contact with the underneath layers and form a firm adherent shield with the wounds, preventing any contamination and enabling lymphatic integrity at this site, as hypothesized by Copra et al. [111].
Extracellular matrix (ECM) component of Amnio-M
The 2D monolayer cell growth lacks faithful mimicry of the biological tissue complexity [112]. 3D natural scaffolds, such as the Amnio-M, or synthetic scaffolds, such as polymer-based scaffolds, play a critical role in supporting cell growth, proliferation, and differentiation [113]. The Amnio-M ECM comprises a cross-linked network of dynamic macromolecules, provides structural support, and acts as a physical scaffold for cells in various body tissues [114]. The Amnio-M possesses unique biophysical and biochemical characteristics that modulate various cell functions such as wound healing and vascularization [115, 116]. In addition, it organizes cells in the space of tissues, controls cell regulation by environmental signals, and activates intracellular signaling by binding with specific transmembrane receptors [117, 118].
Chemical composition of the ECM
Cell attachment to a specific scaffold is controlled by various components of the ECM [119]. The absence of specific ECM molecules, such as laminin, fibronectin, and collagen within the scaffold's basement membrane, has a significant impact on cell growth and adhesion [120]. The ECM’s multiple components act as adhesion and signaling ligands and have a significant role in cell proliferation, migration, and differentiation [116].
The Amnio-M comprises three main layers: an epithelial monolayer, a thick basement membrane, and an avascular stroma [121]. The AECs secrete collagen types I, III, IV, V, VII and non-collagenous glycoproteins, including fibronectin, laminin, and nidogen, all of which constitute the basement membrane of the Amnio-M [119, 122]. On the other hand, a non-fibrillar network of type III collagen, hydrated glycoproteins, and proteoglycans is commonly found in the spongy layer of the stromal part of the amnion [123, 124]. Non-sulfated glycosaminoglycans, such as HA, multiple types of cytokines, proteases, and protease inhibitors, are all significant factors in wound healing [125]. Furthermore, Amnio-M was reported to contain an abundant number of heavy chains of inter-α-inhibitor (HC·HA) combined with human pentraxin 3 (PTX3, TNF-inducible gene 14 protein) [126, 127]. Additionally, perlecan, a large heparan sulfate proteoglycan, is a crucial component of the basement membrane [128, 129]. Perlecan has an essential role in growth factor binding and interactions with many extracellular proteins and molecules responsible for cell adhesion [130].
Mechanical properties of the ECM of the Amnio-M
The mechanical properties of the Amnio-M, such as elasticity, stiffness, and other biomechanical characteristics, are attributed to its ECM, which depends on the variation in its components, including proteoglycan, elastin, and collagen [131]. The Amnio-M exhibits a time-dependent mechanical response and viscoelastic properties [132]. These mechanical properties vary depending on the stage of the Amnio-M. For example, the preterm (26–36 weeks) Amnio-M was found to possess higher mechanical integrity compared to full term Amnio-M (36–40 weeks). However, the stiffness of the term Amnio-M was more adaptable for most tissue engineering applications [119].
The utility of the of the Amnio-M in tissue engineering is highly dependent on its elastic characteristics. Elasticity is defined as the material’s ability to withstand a distorting force and to return to its original shape and size after that force is removed. It is characterized by Young’s modulus, which is the ratio of applied stress to strain and measured in Pascals (= N/m2) and can be found using the following formula E = α/ε, where E is Young’s modulus, α is applied stress, and ε is the strain [133]. Young’s modulus of preterm human Amnio-M is reported to be 3.6 × 106 Pascal (3.6 MPa) and about 2.29 × 106 (2.29 MPa) for full-term human Amnio-M [119]. Benson-Martin et al. reported an inverse relationship between the thickness of Amnio-M and the elastic modulus (stiffness of the material). The thicker (proximal, adjacent to placental disk) Amnio-M has lower Young’s modulus (less stiff) compared to the thinner (distal, handbreadth from the placental disk) Amnio-M (more stiff). One possible explanation may be attributed to variance of the alignment of the collagen fibers, which constitute structure bulk of the Amnio-M [134]. Recently, the distal Amnio-M was shown to possess a higher degree of anisotropy (increase the stiffness of fibrillar materials) within its collagen fiber arrangements than the proximal Amnio-M [134]. This change in mechanical properties may be attributed to the content of collagen. It is also worth mentioning that elastin present in the fetal amnion was reported to provide the molecular basis for Amnio-M elasticity [135].
Essential considerations for biomedical applications of the Amnio-M
The source of the Amnio-M
The source of the Amnio-M source, whether after natural delivery or via Cesarean section, was found to impact its physiology, integrity, growth factors content, and availability. The Amnio-M donor should be screened to avoid transmission of infectious diseases [136], and especially after the COVID-19 pandemic due to fears of vertical transmission [137]. Litwiniuk, Radowicka [138] reported that Cesarean section-derived cervical portion of the Amnio-M stimulated the proliferation of keratinocytes more than that of fibroblasts. These data were valuable in the application of the Amnio-M grafting for ocular defects. Furthermore, natural delivery is associated with decreasing the Amnio-M tensile strength just before labor due to the EMT process. This results in the loss of cytoskeleton organization and intercellular adhesion molecules as described above. The thickness of the membrane also varies from 0.02 to 0.5 mm according to the anatomical site, which may impact its clinical applications [139]. The thickest part lies toward the umbilical cord (placental amnion), while the opposite part is thinner and more transparent (peripheral amnion), which lends itself to superior corneal grafts (Fig. 5) [140].
Processing, preservation and sterilization of the Amnio-M
Over the past century, the Amnio-M use has benefited from technological advantages, preparation techniques, and sterilization methodologies. At the beginning of the twentieth century, the Amnio-M was used immediately after harvesting during delivery to cover skin lesions [7]. Because of the immunogenic response, and the lack of amnion banks, it became necessary to find other methods for preserving and processing the Amnio-M. In 1938, Chao, Humphreys [9] proposed to dry the Amnio-M before usage to avoid the irritation induced by fresh samples. The membrane was air or oven-dried, then sterilized by either autoclaving or boiling. However, the latter treatment was quickly discounted as it resulted in shrinkage and disruption of the membrane. Drying the Amnio-M was a turning point in its usage in tissue reconstruction, as it proved to be safe, effective and solved the storage deficiency of the fresh membrane. With the advances in sterilization techniques, Rao and Chandrasekharam [141] used ultraviolet (UV) sterilization. Their data showed that irradiation did not affect the biological and physical properties of the Amnio-M. Other methods for sterilization of the Amnio-M include the use of peracetic acid and organic peroxides. These chemical factors were shown to be effective and also safe compared to sterilization by irradiation, with minimum effect on collagen content [142].
In the nineties, Kim and Tseng [12] proposed cryopreservation of the Amnio-M by storing it in − 80 °C using a storage medium composed of glycerol in Dulbecco’s Modified Eagle Medium (DMEM) (1:1). The advantages of cryopreservation were most evident in maintaining the integrity of the ECM. However, glycerol was reported to maintain cell viability, as well as high bFGF production for no more than 3 months of storage [143]. More investigations are needed to find an optimal cryo-preservative that can maintain the Amnio-M biological content and physical properties for more extended periods. In 2004, Nakamura and Yoshitani [144] proposed a new preservation technique to freeze-dry the Amnio-M (FDAM) by incubating the membrane with EDTA for 2 h then freeze-drying it under vacuum at room temperature. This technique was as effective as cryopreservation in effectively retaining the biological, physical, and histological properties of the Amnio-M. Compared to the dried Amnio-M, the fresh-frozen membrane showed negligible differences in the membrane stability, although the content of the epidermal growth factor (EGF) was shown to be higher in the dried membrane [145].
Recent attempts to prepare the Amnio-M in an injectable solution has been promising to reduce its grafting procedure's invasiveness, especially for corneal ulcers and osteoarthritis. This suspension could be marketed either in the form of an amnion cytokine extract (ACE) or amniotic membrane extract eye drops (AMEED). ACE was reported to reduce the clinical symptoms of dry eyes [146]. In contrast, AMEED was reported to efficiently treat dry eyes, chemical ulcers, and diffuse limbal stem cell deficiency (LSCD) [147]. In osteoarthritis, the Amnio-M was a part of μ-dam (EpiFix®) product, which showed promising efficacy in ameliorating the arthritis symptoms [16, 148].
Other forms of the Amnio-M include gel and sponge, both used for cartilage regeneration [149]. Gel formation was performed by collagen extraction from the Amnio-M after 24 h incubation with guanidine solution (4 M) suspended in Tris buffer. The sponge scaffold was fabricated by precipitation collagen type I using acetic acid followed by freezing and drying. The extracted collagen in this study has shown high hydrophilicity, biocompatibility, and induced cartilage formation [149]. Other similar components were extracted from the Amnio-M, such as hyaluronic acid and PTX3, both of which had well-known effect on healing and reducing scar formation. Tseng and colleagues [126] purified HC·HA from the Amnio-M. This active component has shown a crucial role in both reducing scar formation and inflammation, which were attributed to suppression of TGF-β1 and inducing macrophage death. Later, human PTX3 was reported to be integrated with HC·HA to form AM HC-HA-PTX3 and was efficiently extracted from the Amnio-M using agarose overlay [127]. Interestingly, PTX3 has been reported to play a role in polarization of M2 macrophages which is linked to phagocytosis of apoptotic cells [127, 150]. In summary, new advanced technology helped in providing the Amnio-M in different forms, rather than the fresh membrane, as cryopreserved Amnio-M, FDAM, Amnio-M suspension, gel and sponge form (Table 2). Also, several components have been extracted to be used in regenerative medicine as collagen, HC·HA and HC-HA-PTX3.
Enhancement of the Amnio-M biomaterial (3D) properties
There is a complex set of requirements that must be taken into consideration when choosing the suitable scaffold to meet the morphology and functionality of the native tissues. Many attempts were reported to modify the Amnio-M to match the ideal scaffold characteristics regarding degradability, porosity, surface roughness, hydrophilicity, delivering bio-active molecule, biocompatibility, deliverability (easy to deliver), and mechanical reliability [151, 152].
Cellularity
To ensure biocompatibility, the decellularization strategy of the Amnio-M evolved to decrease the immunogenic response generated by the in vivo implantation of the membrane. The Amnio-M’s decellularization (removal of the cellular compartment) process was reported to have no adverse effect on intact collagen types I, III, and IV, which will favor biocompatibility [153]. Of note, decellularization results in loss of the stem cell content of the Amnio-M, leading to a lower content of growth factors and cytokines. This encouraged many researchers to use the non-decellularized Amnio-M in preparing Amnio-M extracts or even the Amnio-M powder [154].
Biodegradability
Cross-linking
The fresh cryopreserved membranes take about seven days to degrade by enzymatic digestion [153]. This fast degradation is considered a serious limitation in its usage for skin regeneration, as skin substitutes should stay at least two weeks to vascularize sufficiently [155]. Importantly, many tissue defects required a long-lasting scaffold until complete recovery. To satisfy these requirements, in 1994, Spira et al. investigated the cross-linking of Amnio-M extracted collagen using radiation. This cross-linking resulted in the detection of almost 70% of the Amnio-M collagen after 12 months when implanted subcutaneously in a rat model [156]. Similarly, in 1999, the Amnio-M was cross-linked with either chemicals or radiation, which not only resulted in a highly stable membrane, (for up to 90 days) but also enhanced the mechanical properties of the membrane without any adverse effects on cell attachment or viability [157]. Furthermore, cross-linking using radiation by γ-ray, UV irradiation, and electron beams was shown to be effective and safe [158, 159]. Later on, a larger set of chemicals was shown to be safe and effective in cross-linking the Amnio-M. For example, aluminum sulfate (Al2(SO4)3) showed a significant increase in its tensile strength [160]. Also, carbodiimide enhanced the physicochemical properties of Amnio-M, enhancing the differentiation potential of human limbal epithelial progenitor cells [161]. A combination of carbodiimide and N‐hydroxy‐succinimide caused effective cross-linking sufficient for promoting optimum mechanical and optical properties for corneal regeneration [162]. Recently, the natural extract genipin has been used to cross-link Amnio-M and enhance biostability and biocompatibility [163]. Table 3. summarizes the cross-linking techniques to control the Amnio-M biodegradation rate.
Integration with other material
3D scaffold production was developed to overcome the biodegradability issue by means of fabrication of Amnio-M. In 2011, a preliminary study was conducted to produce a fabricated Amnio-M-fibrin scaffold that effectively enhanced chondrogenic differentiation with a slow degradation rate [164]. Nanofibrous silk fibroin was also tested to produce a 3D bioscaffold, which was shown to extend the degradation time to two weeks [131]. Microfabricated electrospun membranes composed of poly-lactic co-glycolic acid (PLGA) and Amnio-M in equal amounts showed a delay in the degradation rate, pushing it to completely degrade after four weeks [165]. Integration with other material was also indicated to overcome the weak mechanical properties of Amnio-M. Adamowicz, Pokrywczyńska [166] proposed covering the Amnio-M with electrospun poly-L-lactide-co-E-caprolactone (PLCL) from both sides to be grafted in the wall of urinary bladder. The degradation of this biomaterial in vivo ranged between 8 and 10 weeks. Also, higher elasticity similar to normal biomechanical properties of the urinary bladder wall was reported.
Orchestrating tissue healing and regeneration
Biomaterials used in tissue engineering for clinical applications should display desirable effects such as suppressing inflammation, reducing scar formation, enhancing vascularization, preventing infection, and recruiting native resident stem cells to the site of injury. The content of proteins, cytokines and growth factors in the Amnio-M propose its use as an optimal biomaterial for wound healing. Using the Amnio-M as a single or even double sheet has a wide range of applications for covering injuries at the outer exposed surfaces of the body, such as the skin and the cornea. Moreover, the Amnio-M can also act as a protective barrier for internal organs. For example, wrapping of peripheral nerves, the spinal cord, the peritoneal cavity, and tendon with the Amnio-M could prevent adhesion and reduce scar formation [167,168,169,170].
Importantly, the regenerative potential of the Amnio-M is not limited to simple usage as a coverage bandage but extends to include its high content of regenerative key factors. Advanced technologies supported the transformation of the Amnio-M into 3D scaffold that can fit appropriately into a defect. They have also helped its reintegration with other natural and synthetic biomaterials as shown in Table 3. This integration could allow for superior applications. For example, the gel form of the Amnio-M would be advantageous over powder or cryopreserved Amnio-M in wound healing as it will provide a hydrating wound barrier, overcome the wound contractility, and control the rate of release of therapeutic components [154].
In a recent study, human solubilized Amnio-M (prepared by lyophilization) was combined with hyaluronic acid and cross-linked to evaluate its application in skin wound regeneration [171]. Accelerated healing and reepithelization, as well as noticeable neovascularization, were achieved. Similarly, combination of Amnio-M powder with methacrylated gelatin (GelMA) hydrogel could enhance mucosa regeneration through enhancing vascularization [172]. The same group used GelMA-Amnio-M composite in skin regeneration, showing adequate collagen deposition, as well as proper mechanical properties [173]. Integration of the dAmnio-M with nano-fibrous fibroin enhanced the in vitro differentiation of menstrual blood MSCs into keratinocyte [174]. Furthermore, Amnio-M powder mixed with Aloe vera gel enhanced in vitro proliferation, migration and adhesion of fibroblast and keratinocytes. In vivo studies showed that the gel did not cause irritation and accelerated the wound healing with minimal scar formation. However, precautions should be taken for high Aloe vera concentration as it showed cytotoxic effects in vitro [175].
One of the most exciting developments of the Amnio-M is its optimization in producing sutureless membranes to facilitate its applications, especially in the corneal defects. Fibrin glue has been proposed by Szurman, Warga [176] as a bio-adhesive to stabilize the Amnio-M over the corneal surface. However, in some cases, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) which require covering the entire cornea, the conjunctiva, as well as eyelid, securing a large sheet of Amnio-M was challenging. Shanbhag, Chodosh [177] proposed cyanoacrylate glue to fix Amnio-M into the eyelid skin alongside using a silicon ring to stabilize it over the cornea. Another study on the treatment of recurrent retinal detachment using Amnio-M has shown that adding platelet-rich plasma (PRP) increased the success rate of sealing the retinal hole [178].
Recently, the drug reservoir properties of the Amnio-M have been investigated. They were shown to effectively deliver bioactive molecules such as cefazolin and moxifloxacin, where the Amnio-M could sustain their release for up to 7 weeks [179, 180]. Furthermore, the Amnio-M was loaded with calcium and phosphate using the double diffusion method to develop a mineralized membrane capable of bone regeneration [181]. It is worth mentioning that Amnio-M was investigated for effectively acting as a carrier for stem cells delivery from different sources (Table 3). These include the bone marrow, adipose tissue, dental pulp, and menstrual blood [174, 182,183,184,185]. Decellularized Amnio-M provided a biocompatible ECM for culturing DP-derived cells and retaining their properties and provided cell sheet that favors its application in periodontal tissue regeneration [182]. The dAmnio-M loaded ASCs have shown potent anti-inflammatory effects and fastened skin wound healing in burn animal models [184]. Similarly, dehydrated Amnio-M loaded with genetically modified TGF-β3 BMSCs significantly reduced scar formation and improved the cosmetic appearance in full-thickness wounds [183].
Conclusions
According to the tissue engineering pyramid, successful tissue engineering and regeneration can be accomplished by integrating several factors including scaffolds, cells, vascularization, growth factors, and chemical and physical cues. The Amnio-M cover most of the tissue engineering pyramid component as it can provide appropriate ECM, cells and different kinds of growth factors [152]. This wide range of cover in tissue engineering encouraged researchers to develop the membrane using advanced technologies to modify and enhance these unique and valuable properties. These modifications aimed to increase biocompatibility by decellularizing the membrane and facilitating the deliverability through producing Amnio-M suspension as AMEED and μ-dHACM that can be injected rather than sutured. Furthermore, it helps in controlling biodegradability and enhancing the mechanical properties by cross-linking and fabrication. Moreover, advanced drug reservoir technology broadens its potential for use in sustained drug release, such as cefazolin and Moxifloxacin biomolecules. The Amnio-M’s content of unique types of stem cells significantly enhances its value as a rich biomaterial for tissue regeneration. In conclusion, advanced technology has significantly enhanced the applications of the Amnio-M in regenerative therapy by both enhancing its forms and delivery methods..
Future perspectives
The amniotic membrane has many beneficial usages as a natural biocompatible material for tissue engineering applications; many of which have not been thoroughly investigated. It also has some drawbacks, which, if appropriately addressed, can substantially enhance its applications. These drawbacks include rapid degradation, poor mechanical properties, and inconvenient forms. More investigations are thus needed to prepare proper scaffolds forms of Amnio-M in combination with either natural materials, synthetic materials, or hybrids. In addition, the different physicochemical and biomedical properties of these material integrated with the Amnio-M should be thoroughly investigated both in vitro and in vivo to gain insightful information about their interaction with the living cells.
Although the notion of sutureless Amnio-M aimed to decrease the invasiveness of its application in delicate tissue such as the cornea, the use of alternative traditional methods such as glue was not satisfying. Nanotechnology approaches could be superior to conventional glues in providing an adhesive membrane to be directly implanted at the defect site, either in the skin or cornea. By applying new available technologies such as 3D printing, the Amnio-M products are expected to witness significant modifications through integration with different polymers, leading to expanding their clinical applications.
Data availability
All data presented in this review are totally available and present in the text.
Code availability
Not applicable.
Abbreviations
- 3D:
-
Three-dimensional
- ACE:
-
Amnion cytokine extract
- AECs:
-
Amniotic epithelial cells
- AF:
-
Amniotic fluid
- AF-AECs:
-
Human amniotic fluid epithelial cells
- AF-CM:
-
Amniotic fluid conditioned media
- AFCs:
-
Amniotic fluid stem cells
- Al2(SO4)3 :
-
Aluminum sulfate
- AMEED:
-
Amniotic membrane extract eye drops
- Amnio-M:
-
Amniotic membrane
- AMSCs:
-
Amniotic mesenchymal stromal cells
- BM:
-
Bone marrow
- DMEM:
-
Dulbecco's Modified Eagle Medium
- DP:
-
Dental pulp
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial-mesenchymal transition
- ESCs:
-
Embryonic stem cells
- FDAM:
-
Freeze-dry Amnio-M
- FGF:
-
Fibroblast growth factor
- GelMA:
-
Methacrylated gelatin
- HA:
-
Hyaluronic acid
- IGFBP-4:
-
Insulin-like growth factor binding protein 4
- IL-6:
-
Interleukin 6
- LSCD:
-
Limbal stem cell deficiency
- M-CSFR:
-
Macrophage colony-stimulating factor receptor
- MMP:
-
Matrix metalloproteinase
- MPO:
-
Neutrophil myeloperoxidase
- NT-4:
-
Neurotrophin-4
- OA:
-
Osteoarthritis
- PDGF:
-
Platelet-derived growth factor
- PLCL:
-
Poly-(l-lactide-co-E-caprolactone)
- PLGA:
-
Poly-lactic co-glycolic acid
- PRP:
-
Platelet-rich plasma
- PTX3:
-
Pentraxin 3
- SDF-1:
-
Stromal cell-derived factor-1
- SJS:
-
Stevens-Johnson syndrome
- SNAIL1:
-
Zinc finger protein of Snail1
- TEN:
-
Toxic epidermal necrolysis
- TGF-β:
-
Transforming growth factor beta
- TIMP:
-
Tissue inhibitors of metalloproteases
- TNF-α:
-
Tumor necrosis factor alpha
- UC-MSCs:
-
Umbilical cord MSCs
- UV:
-
Ultraviolet
- VEGF:
-
Vascular endothelial growth factor
- ZEB:
-
Zinc finger E-box
- μ-dHACM:
-
Micronized dehydrated human amnion/chorion membrane
References
Pereira PNG, Dobreva MP, Graham L, Huylebroeck D, Lawson KA, Zwijsen AN. Amnion formation in the mouse embryo: the single amniochorionic fold model. BMC Dev Biol. 2011;11(1):48.
Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011;32(Suppl 4):S320–5.
Salah RA, Elkhenany H, El-Badri N. Scaffold engineering using the amniotic membrane. In: El-Badri N, editor. Regenerative medicine and stem cell biology. Cham: Springer; 2020. p. 323–46.
Díaz-Prado S, Muiños-López E, Hermida-Gómez T, Cicione C, Rendal-Vázquez ME, Fuentes-Boquete I, et al. Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation. 2011;81(3):162–71.
Farhadihosseinabadi B, Farahani M, Tayebi T, Jafari A, Biniazan F, Modaresifar K, et al. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol. 2018;46(sup2):431–40.
Grzywocz Z, Pius-Sadowska E, Klos P, Gryzik M, Wasilewska D, Aleksandrowicz B, et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem Cytobiol. 2014;52(3):163–70.
Davis JS. Skin transplantation. Johns Hopkins Hosp Rep. 1910;15(307):96.
De Rötth A. Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol. 1940;23(3):522–5.
Chao Y-C, Humphreys S, Penfield W. A new method of preventing adhesions. The use of amnioplastin after craniotomy. Br Med J. 1940;1(4134):517.
Bennett J, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980;315(8179):1153–6.
Morton KE, Dewhurst C. Human amnion in the treatment of vaginal malformations. BJOG Int J Obstet Gynaecol. 1986;93(1):50–4.
Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14(5):473–84.
Lee S-H, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123(3):303–12.
Güler R, Ercan M, Ulutuncel N, Devrim H, Uran N. Measurement of blood flow by the 133Xe clearance technique to grafts of amnion used in vestibuloplasty. Br J Oral Maxillofac Surg. 1997;35(4):280–3.
Bonci P, Bonci P, Lia A. Suspension made with amniotic membrane: clinical trial. Eur J Ophthalmol. 2005;15(4):441–5.
Reece DS, Burnsed OA, Parchinski K, Marr EE, Salazar-Noratto GE, Lin ASP, et al. Reduced size profile of amniotic membrane particles decreases osteoarthritis therapeutic efficacy. Tissue Eng Part A. 2019;26:28–37.
Salazar-Noratto GE, Nations CC, Stevens HY, Guldberg RE. Localized osteoarthritis disease-modifying changes due to intra-articular injection of micronized dehydrated human amnion/chorion membrane. Regen Eng Transl Med. 2019;5(2):210–9.
Cazzell S, Stewart J, Agnew PS, Senatore J, Walters J, Murdoch D, et al. Randomized controlled trial of micronized dehydrated human amnion/chorion membrane (dHACM) injection compared to placebo for the treatment of plantar fasciitis. Foot Ankle Int. 2018;39(10):1151–61.
Hawkins B. The use of micronized dehydrated human amnion/chorion membrane allograft for the treatment of diabetic foot ulcers: a case series. Wounds. 2016;28(5):152–7.
Buday MC, Ozturk M. Evaluation of folded amniotic membrane and injectable amniotic membrane pieces as soft tissue filler materials. Auris Nasus Larynx. 2019;46(3):451–6.
Davis A, Augenstein A. Amniotic allograft implantation for midface aging correction: a retrospective comparative study with platelet-rich plasma. Aesth Plast Surg. 2019;43:1345–52.
Khalil S, El-Badri N, El-Mokhtaar M, Al-Mofty S, Farghaly M, Ayman R, et al. A cost-effective method to assemble biomimetic 3D cell culture platforms. PLoS ONE. 2016;11(12):e0167116.
Richardson L, Jeong S, Kim S, Hart A, Menon R. Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. FASEB J. 2019;33(8):8945–60.
Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med. 2009;4(3):423–33.
Cai J, Li W, Su H, Qin D, Yang J, Zhu F, et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem. 2010;285(15):11227–34.
Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300–11.
Sakuragawa N, Kakinuma K, Kikuchi A, Okano H, Uchida S, Kamo I, et al. Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J Neurosci Res. 2004;78(2):208–14.
Gawryluk A. Properties and clinical application of human amniotic epithelial cells (HAEC). Curr Gynecol Oncol. 2015;13(2):123–35.
Hu J, Cai Z, Zhou Z. Progress in studies on the characteristics of human amnion mesenchymal cells. Prog Nat Sci. 2009;19(9):1047–52.
Klemmt PA, Vafaizadeh V, Groner B. The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin Biol Ther. 2011;11(10):1297–314.
Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem cells. 2005;23(10):1549–59.
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100.
Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577–88.
Zhang S, Geng H, Xie H, Wu Q, Ma X, Zhou J, et al. The heterogeneity of cell subtypes from a primary culture of human amniotic fluid. Cell Mol Biol Lett. 2010;15(3):424.
Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19(6):1450–6.
Kim EY, Lee K-B, Kim MK. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014;47(3):135.
Torricelli F, Brizzi L, Bernabei P, Gheri G, Di Lollo S, Nutini L, et al. Identification of hematopoietic progenitor cells in human amniotic fluid before the 12th week of gestation. Ital J Anat Embryol. 1993;98(2):119–26.
Kakishita K, Nakao N, Sakuragawa N, Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 2003;980(1):48–56.
int Anker PS, Scherjon SA, Kleijburg-Van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–9.
Xu H, Zhang J, Tsang KS, Yang H, Gao W-Q. Therapeutic potential of human amniotic epithelial cells on injuries and disorders in the central nervous system. Stem Cells Int. 2019;2019:5432301.
Hua J, Zhou Z. Progress in studies on the characteristics of human amnion mesenchymal cells. Progress Nat Sci. 2009;19(10):1047–52.
Campos LL, Landim-Alvarenga FC, Ikeda TL, Monteiro BA, Maia L, Freitas-Dell’aqua CP, et al. Isolation, culture, characterization and cryopreservation of stem cells derived from amniotic mesenchymal layer and umbilical cord tissue of bovine fetuses. Pesqui Vet Bras. 2017;37(3):278–86.
Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21–45.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Canciello A, Russo V, Berardinelli P, Bernabo N, Muttini A, Mattioli M, et al. Progesterone prevents epithelial-mesenchymal transition of ovine amniotic epithelial cells and enhances their immunomodulatory properties. Sci Rep. 2017;7(1):3761.
Roche J. The epithelial-to-mesenchymal transition in cancer. Cancers (Basel). 2018;10(2):52.
Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through β-catenin–T-cell factor-4-dependent expression of transforming growth factor-β3. Mol Biol Cell. 2008;19(11):4875–87.
Janzen C, Sen S, Lei MY, Gagliardi de Assumpcao M, Challis J, Chaudhuri G. The role of epithelial to mesenchymal transition in human amniotic membrane rupture. J Clin Endocrinol Metab. 2017;102(4):1261–9.
Stallmach T, Hebisch G, Joller-Jemelka HI, Orban P, Schwaller J, Engelmann M. Cytokine production and visualized effects in the feto-maternal unit. Quantitative and topographic data on cytokines during intrauterine disease. Lab Investig J Tech Methods Pathol. 1995;73(3):384–92.
Denison FC, Kelly RW, Calder AA, Riley SC. Cytokine secretion by human fetal membranes, decidua and placenta at term. Hum Reprod (Oxford, England). 1998;13(12):3560–5.
Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41(4):675–82.
Iravani K, Mehravar S, Bahador M, Azarpira N. The healing effect of amniotic membrane in laryngeal defects in rabbit model. Laryngoscope. 2021;131(2):E527–33.
Fuchs JR, Kaviani A, Oh J-T, LaVan D, Udagawa T, Jennings RW, et al. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg. 2004;39(6):834–8.
Kunisaki SM, Fuchs JR, Kaviani A, Oh J-T, LaVan DA, Vacanti JP, et al. Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte—and myoblast-based constructs. J Pediatr Surg. 2006;41(1):34–9.
Steigman SA, Ahmed A, Shanti RM, Tuan RS, Valim C, Fauza DO. Sternal repair with bone grafts engineered from amniotic mesenchymal stem cells. J Pediatr Surg. 2009;44(6):1120–6.
Klein JD, Turner CG, Ahmed A, Steigman SA, Zurakowski D, Fauza DO. Chest wall repair with engineered fetal bone grafts: an efficacy analysis in an autologous leporine model. J Pediatr Surg. 2010;45(6):1354–60.
Schmidt D, Achermann J, Odermatt B, Breymann C, Mol A, Genoni M, et al. Prenatally fabricated autologous human living heart valves based on amniotic fluid–derived progenitor cells as single cell source. Circulation. 2007;116(11_supplement):I64–70.
Schmidt D, Achermann J, Odermatt B, Genoni M, Zund G, Hoerstrup SP. Cryopreserved amniotic fluid-derived cells: a lifelong autologous fetal stem cell source for heart valve tissue engineering. J Heart Valve Dis. 2008;17(4):446–55 (discussion 55).
Weber B, Emmert MY, Behr L, Schoenauer R, Brokopp C, Drögemüller C, et al. Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials. 2012;33(16):4031–43.
Chen M, Wang X, Ye Z, Zhang Y, Zhou Y, Tan W-S. A modular approach to the engineering of a centimeter-sized bone tissue construct with human amniotic mesenchymal stem cells-laden microcarriers. Biomaterials. 2011;32(30):7532–42.
Barboni B, Russo V, Gatta V, Bernabò N, Berardinelli P, Mauro A, et al. Therapeutic potential of hAECs for early Achilles tendon defect repair through regeneration. J Tissue Eng Regen Med. 2018;12(3):e1594–608.
Manuelpillai U, Tchongue J, Lourensz D, Vaghjiani V, Samuel CS, Liu A, et al. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated mice. Cell Transplant. 2010;19(9):1157–68.
Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS ONE. 2011;6(2):e16789.
Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79(5):528–35.
Tsuji H, Ikegami Y, Miyoshi S, Hida N, Nishiyama N, Togashi I, et al. Xenografted human amniotic membrane-derived mesenchymal stem cells acquired immune tolerance and transdifferentiated into cardiomyocytes in vivo. Am Heart Assoc. 2008;118:S_420.
Fujimoto KL, Miki T, Liu LJ, Hashizume R, Strom SC, Wagner WR, et al. Naive rat amnion-derived cell transplantation improved left ventricular function and reduced myocardial scar of postinfarcted heart. Cell Transplant. 2009;18(4):477–86.
Blume GG, Machado-Júnior PAB, Paludo Bertinato G, Simeoni RB, Francisco JC, Guarita-Souza LC. Tissue-engineered amniotic membrane in the treatment of myocardial infarction: a systematic review of experimental studies. Am J Cardiovasc Dis. 2021;11(1):1–11.
Wei JP, Zhang TS, Kawa S, Aizawa T, Ota M, Akaike T, et al. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 2003;12(5):545–52.
Hou Y, Huang Q, Liu T, Guo L. Human amnion epithelial cells can be induced to differentiate into functional insulin-producing cells. Acta Biochim Biophys Sin. 2008;40(9):830–9.
Kadam SS, Sudhakar M, Nair PD, Bhonde RR. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12(8):982–91.
Sakuragawa N, Thangavel R, Mizuguchi M, Hirasawa M, Kamo I. Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neurosci Lett. 1996;209(1):9–12.
Elwan MA, Sakuragawa N. Evidence for synthesis and release of catecholamines by human amniotic epithelial cells. NeuroReport. 1997;8(16):3435–8.
Roh D-H, Seo M-S, Choi H-S, Park S-B, Han H-J, Beitz AJ, et al. Transplantation of human umbilical cord blood or amniotic epithelial stem cells alleviates mechanical allodynia after spinal cord injury in rats. Cell Transplant. 2013;22(9):1577–90.
Rosner M, Schipany K, Shanmugasundaram B, Lubec G, Hengstschläger M. Amniotic fluid stem cells: future perspectives. Stem cells Int. 2012;2012:1–6.
Jezierski A, Rennie K, Tremblay R, Zurakowski B, Gruslin A, Sikorska M, et al. Human amniotic fluid cells form functional gap junctions with cortical cells. Stem Cells Int. 2012;2012:1–6.
Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5(6):933–46.
De Feo D, Merlini A, Laterza C, Martino G. Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection. Curr Opin Neurol. 2012;25(3):322–33.
Dai W, Kay GL, Jyrala AJ, Kloner RA. Experience from experimental cell transplantation therapy of myocardial infarction: what have we learned? Cell Transplant. 2013;22(3):563–8.
Mirabella T, Cilli M, Carlone S, Cancedda R, Gentili C. Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model. Biomaterials. 2011;32(15):3689–99.
Kim HG, Choi OH. Neovascularization in a mouse model via stem cells derived from human fetal amniotic membranes. Heart Vessels. 2011;26(2):196–205.
Yoon BS, Moon J-H, Jun EK, Kim J, Maeng I, Kim JS, et al. Secretory profiles and wound healing effects of human amniotic fluid–derived mesenchymal stem cells. Stem Cells Dev. 2009;19(6):887–902.
Mirabella T, Hartinger J, Lorandi C, Gentili C, van Griensven M, Cancedda R. Proangiogenic soluble factors from amniotic fluid stem cells mediate the recruitment of endothelial progenitors in a model of ischemic fasciocutaneous flap. Stem Cells Dev. 2012;21(12):2179–88.
Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62(4):585–90.
Moorefield EC, McKee EE, Solchaga L, Orlando G, Yoo JJ, Walker S, et al. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS ONE. 2011;6(10):e26535.
Kamiya K, Wang M, Uchida S, Amano S, Oshika T, Sakuragawa N, et al. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. 2005;80(5):671–9.
Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118(1):11–7.
Elkhenany H, Shekshek A, Abdel-Daim M, El-Badri N. Stem cell therapy for hepatocellular carcinoma: future perspectives. In: Turksen K, editor. Cell biology and translational medicine, volume 7: Stem cells and therapy: emerging approaches. Springer International Publishing: Cham; 2020. p. 97–119.
Mamede AC, Laranjo M, Carvalho MJ, Abrantes AM, Pires AS, Brito AF, et al. Effect of amniotic membrane proteins in human cancer cell lines: an exploratory study. J Membr Biol. 2014;247(4):357–60.
Mamede A, Guerra S, Laranjo M, Santos K, Carvalho M, Carvalheiro T, et al. Oxidative stress, DNA, cell cycle/cell cycle associated proteins and multidrug resistance proteins: targets of human amniotic membrane in hepatocellular carcinoma. Pathol Oncol Res. 2016;22(4):689–97.
Paradowska E, Blach-Olszewska Z, Gejdel E. Constitutive and induced cytokine production by human placenta and amniotic membrane at term. Placenta. 1997;18(5–6):441–6.
Ito T, Williams JD, Fraser DJ, Phillips AO. Hyaluronan regulates transforming growth factor-β1 receptor compartmentalization. J Biol Chem. 2004;279(24):25326–32.
Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111–21.
Tseng SCG, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, et al. How does amniotic membrane work? Ocul Surf. 2004;2(3):177–87.
Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20(4):408–13.
LeBleu VS, Kalluri R. Exosomes exercise inhibition of anti-tumor immunity during chemotherapy. Immunity. 2019;50(3):547–9.
Tan JL, Lau SN, Leaw B, Nguyen HPT, Salamonsen LA, Saad MI, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7(2):180–96.
Magatti M, Vertua E, Cargnoni A, Silini A, Parolini O. The immunomodulatory properties of amniotic cells: the two sides of the coin. Cell Transplant. 2018;27(1):31–44.
Pratama G, Vaghjiani V, Tee JY, Liu YH, Chan J, Tan C, et al. Changes in culture expanded human amniotic epithelial cells: implications for potential therapeutic applications. PLoS ONE. 2011;6(11):e26136.
Li J, Qiu C, Wei Y, Yuan W, Liu J, Cui W, et al. Human amniotic epithelial stem cell-derived retinal pigment epithelium cells repair retinal degeneration. Front Cell Dev Biol. 2021;9(2688):1–13.
Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42(7):1539–46.
Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D, et al. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009;18(4):405–22.
Hao Y, Ma DH-K, Hwang DG, Kim W-S, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348–52.
Parolini O, Soncini M, Evangelista M, Schmidt D. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med. 2009;4(2):275–91.
Niknejad H, Khayat-Khoei M, Peirovi H, Abolghasemi H. Human amniotic epithelial cells induce apoptosis of cancer cells: a new anti-tumor therapeutic strategy. Cytotherapy. 2014;16(1):33–40.
Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135(6 Pt 1):1633–42.
Tsai S-H, Liu Y-W, Tang W-C, Zhou Z-W, Hwang C-Y, Hwang G-Y, et al. Characterization of porcine arterial endothelial cells cultured on amniotic membrane, a potential matrix for vascular tissue engineering. Biochem Biophys Res Commun. 2007;357(4):984–90.
Gilbert RW, Vickaryous MK, Viloria-Petit AM. Signalling by transforming growth factor beta isoforms in wound healing and tissue regeneration. J Dev Biol. 2016;4(2):21.
Zare-Bidaki M, Sadrinia S, Erfani S, Afkar E, Ghanbarzade N. Antimicrobial properties of amniotic and chorionic membranes: a comparative study of two human fetal sacs. J Reprod infertil. 2017;18(2):218.
Tehrani FA, Modaresifar K, Azizian S, Niknejad H. Induction of antimicrobial peptides secretion by IL-1β enhances human amniotic membrane for regenerative medicine. Sci Rep. 2017;7(1):17022.
Eskandarlou M, Azimi M, Rabiee S, Rabiee MAS. The healing effect of amniotic membrane in burn patients. World J Plast Surg. 2016;5(1):39.
Chopra A, Thomas BS. Amniotic membrane: a novel material for regeneration and repair. J Biomim Biomater Tissue Eng. 2013;18(1):1–8.
Wang R, Wang Y, Yao B, Hu T, Li Z, Huang S, et al. Beyond 2D: 3D bioprinting for skin regeneration. Int Wound J. 2019;16(1):134–8.
Taghiabadi E, Nasri S, Shafieyan S, Jalili Firoozinezhad S, Aghdami N. Fabrication and characterization of spongy denuded amniotic membrane based scaffold for tissue engineering. Cell J. 2015;16(4):476–87.
Barnes P, Drazen J, Rennard S, Thomson N. Asthma and COPD second edition. Basic mechanisms and clinical management. San Diego: Academic Press; 2009.
Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9.
Pardo-Saganta A, Calvo IA, Saez B, Prosper F. Role of the extracellular matrix in stem cell maintenance. Curr Stem Cell Rep. 2019;5(1):1–10.
Hubbell JA. Chapter 21—matrix effects. In: Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. 4th ed. Boston: Academic Press; 2014. p. 407–21.
Muncie JM, Weaver VM. Chapter one—the physical and biochemical properties of the extracellular matrix regulate cell fate. In: Litscher ES, Wassarman PM, editors. Current topics in developmental biology, vol. 130. New York: Academic Press; 2018. p. 1–37.
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater. 2008;15:88–99.
Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105(3):215–28.
Salah RA, Mohamed IK, El-Badri N. Development of decellularized amniotic membrane as a bioscaffold for bone marrow-derived mesenchymal stem cells: ultrastructural study. J Mol Histol. 2018;49:289–301.
Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–7.
Zhou M. Amniotic membrane as a scaffold for human adipose stem cells and umbilical vein endothelial cells. 2018.
Nabi AT, Huda I, Pratap A, Kumari S, Singh PK, Singh P. Amniotic membrane-a new vista in periodontics. J Adv Med Dent Sci Res. 2018;6(7):111–4.
Lei J, Priddy LB, Lim JJ, Massee M, Koob TJ. Identification of extracellular matrix components and biological factors in micronized dehydrated human amnion/chorion membrane. Adv Wound Care. 2017;6(2):43–53.
He H, Li W, Tseng DY, Zhang S, Chen S-Y, Day AJ, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-α-inhibitor (HC·HA) purified from extracts of human amniotic membrane*. J Biol Chem. 2009;284(30):20136–46.
Zhang S, Zhu Y-T, Chen S-Y, He H, Tseng SCG. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex*. J Biol Chem. 2014;289(19):13531–42.
Zhang L, Zou D, Li S, Wang J, Qu Y, Ou S, et al. An ultra-thin amniotic membrane as carrier in corneal epithelium tissue-engineering. Sci Rep. 2016;6(1):21021.
Lord MS, Tang F, Rnjak-Kovacina J, Smith JG, Melrose J, Whitelock JM. The multifaceted roles of perlecan in fibrosis. Matrix Biol. 2018;68:150–66.
Martinez JR, Grindel BJ, Hubka KM, Dodge GR, Farach-Carson MC. Perlecan/HSPG2: signaling role of domain IV in chondrocyte clustering with implications for Schwartz-Jampel Syndrome. J Cell Biochem. 2019;120(2):2138–50.
Gholipourmalekabadi M, Samadikuchaksaraei A, Seifalian AM, Urbanska AM, Ghanbarian H, Hardy JG, et al. Silk fibroin/amniotic membrane 3D bi-layered artificial skin. Biomed Mater. 2018;13(3):035003.
Jin H, Yang Q, Ji F, Zhang Y, Zhao Y, Luo M. Human amniotic epithelial cell transplantation for the repair of injured brachial plexus nerve: evaluation of nerve viscoelastic properties. Neural Regen Res. 2015;10(2):260.
Hughes D, Babbs CF, Geddes L, Bourland J. Measurements of Young’s modulus of elasticity of the canine aorta with ultrasound. Ultrason Imaging. 1979;1(4):356–67.
Chen B, Jones RR, Mi S, Foster J, Alcock SG, Hamley IW, et al. The mechanical properties of amniotic membrane influence its effect as a biomaterial for ocular surface repair. Soft Matter. 2012;8(32):8379–87.
George AK, Cherian SA. An in vitro investigation and comparison of the mechanical properties of human amnion and bovine collagen membrane. Braz J Periodontol-December. 2018;28(04):7–12.
Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193–204.
Yeung TLM, Liu S, Li BCY, Mok KM, Li KKW. Amniotic membrane harvesting during COVID-19 pandemic. Eye (London, England). 2021;35(3):1019.
Litwiniuk M, Radowicka M, Krejner A, Grzela T. The influence of amniotic membrane extracts on cell growth depends on the part of membrane and childbirth mode selected: a proof-of-concept study. J Wound Care. 2017;26(8):498–503.
Danforth DN, Hull RW. The microscopic anatomy of the fetal membranes with particular reference to the detailed structure of the amnion. Am J Obstet Gynecol. 1958;75(3):536–50.
Dua HS, Maharajan VS, Hopkinson A. Controversies and limitations of amniotic membrane in ophthalmic surgery. Cornea and external eye disease. Berlin: Springer; 2006. p. 21–33.
Rao TV, Chandrasekharam V. Use of dry human and bovine amnion as a biological dressing. JAMA Surg. 1981;116(7):891–6.
von Versen-Höynck F, Syring C, Bachmann S, Möller D. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts—light and scanning electron microscopic studies. Cell Tissue Bank. 2004;5(1):45–56.
Wagner M, Walter P, Salla S, Johnen S, Plange N, Rütten S, et al. Cryopreservation of amniotic membrane with and without glycerol additive. Graefes Arch Clin Exp Ophthalmol. 2018;256(6):1117–26.
Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, et al. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45(1):93–9.
Kim TG, Do Ki K, Lee MK, So JW, Chung SK, Kang J. Comparison of cytokine expression and ultrastructural alterations in fresh-frozen and dried electron beam-irradiated human amniotic membrane and chorion. Cell Tissue Bank. 2019;20(2):163–72.
Yeu E, Goldberg DF, Mah FS, Beckman KA, Luchs JI, Solomon JD, et al. Safety and efficacy of amniotic cytokine extract in the treatment of dry eye disease. Clin Ophthalmol. 2019;13:887–94.
Murri MS, Moshirfar M, Birdsong OC, Ronquillo YC, Ding Y, Hoopes PC. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clin Ophthalmol (Auckland, NZ). 2018;12:1105–12.
Willett NJ, Thote T, Lin AS, Moran S, Raji Y, Sridaran S, et al. Intra-articular injection of micronized dehydrated human amnion/chorion membrane attenuates osteoarthritis development. Arthritis Res Ther. 2014;16(1):R47.
Park S-H, Song T, Bae TS, Khang G, Choi BH, Park SR, et al. Comparative analysis of collagens extracted from different animal sources for application of cartilage tissue engineering. Int J Precis Eng Manuf. 2012;13(11):2059–66.
He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem. 2013;288(36):25792–803.
Elkhenany H, Bourdo S, Biris A, Anderson D, Dhar M. Important considerations in the therapeutic application of stem cells in bone healing and regeneration. Stem Cells Toxicol Med. 2016; 458–80.
Elkhenany H, Abd Elkodous M, Newby SD, El-Derby AM, Dhar M, El-Badri N. Tissue engineering modalities and nanotechnology. Regenerative medicine and stem cell biology. Berlin: Springer; 2020. p. 289–322.
Gholipourmalekabadi M, Mozafari M, Salehi M, Seifalian A, Bandehpour M, Ghanbarian H, et al. Development of a cost-effective and simple protocol for decellularization and preservation of human amniotic membrane as a soft tissue replacement and delivery system for bone marrow stromal cells. Adv Healthc Mater. 2015;4(6):918–26.
Murphy SV, Skardal A, Nelson RA Jr, Sunnon K, Reid T, Clouse C, et al. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. Stem Cells Transl Med. 2020;9(1):80–92.
Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma. 2018;6:4.
Spira M, Liu B, Xu Z, Harrell R, Chahadeh H. Human amnion collagen for soft tissue augmentation–biochemical characterizations and animal observations. J Biomed Mater Res. 1994;28(1):91–6.
Fujisato T, Tomihata K, Tabata Y, Iwamoto Y, Burczak K, Ikada Y. Cross-linking of amniotic membranes. J Biomater Sci Polym Ed. 1999;10(11):1171–81.
Spoerl E, Wollensak G, Reber F, Pillunat L. Cross-linking of human amniotic membrane by glutaraldehyde. Ophthalmic Res. 2004;36(2):71–7.
Lai JY. Photo-cross-linking of amniotic membranes for limbal epithelial cell cultivation. Mater Sci Eng C Mater Biol Appl. 2014;45:313–9.
Sekar S, Sasirekha K, Krishnakumar S, Sastry TP. A novel cross-linked human amniotic membrane for corneal implantations. Proc Inst Mech Eng H. 2013;227(3):221–8.
Ma DH, Chen HC, Ma KS, Lai JY, Yang U, Yeh LK, et al. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta Biomater. 2016;31:144–55.
Tanaka Y, Kubota A, Yokokura S, Uematsu M, Shi D, Yamato M, et al. Optical mechanical refinement of human amniotic membrane by dehydration and cross-linking. J Tissue Eng Regen Med. 2012;6(9):731–7.
Gobinathan S, Zainol SS, Azizi SF, Iman NM, Muniandy R, Hasmad HN, et al. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J Biomater Sci Polym Ed. 2018;29(17):2051–67.
Hussin I, Pingguan-Murphy B, Osman S, editors. The fabrication of human amniotic membrane based hydrogel for cartilage tissue engineering applications: a preliminary study. In: 5th Kuala Lumpur international conference on biomedical engineering, vol 2011; 2011. Springer.
Ramachandran C, Sangwan VS, Ortega I, Bhatnagar U, Mulla SMA, McKean R, et al. Synthetic biodegradable alternatives to the use of the amniotic membrane for corneal regeneration: assessment of local and systemic toxicity in rabbits. Br J Ophthalmol. 2019;103(2):286–92.
Adamowicz J, Pokrywczyńska M, Tworkiewicz J, Kowalczyk T, van Breda SV, Tyloch D, et al. New amniotic membrane based biocomposite for future application in reconstructive urology. PLoS ONE. 2016;11(1):e0146012.
Meng H, Li M, You F, Du J, Luo Z. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci Lett. 2011;496(1):48–53.
Barbuto RC, de Araujo ID, Bonomi Dde O, Tafuri LS, Calvão Neto A, Malinowski R, et al. Use of the amniotic membrane to cover the peritoneal cavity in the reconstruction of the abdominal wall with polypropylene mesh in rats. Rev Col Bras Cir. 2015;42(1):49–55.
Prakash S, Kalra P, Dhal A. Correction to: Flexor tendon repair with amniotic membrane. Int Orthop. 2020;44(10):2047.
Walker CT, Godzik J, Kakarla UK, Turner JD, Whiting AC, Nakaji P. Human amniotic membrane for the prevention of intradural spinal cord adhesions: retrospective review of its novel use in a case series of 14 patients. Neurosurgery. 2018;83(5):989–96.
Murphy SV, Skardal A, Song L, Sutton K, Haug R, Mack DL, et al. Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med. 2017;6(11):2020–32.
Zhang Q, Qian C, Xiao W, Zhu H, Guo J, Ge Z, et al. Development of a visible light, cross-linked GelMA hydrogel containing decellularized human amniotic particles as a soft tissue replacement for oral mucosa repair. RSC Adv. 2019;9(32):18344–52.
Zhang Q, Chang C, Qian C, Xiao W, Zhu H, Guo J, et al. Photo-crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater. 2021;125:197–207.
Fard M, Akhavan-Tavakoli M, Khanjani S, Zare S, Edalatkhah H, Arasteh S, et al. Bilayer amniotic membrane/nano-fibrous fibroin scaffold promotes differentiation capability of menstrual blood stem cells into keratinocyte-like cells. Mol Biotechnol. 2018;60(2):100–10.
Rahman MS, Islam R, Rana MM, Spitzhorn LS, Rahman MS, Adjaye J, et al. Characterization of burn wound healing gel prepared from human amniotic membrane and Aloe vera extract. BMC Complement Altern Med. 2019;19(1):115.
Szurman P, Warga M, Grisanti S, Roters S, Rohrbach JM, Aisenbrey S, et al. Sutureless amniotic membrane fixation using fibrin glue for ocular surface reconstruction in a rabbit model. Cornea. 2006;25(4):460–6.
Shanbhag SS, Chodosh J, Saeed HN. Sutureless amniotic membrane transplantation with cyanoacrylate glue for acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul Surf. 2019;17(3):560–4.
Di Tizio F, Gattazzo I, Di Staso F, Di Pippo M, Guglielmelli F, Scuderi G. Human amniotic membrane patch and platelet-rich plasma to promote retinal hole repair in a recurrent retinal detachment. Eur J Ophthalmol. 2021;31(3):1479–82.
Sara SH, Prajna NV, Senthilkumari S. Human amniotic membrane as a drug carrier—an in-vitro study using fortified cefazolin ophthalmic solution. Indian J Ophthalmol. 2019;67(4):472–5.
Yelchuri ML, Madhavi B, Gohil N, Sajeev HS, Venkatesh Prajna N, Srinivasan S. In vitro evaluation of the drug reservoir function of human amniotic membrane using moxifloxacin as a model drug. Cornea. 2017;36(5):594–9.
Sabouri L, Farzin A, Kabiri A, Milan PB, Farahbakhsh M, Mehdizadehkashi A, et al. Mineralized human amniotic membrane as a biomimetic scaffold for hard tissue engineering applications. ACS Biomater Sci Eng. 2020;6(11):6285–98.
Honjo K, Yamamoto T, Adachi T, Amemiya T, Mazda O, Kanamura N, et al. Evaluation of a dental pulp-derived cell sheet cultured on amniotic membrane substrate. Bio-Med Mater Eng. 2015;25(2):203–12.
Samadikuchaksaraei A, Mehdipour A, Habibi Roudkenar M, Verdi J, Joghataei MT, As’adi K, et al. A dermal equivalent engineered with TGF-beta3 expressing bone marrow stromal cells and amniotic membrane: cosmetic healing of full-thickness skin wounds in rats. Artif Organs. 2016;40(12):E266–79.
Motamed S, Taghiabadi E, Molaei H, Sodeifi N, Hassanpour SE, Shafieyan S, et al. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am J Surg. 2017;214(4):762–9.
Dziedzic DSM, Francisco JC, Mogharbel BF, Irioda AC, Stricker PEF, Floriano J, et al. Combined biomaterials: amniotic membrane and adipose tissue to restore injured bone as promoter of calcification in bone regeneration: preclinical model. Calcif Tissue Int. 2021;108(5):667–79.
Singh R, Chacharkar MP. Dried gamma-irradiated amniotic membrane as dressing in burn wound care. J Tissue Viability. 2011;20(2):49–54.
Suroto H, Aryawan DM, Prakoeswa CA. The Influence of the preservation method and gamma irradiation sterilization on TGF-β and bFGF levels in freeze-dried amnion membrane (FD-AM) and amnion sponge. Int J Biomater. 2021;2021:1–9.
Thomasen H, Pauklin M, Steuhl K-P, Meller D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1691–700.
Fenelon M, Maurel DB, Siadous R, Gremare A, Delmond S, Durand M, et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Mater Sci Eng C. 2019;104:109903.
Ma DH-K, Lai J-Y, Cheng H-Y, Tsai C-C, Yeh L-K. Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells. Biomaterials. 2010;31(25):6647–58.
Kim H, Son D, Choi TH, Jung S, Kwon S, Kim J, et al. Evaluation of an amniotic membrane-collagen dermal substitute in the management of full-thickness skin defects in a pig. Arch Plast Surg. 2013;40(1):11–8.
Li W, Fu Y, Jiang B, Lo AY, Ameer GA, Barnett C, et al. Polymer-integrated amnion scaffold significantly improves cleft palate repair. Acta Biomater. 2019;92:104–14.
Che X, Wu H, Jia C, Sun H, Ou S, Wang J, et al. A Novel Tissue-engineered corneal stromal equivalent based on amniotic membrane and keratocytes. Invest Ophthalmol Vis Sci. 2019;60(2):517–27.
Cho WH, Sung MT, Lin PW, Yu HJ. Progressive large pediatric corneal limbal dermoid management with tissue glue-assisted monolayer amniotic membrane transplantation: a case report. Medicine. 2018;97(46):e13084.
Lai JY. Carbodiimide cross-linking of amniotic membranes in the presence of amino acid bridges. Mater Sci Eng C Mater Biol Appl. 2015;51:28–36.
Acknowledgements
Not applicable.
Funding
This work was supported by grant # 7304 from the Egyptian Academy of Scientific Research and Technology (ASRT).
Author information
Authors and Affiliations
Contributions
NEB and HEK contributed to conceptualization. HEK performed the selection of literature, drafted the manuscript, and prepared the figures. AED, MAE and RAS drafted the manuscript, collected the related references, and participated in the discussion. AL collected the related references, reviewed, and edited the manuscript. NEB carried out the design and language revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elkhenany, H., El-Derby, A., Abd Elkodous, M. et al. Applications of the amniotic membrane in tissue engineering and regeneration: the hundred-year challenge. Stem Cell Res Ther 13, 8 (2022). https://doi.org/10.1186/s13287-021-02684-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-021-02684-0