Abstract
Background
Cardiac autonomic neuropathy is a highly prevalent pathology in the diabetic population, and is the leading cause of death in this population. Orthostatic hypotension is the main clinical manifestation of the disease. In some patients, this orthostatic hypotension is associated with supine hypertension, posing a therapeutic challenge since treatment of one entity may aggravate the other. The challenge is to manage each of these two hemodynamic opposites without exposing the patient to a life-threatening risk of severe hypotension or hypertension.
Case presentation
We report a case of a 62-year-old ethnic Moroccan woman who has cardiovascular risk factors such as type 2 diabetes, arterial hypertension, and dyslipidemia. The patient’s symptoms included dizziness, tremors, morning sickness, palpitations, and intolerance to exertion. Given her symptomatology, the patient benefited from an exploration of the autonomic nervous system through cardiovascular reactivity tests (Ewing tests), which confirmed the diagnosis of cardiac autonomic neuropathy. In addition to orthostatic hypotension, our patient had supine arterial hypertension, complicating management. To treat orthostatic hypotension, we advised the patient to avoid the supine position during the day, to raise the head of the bed during the night, and to have a sufficient fluid intake, with a gradual transition from decubitus to orthostatism and venous restraint of the lower limbs. Supine hypertension was treated with transdermal nitrates placed at bedtime and removed 1 hour before getting up. One week after the introduction of treatment, the patient reported a clear regression of functional symptoms, with an improvement in her quality of life. Improvement in symptomatology was maintained during quarterly follow-up consultations.
Conclusions
Cardiac autonomic neuropathy is a very common pathology in diabetic patients. It is a serious condition with a life-threatening prognosis. Its management must be individualized according to the symptomatology and profile of each patient. The treatment of patients with orthostatic hypotension and supine hypertension requires special attention to ensure that each entity is treated without aggravating the other.
Similar content being viewed by others
Background
Cardiac autonomic neuropathy (CAN) is the most common autonomic nervous system abnormality in diabetes. It corresponds to an alteration of the autonomic nerve fibers of the heart and blood vessels, leading to a dysregulation of the sympathovagal balance, resulting in abnormalities in the control of heart rate and blood pressure.
The prevalence of CAN confirmed in people with type 1 and type 2 diabetes is about 20%; this prevalence can increase with age and duration of diabetes and reach up to 65% [1]. CAN is often latent but severe, linked to excessive mortality related to the occurrence of cardiovascular complications including silent myocardial infarction, permanent tachycardia with shortened diastole and reduced myocardial viability, QT interval prolongation with risk of arrhythmia, and sudden death [2].
Despite these deadly consequences, CAN remains poorly studied on a practical level, and its detection, quantification, and treatment remain insufficient. The reduction of heart rate variability is one of the first signs of CAN but is generally unknown unless it is specifically sought in autonomic nervous system tests [3].
CAN is most often revealed by orthostatic hypotension (OH) defined by a decrease in systolic blood pressure of more than 20 mmHg and/or diastolic blood pressure of more than 10 mmHg within 3 minutes of decubitus [4].
In some patients, this orthostatic hypotension can coexist with supine hypertension, presenting a therapeutic challenge with the need to manage each of these two components without aggravating the other.
Case presentation
We report a case of a 62-year-old ethnic Moroccan woman, who has cardiovascular risk factors such as type 2 diabetes, arterial hypertension, and dyslipidemia. The patient presented with a symptomatology made of vertigo and tremor, associated with a malaise when getting up in the morning. Her symptomatology was aggravated in warm atmospheres, with a feeling of well-being in cold climates. In addition, the patient presented palpitations with intolerance to the effort.
The clinical examination found a blood pressure of 159/101 mmHg in the right arm and 157/99 mmHg in the left arm, and a heart rate of 94 beats/minute. The cardiovascular examination was without special features, and the patient’s echocardiography was normal. The electrocardiogram showed a regular sinus rhythm at 94 beats/minute, and a fixed PR (PR is time between onset of atrial activation (P wave) and onset of ventricular myocardial activation (onset of QRS complex)) at 0.18 seconds without secondary repolarization disorders.
Given her symptomatology, the patient benefited from an exploration of the autonomic nervous system through cardiovascular reactivity tests (Ewing tests).
During this exploration, the patient was first placed in a calm atmosphere, in dorsal decubitus position on an examination table. Blood pressure and heart rate was measured every 5 minutes for 30 minutes, then the patient performed five tests of the autonomic nervous system, interspersed with periods of rest.
The first test: deep breathing test
During this test, the patient performed six deep breaths for 1 minute. The patient’s calculated vagal response was 7% [normal (N): 30%], indicating a vagal deficiency.
The second test: hand grip test
This test is a manual contraction effort performed to determine changes in blood pressure and heart rate at the static effort. In the patient, the vagal response was 6.5% (N: 10%), indicating a vagal deficiency
The peripheral sympathetic alpha response was 11% (N: 10%), reflecting peripheral sympathetic alpha hyperactivity.
The third test: hyperventilation
This test corresponds to the patient performing shallow breathing for 15 seconds. The heart rate increased from 91 beats/minute to 97 beats/minute during this test. Blood pressure decreased from 171/100 mmHg to 144/86 mmHg.
The fourth test: the mental stress test
This test consists of successive mental calculations leading to an increase in heart rate and blood pressure by increasing central sympathetic activity. The central alpha sympathetic response in the patient was 22% (N: 10%), indicating alpha sympathetic hyperactivity.
The fifth test: orthostatic test
This test consisted of measuring the variation in heart rate and blood pressure in response to active lifting for at least 5 seconds after a period of rest in a supine position.
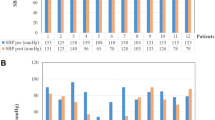
The patient’s blood pressure decreased from 159/99 mmHg to 128/80 mmHg, indicating the existence of orthostatic hypotension.
After the end of the orthostatic test, the shift to the supine position was associated with an increase in blood pressure to 173/110 mmHg in relation to supine hypertension (Fig. 1).
Table 1 summarizes the cardiovascular autonomic profile obtained from the cardiovascular reactivity tests (Ewing test):
We note high blood pressure and heart rate in the basic state, a vagus deficiency in deep breathing and orthostatic test, sympathetic central and peripheral hyperactivity, and orthostatic hypotension associated with supine high blood pressure.
This autonomic profile is in favor of an autonomic cardiac neuropathy syndrome combining vagal deficiency and sympathetic hyperactivity.
Discussion
CAN is a peripheral vegetative neuropathy, predominantly in small-caliber nerve fibers with little or no myelin. Representing one of the most frequent complications of diabetes, its prevalence increases with the duration of its evolution and the glycemic imbalance [5]. CAN induces an increase in cardiovascular morbidity and mortality; its symptomatology most often consists of orthostatic or postprandial hypotension, permanent tachycardia, silent myocardial ischemia, and sudden death secondary to severe cardiac rhythm disorders. Only 24% of patients present signs suggestive of dysautonomia, hence the interest to systematically search for CAN in diabetic patients [5].
Its diagnosis is based on clinical tests of the autonomic nervous system showing a vagal deficiency, which is often associated with sympathetic hyperactivity.
The resting heart rate is approximately 10 beats/minute higher in diabetic subjects with CAN than in those without CAN. This resting tachycardia can be explained by parasympathetic damage, which generally precedes orthosympathetic damage. Dynamic tests also show a decrease in heart rate variability in these patients, and therefore an abnormality in the adaptation of the heart rate to circadian variations, stress, and exercise, which is the cause of the exercise intolerance frequently experienced by patients with CAN [6,7,8].
Orthostatic hypotension is a later sign that can be isolated or associated with the occurrence of a sensation of fatigue, dizziness, lipothymia, or even syncope. This symptomatology is due to an abnormality in the functioning of the baroreflex arc with a defect in the ascent of the heart rate and myocardial ionotropic during the transition to the standing position [4].
In some patients, this orthostatic hypotension may coexist with supine hypertension, which poses a therapeutic problem because the treatment of one entity may aggravate the other.
Management of combined orthostatic hypotension and supine hypertension: in most patients with orthostatic hypotension with supine hypertension, there are good reasons to prioritize the treatment of orthostatic hypotension over supine hypertension [9, 10].
Symptomatic orthostatic hypotension is accompanied by various debilitating symptoms, including postural dizziness, syncope, fatigue, weakness, and visual disturbances. All of these symptoms can contribute to an increased frequency of falls with numerous complications and risk of death [11].
Fludrocortisone should be avoided when treating OH in patients with an association of OH with supine hypertension [11]. The use of midodrine, which is a peripheral and selective alpha adrenergic receptor agonist that acts by increasing arterial resistance and venous return, carries a risk of significant supine hypertension, so it is recommended that patients do not take midodrine within 5 hours before bedtime [9,10,11,12] and do not rest or sleep in the supine position; on the contrary, decubitus should always be assumed in the head-up position [13].
Expert recommendations for the management of supine hypertension in the setting of orthostatic hypotension suggest that supine hypertension requires intervention if systolic blood pressure exceeds the range of 160–180 mmHg. Many medications used to treat orthostatic hypotension can cause or exacerbate supine hypertension, given that orthostatic hypotension and supine hypertension are hemodynamic opposites, amelioration of one may worsen the other [11].
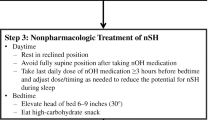
All patients with OH and supine hypertension should be advised to avoid the supine position during the day and elevate the head of the bed as tolerated at night. Clinicians should manage patients with significant supine hypertension with short-acting antihypertensive drugs such as captopril, clonidine, hydralazine, and losartan administered at bedtime and not during the day to avoid the worsening of OH during the day [14].
The use of transdermal nitrates is the treatment of choice for supine hypertension [15]. It is recommended that the nitrate patch be applied at bedtime and removed 1–2 hours before getting up [11].
In our patient, we indicated hygienic and dietetic measures to treat orthostatic hypotension, in particular sufficient fluid intake, a gradual transition from decubitus to orthostasis, venous restraint of the lower limbs, and elevation of the head of the bed by 5° to 10°. For the treatment of supine hypertension, we opted for transdermal nitrates placed at bedtime and removed 1 hour before getting up.
One week after the introduction of treatment, the patient reported a clear regression of functional symptoms, with an improvement in her quality of life. Improvement in symptomatology was maintained during quarterly follow-up consultations.
Conclusions
Cardiac autonomic neuropathy is a highly responsive pathology in diabetic patients occurring in the medium or long term during the course of diabetes, corresponding to an alteration of the cardiovascular system’s sympathetic and parasympathetic autonomic control.
The alteration of the functioning of the baroreflex arc during CAN is responsible for the appearance of orthostatic hypotension, which is added in 50% of the patients to supine hypertension, exposing a therapeutic problem because the treatment of one entity can aggravate the other.
In these patients, orthostatic hypotension management consists of adopting hygienic and dietetic measures (abundant fluid intake, a progressive transition from decubitus to orthostatism, venous restraint of the lower limbs, and so on). Avoidance of diuretics and long-acting antihypertensive drugs is necessary. Midodrine should not be taken within 5 hours before bedtime to avoid the aggravation of supine hypertension.
As for the management of supine hypertension, short-acting antihypertensive drugs such as nitrates, captopril, losartan, and clonidine should be administered at bedtime and not during the day to avoid the worsening of OH.
Availability of data and materials
Non-applicable.
Abbreviations
- CAN:
-
Cardiac autonomic neuropathy
- OH:
-
Orthostatic hypotension
- N:
-
Normal
References
Valensi P, et al. Autonomic neuropathy in diabetes. Diabetes Metab. 1997;23:1–8.
Aring AM, Jones DE, Falko JM. Evaluation and prevention of diabetic neuropathy. Am Fam Physician. 2005;71:2123–30.
Philips JC, Marchand M, Scheen AJ. Diabetic cardiac autonomic neuropathy. Rev Med Liège. 2005;60:498–504.
Purewal TS, Watkins PJ. Postural hypotension in diabetic autonomic neuropathy: a review. Diabetic Med. 1995;12:1992–200.
Boulton A, Valensi P, Tesfaye S. The diabetic neuropathies: reports from the Diabetic Neuropathy Expert Panel Meeting on Neuropathy, Toronto, October 2009. Introduction.
Maser RE, Mitchell BD, Vinik AI, et al. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–901.
Vinik A, Maser R, Mitchell B, et al. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–79.
Maser RE, Lenhard MJ. Review: cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab. 2005;90:5896–903.
Jankovic J, Gilden JL, Hiner BC, Kaufmann H, Brown DC, Coghlan CH, Rubin M, Fouad-Tarazi FM. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrine. Am J Med. 1993;95(1):38–48.
Jordan J, Biaggioni I. Diagnosis and treatment of supine hypertension in autonomic failure patients with orthostatic hypotension. J Clin Hypertens (Greenwich). 2002;4(2):139–45.
Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567–82.
Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA. A double-blind dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51(1):120–4.
Parsaik AK, Singh B, Altayar O, Mascarenhas SS, Singh SK, Erwin PJ, Murad MH. Midodrine for orthostatic hypotension: a systematic review and meta-analysis of clinical trials. J Gen Intern Med. 2013;28(11):1496–503.
Ahmad A, Grubb B, Zaman L, Kanjwal K. Syndrome of supine hypertension with orthostatic hypotension: a nightmare for physicians. J Innov Cardiac Rhythm Manag. 2016;7:1–4.
Lamarre-Cliché M. Orthostatic hypotension and supine hypertension in the patient with autonomic failure. Can J Gen Intern Med. 2014;9(3):91–5.
Acknowledgements
Not applicable.
Funding
There has been no significant financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Contributions
JD, HK, and SM designed the research. HR, DA, and AL contributed to the bibliographical research. HM contributed to the figures' formation. JD wrote the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was conducted in accordance with the declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a health insurance portability and accountability Act. we know of no conflicts of interest associated with this publication.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dounia, J., Kamal, H., Marouane, S. et al. A therapeutic challenge relating to the association of orthostatic hypotension and supine hypertension in a patient with cardiac autonomic neuropathy: a case report. J Med Case Reports 18, 102 (2024). https://doi.org/10.1186/s13256-024-04346-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04346-0