Abstract
Objective
To investigate whether commercially available deep learning (DL) software improves the Prostate Imaging-Reporting and Data System (PI-RADS) scoring consistency on bi-parametric MRI among radiologists with various levels of experience; to assess whether the DL software improves the performance of the radiologists in identifying clinically significant prostate cancer (csPCa).
Methods
We retrospectively enrolled consecutive men who underwent bi-parametric prostate MRI at a 3 T scanner due to suspicion of PCa. Four radiologists with 2, 3, 5, and > 20 years of experience evaluated the bi-parametric prostate MRI scans with and without the DL software. Whole-mount pathology or MRI/ultrasound fusion-guided biopsy was the reference. The area under the receiver operating curve (AUROC) was calculated for each radiologist with and without the DL software and compared using De Long’s test. In addition, the inter-rater agreement was investigated using kappa statistics.
Results
In all, 153 men with a mean age of 63.59 ± 7.56 years (range 53–80) were enrolled in the study. In the study sample, 45 men (29.80%) had clinically significant PCa. During the reading with the DL software, the radiologists changed their initial scores in 1/153 (0.65%), 2/153 (1.3%), 0/153 (0%), and 3/153 (1.9%) of the patients, yielding no significant increase in the AUROC (p > 0.05). Fleiss’ kappa scores among the radiologists were 0.39 and 0.40 with and without the DL software (p = 0.56).
Conclusions
The commercially available DL software does not increase the consistency of the bi-parametric PI-RADS scoring or csPCa detection performance of radiologists with varying levels of experience.
Key points
-
Radiologists with varying experiences assigned the scores with and without the DL.
-
The radiologists infrequently changed their initial PI-RADS scores.
-
The DL software did not improve the PI-RADS scoring consistency.
-
The DL software did not improve the performance in identifying csPCa.
Similar content being viewed by others
Introduction
Magnetic resonance imaging (MRI) is the backbone imaging modality for assessing prostate cancer (PCa). The role of MRI in evaluating PCa has recently heightened as the new evidence suggests the benefits of pre-biopsy MRI in all men with a suspicion of PCa [1,2,3]. As the importance of MRI in PCa diagnosis increased, the Prostate Imaging-Reporting and Data System (PI-RADS) and its following versions were introduced to bring standardization [4]. The PI-RADS provides guidelines for acquiring and interpreting prostate MRI, and the benefits of the system have been demonstrated in large-scale multi-center studies [5]. However, despite the PI-RADS, there are still non-negligible intra-reader and inter-reader differences in interpreting prostate MRI [6]. Furthermore, the inconsistencies appear to be more prominent with the less-experienced readers, hindering the standardization efforts [6].
Deep learning (DL) has shown remarkable performance on prostate MRI in recent years, including PCa detection, classification, and segmentation [7,8,9]. Nevertheless, a few studies have explicitly investigated whether DL benefits in standardizing the PI-RADS scoring among radiologists [10,11,12]. Further, prior studies have used in-house algorithms or prototype DL software; hence, there is a need for evidence for the yields of regulatory body-approved commercially available DL software in standardizing the PI-RADS scores and improving the performance of radiologists in identifying clinically significant PCa (csPCa).
The aims of this study were twofold: First, to investigate whether the commercially available DL software increases the PI-RADS scoring consistency on bi-parametric MRI among radiologists with various experience levels; Second, to assess whether the DL software improves the performance of radiologists in identifying csPCa.
Methods
Acibadem University Review board approved this retrospective study (ID: 2022-05/08) and waived the need for informed consent for the retrospective analysis of anonymized medical data. We reviewed consecutive patients who underwent a prostate MRI scan due to suspicion of PCa (i.e., increased prostate-specific antigen or suspicious digital rectal examination) or active surveillance between January 2019 and December 2020.
The inclusion criteria were the followings: (1) having whole-mount pathology or biopsy for patients with a PI-RADS ≥ 3 score assigned during routine clinical reading; (2) having a prostate MRI scan obtained at 3 T without an endorectal coil following PI-RADS version 2; and (3) ≥ 18 months of follow-up without any clinical, laboratory, or imaging evidence of PCa for patients with a PI-RADS score ≤ 2 [13].
The following patients were excluded from the study: (1) patients who underwent prostate MRI at 1.5 T; (2) patients who underwent prostate MRI with an endorectal coil; (3) patients with PI-RADS ≥ 3 examinations without any histopathological confirmation; and (4) history of any treatment for PCa.
MRI acquisitions
All patients underwent prostate MRI on one of our 3 Tesla MRI units (Vida or Skyra, Siemens Healthcare) using an 18-channel phased-array surface coil. The MRI protocol was consistent with PI-RADS version 2, as version 2.1 was unavailable during the study period [4]. To minimize bowel movements, Butylscopolamine bromide (Buscopan, Bohringer Ingelheim) was given to the patients.
The bi-parametric prostate MRI protocol encompassed tri-planar T2-weighted imaging and diffusion-weighted imaging. The diffusion-weighted imaging was performed with echo-planar imaging in axial planes at b-values of 0, 50, 500, and 1000 s/mm2. We excluded dynamic contrast-enhanced images since the DL software could not process them. The detailed parameters of the MRI protocol are given in Table 1.
DL software
The DL software (Prostate AI, Version Syngo.Via VB60, Siemens Healthcare) used in this study has three modules: (i) preprocessing module, (ii) DL-based lesion detection module, and (iii) DL-based lesion classification module. In this study, we did not perform any model training or fine-tuning and only used the model for performance testing.
Preprocessing module
The preprocessing module parses the DICOM files to select the axial T2-weighted and DWI with various b-values (e.g., 0 s/mm2 and 800 s/mm2). Then, the preprocessing module computes the ADC maps and synthetic DWI with a b-value of 2000 s/mm2 using a linear least-square fitting with all acquired b-values (i.e., b-values of 0, 50, 500, and 1000 s/mm2 for this study). Afterward, it performs prostate segmentation on T2-weighted images using a DL method proposed by Yang et al. [14] and rigid registration of T2-weighted and DWI.
DL-based lesion detection module
Preprocessed images are propagated into the DL-based lesion detection module. This module has two subcomponents: (1) DL-based lesion candidate detection model and (2) multi-scale false-positive reduction network.
DL-based lesion candidate detection model is a simple 2D U-Net consisting of descending and ascending pathways inter-connected with skip connections at different levels and convolutional blocks at the bottom, resembling a U shape. This model takes 3D volumes of ADC, DWI with a b-value of 2000 s/mm2, and T2-weighted images but processes them slice by slice. The model outputs 2D heatmaps fused to create 3D connected components (i.e., lesion candidates). The detected lesion candidates then propagated into the false-positive reduction model.
The false-positive reduction model is a 2.5D multi-scale deep network previously trained and validated on radiologists-annotated 2170 bi-parametric prostate MRI scans from 7 institutions. The model takes the patches of ADC, DWI, and T2-weighted images of lesion candidates provided by the DL-based lesion candidate detection model.
A 2D DL model can assess the in-plane information within an image (i.e., x and y axes), while it cannot capture the out-of-plane information (i.e., z-axis). Given that the prostate images contain relevant information in the x, y, and z axes, it is essential to consider the information of all planes in evaluating prostate MRI, particularly for eliminating false-positive lesions. Hence, the false-positive reduction model takes two adjacent slices of a 2D input slice as additional channels, making it a 2.5D network. For instance, a T2-weighted image harboring a lesion is fed to the model along with a slice above and below it. This design allows the network to capture the information z-axis and improves consistency and performance. At the same time, it mitigates the need for using fully 3D DL networks, which are resource intensive. In addition, this model is fed by prostate images with a varying field of view (i.e., multi-scale) to empower the model in capturing additional contextual information.
DL-based lesion classification module
The final module of the DL software is the lesion classification module. This module takes the lesion candidates offered by the preceding lesion detection module and provides the PI-RADS scores of the lesion, if present, as PI-RADS 3, 4, or 5, and highlights the lesions on the axial T2-weighted images. Supplementary Document S1 illustrates the components of the DL software. A further detailed description of the DL software can be found in Yu et al. [15].
Radiologists reading
Four radiologists with varying experience levels interpreted the scans with and without the DL software on a dedicated workstation (Syngo.Via, Siemens Healthcare) equipped with a 6-megapixel diagnostic color monitor (Radiforce RX 660, EIZO). All reviewed images were in Digital Imaging and Communications in Medicine (DICOM) format. The first reader was a radiologist with > 20 years of experience. The remaining three radiologists had 5, 3, and 2 years of prostate MRI experience and were routinely interpreting less than 50 prostate MRI scans yearly (hereafter, these radiologists were denoted as less-experienced radiologists 1, 2, and 3, respectively). All radiologists were briefly instructed about the software before the reading.
The radiologists evaluated the scans following PI-RADS version 2, as the DL software used in this study was developed following PI-RADS version 2. With multiparametric prostate MRI, PI-RADS 3 lesions of the peripheral zone showing focal or early contrast-enhancement are upgraded to PI-RADS 4 (i.e., PI-RADS 3 + 1) following PI-RADS version 2 [4]. However, as the contrast-enhanced sequences are not available in bi-parametric MRI, lesions of the peripheral gland are scored using only the diffusion-weighted sequences. Thus, in this study, none of the PI-RADS 3 lesions of the peripheral zone were upgraded to a higher score.
In the initial readings, the radiologists were provided with bi-parametric MRI scans including high b-value DWI and asked to identify the index lesion (i.e., the lesion with the highest PI-RADS score or the largest lesion if there were ≥ 2 lesions with the same score). First, the radiologists marked the index lesion with its PI-RADS score using the standard prostate reading template [4]. Then the radiologists were provided with the decision of DL software overlaid on a T2-weighted image and asked to re-evaluate the scans to assess whether they changed their initial PI-RADS score. Likewise, the PI-RADS scores of the radiologists with the DL software were recorded in the same template. Supplementary Document S2 shows how radiologists read the cases with and without the DL software step by step.
Whole-mount histopathology and biopsy
All biopsy procedures involved a combination of transrectal 12-core systematic and 3–4-core MRI/ultrasound fusion-guided biopsies (Artemis, Eigen) following up-to-date evidence [16]. Biopsy and whole-mount specimens were prepared and evaluated by a genitourinary pathologist with over 20 years of experience following international guidelines [16]. The lesion with the highest Gleason score was defined as the index lesion. A lesion with a Gleason score ≥ 3 + 4 was defined as a clinically significant PCa following the 2014 International Society of Urological Pathology consensus [17].
Statistical analysis
The statistical analyses were performed using the SciPy library of the Python programming language. The continuous variables are presented using the mean and standard deviations with the minimum and maximum; the categorical and ordinal variables are presented with frequencies and percentages. The PI-RADS scores of the radiologists were calculated and compared on a scan level. The inter-rater agreement among the radiologists in PI-RADS scoring with and without the DL software was evaluated using Fleiss’ kappa [18]; the pair-wise inter-rater agreements were investigated using linearly weighted Cohen’s kappa [19]. The kappa scores were interpreted as follows: a kappa score of < 20, a poor agreement; 21–40, a fair agreement; 41–60, a moderate agreement; 61–80, a good agreement; and 81–100, an excellent agreement. The kappa scores were compared following the prior work [20]. We calculated the area under the receiver operating curve (AUROC) in assessing csPCa and compared the AUROCs using DeLong’s test. A p value less than 0.05 was accepted as significant.
Results
In all, 153 men with a mean age of 63.59 ± 7.56 years (range 53–80) were enrolled in the study. The mean prostate-specific antigen level of the men was 6.42 ± 3.87 ng/ml (range 2–24).
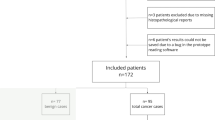
Of 153 men, 113 (75.16%) had a histopathology result, with 45 (29.41%) having clinically significant PCa identified by whole-mount pathology superseding the biopsy, 31 (20.26%) had nonsignificant PCa identified by whole-mount pathology (n = 5, 3.26%) or biopsy (n = 26, 16.99%), and 39 (25.49%) had a benign disease as identified by biopsy. The remaining 38 men (24.83%) had a PI-RADS score of ≤ 2 on MRI and ≥ 18 months of follow-up without any clinical, laboratory, or imaging evidence of PCa (Fig. 1).
The inter-rater agreement among the radiologist with and without the DL software
The PI-RADS scores assigned by the radiologists with and without DL are given in Fig. 2. Notably, the radiologists changed their initial PI-RADS scores in 1/153 (0.65%), 2/153 (1.3%), 0/153 (0%), and 3/153 (1.9%) of the patients with the DL software.
Fleiss’ kappa Score among the radiologists without the DL software was 0.39, equating to a fair agreement. Fleiss’ kappa Score among the radiologists increased from 0.39 to 0.40 with the DL software, not representing a significant difference (p = 0.56). The pair-wise kappa scores among radiologists with and without the DL software are shown in Fig. 3. Figures 4 and 5 show representative patients with clinically significant PCa.
Radiologists and the DL software in assigning PI-RADS scores. A 64-year-old man with prostate adenocarcinoma with a Gleason Score of 4 + 3 in the right posterolateral peripheral gland at the mid-prostatic level. An axial T2-weighted imaging (a), apparent diffusion coefficient map (b), diffusion-weighted imaging with a high b-value (c), and deep learning decisions overlaid on T2-weighted imaging with a heatmap (d) are shown. The radiologists scored this lesion as PI-RADS 5 with and without the DL software
Radiologists and the DL software in assigning PI-RADS scores. A 55-year-old man with clinically significant prostate adenocarcinoma with a Gleason Score of 4 + 3 in the left posterior peripheral gland at the basal level. An axial T2-weighted imaging (a), apparent diffusion coefficient map (b), diffusion-weighted imaging with a high b-value (c), and deep learning decisions overlaid on T2-weighted imaging with a heatmap (d) are shown. All radiologists scored PI-RADS 5 for the index lesion. However, the deep learning software failed to identify the index lesion
The performance of the radiologists in identifying csPCa with and without DL software
The AUROCs of the experienced radiologist, less-experienced radiologist 1, less-experienced radiologist 2, and less-experienced radiologist 3 without the DL software were 0.917 (95% CI 0.878–0.957), 0.847 (95% CI 0.785–0.909), 0.81 (95% CI 0.733–0.883), 0.782 (95% CI 0.702–0.862). The AUROC of the standalone DL software was 0.756 (95% CI 0.671–0.842). The AUROCs of the experienced radiologist, less-experienced radiologist 1, less-experienced radiologist 2, and less-experienced radiologist 3 with the DL software were 0.917 (95% CI 0.878–0.957), 0.864 (95% CI 0.806–0.921), 0.81 (95% CI 0.733–0.883), and 0.789 (95% CI 0.710–0.868). Figure 6 shows the ROC curves of the radiologists with and without the DL software in predicting clinically significant PCa.
The AUROCs of the experienced radiologist and less-experienced radiologist 1 were significantly higher than that of the DL software (p < 0.0001 and p = 0.04). In contrast, the AUROCs of the remaining less-experienced radiologists 2 and 3 did not significantly differ from that of the DL software (p = 0.63 and p = 0.23). The AUROCs of radiologists in identifying csPCa with and without the DL software did not differ for radiologists (p > 0.05).
Discussion
This study investigated whether DL improves the consistency and performance of radiologists with various levels of experience in assessing bi-parametric prostate MRI. In this study, there was a fair agreement between the radiologists in assigning the bi-parametric PI-RADS scores, and the inter-rater agreement among radiologists did not significantly increase using the DL software. Overall, the radiologists changed their initial PI-RADS scores in ~ 1% of the scans with the DL software, and radiologists with ≥ 5 years of experience provided a statistically higher performance in identifying csPCa than the DL software. Furthermore, the DL software did not improve radiologists’ performance in identifying csPCa.
In this study, the DL software used PI-RADS 3 sparingly while mainly allocating scans as negative or highly suspicious of cancer (i.e., PI-RADS score of ≥ 4). A similar trend was also reported by prior research using the prototype version of the DL software [10, 21]. In contrast, the experienced radiologist assigned a PI-RADS score of 3 to about a quarter of the patients in the present work. Likewise, a recent meta-analysis pooled data across 26 centers showed that approximately 30% of the lesions were assigned a PI-RADS score of 3 by radiologists [5]. We suggest that the potential underlying factors that lead the DL software to assign PI-RADS 3 to only a minority of the patients and whether this tendency is beneficial (e.g., sparing patients from unnecessary biopsy or identifying clinically insignificant cancers) should be investigated in future work.
The experienced radiologist provided an AUROC of 0.917 in identifying significant PCa, compatible with the literature [5, 21]. On the other hand, standalone DL software had a worse performance, with an AUROC of 0.756 in the same task. Furthermore, the radiologists with 5 years of experience also performed better than the DL software, while the radiologist with 3 and 2 years of experience provided a similar performance. These findings might imply that the DL software used in this study might be at the same level as a radiologist with ≤ 3 years of experience, while it fails to match the performance of radiologists with more experience.
At first glance, the low performance of DL software appears to contradict the results from the earlier studies using the prototype version of the same DL software [11, 21]. However, prior studies tested the DL software on in-distribution or ProstateX data containing relatively straightforward cases. Though our MRI scans were obtained with the same manufacturer’s scanner, it might represent out-of-distribution data for the DL software, eventually impairing its performance. Furthermore, our findings, to some extent, align with those from the study by Youn et al., where the authors found that the prototype version of the DL software had a performance between an expert radiologist and radiology residents [21].
Many recent studies have claimed that DL could surpass human radiologists in identifying clinically significant PCa [22,23,24,25,26]. Nevertheless, in a recent large-scale multi-center study, Hosseinzadeh criticized earlier studies and suggested that small test sizes and the comparison with local radiologists’ performance might lead to overestimating the performance of DL [27]. The authors stated that those prostate DL models trained less than 1 k scans and pointed out that a DL model with an expert-level performance could only be achieved by training over ≥ 35 k scans for lung cancer detection on CT and 90 ≥ scans for breast cancer detection on mammography [28, 29]. However, the commercial DL software used in this study trained slightly over 2 k scans [10].
Considering the current DL models that were trained on relatively small data, expecting an expert-level performance might be too optimistic at this moment. Further, despite its drawbacks regarding consistency, the benefits of the PI-RADS scores assigned by human radiologists are much more rigorously documented than DL in PCa diagnostics [5]. Thus, we suggest that creating a DL software that can replace human radiologists might be a longer-term goal, while designing DL models that improve and standardize the PI-RADS scores among radiologists seems to be a more reachable target [30].
Apart from the present work, few other studies specifically investigated the benefits of DL in the context of PI-RADS scoring consistency. For example, in their large-scale study, Sanford et al. evaluated the inter-rater agreement between their in-house deep learning model, U-net, and human experts [12]. The authors documented a moderate level of agreement between the software and experts. However, unlike the present work, the authors did not investigate whether prostate MRI reading with the DL model improves the inter-rater agreement.
Winkel et al. investigated the prototype version of the DL software used in the present work in two consecutive studies [10, 11]. Their preliminary study was small-scale and only examined the inter-rater agreement between radiology reports and DL software, yielding a kappa score of 0.42. Their subsequent study used a similar methodology to the present work, and radiologists with various levels of experience performed prostate MRI readings with and without the DL software. In contrast to the present work, the DL software improved the inter-rater agreement in their research. However, the authors binarized the PI-RADS scores using the cut-off values of PI-RADS 3 and 4. Using PI-RADS ≥ 3 cut-off threshold, the inter-rater agreement increased from kappa of 0.33 to 0.41 with the DL software, while it was increased from kappa of 0.22 to 0.36 using a PI-RADS ≥ 4 cut-off threshold.
This study had several drawbacks that should be acknowledged. First and foremost, the sample size was relatively small, covering prostate MRI scans obtained with the same manufacturer’s 3 T scanner from a single tertiary center.
Second, we used bi-parametric MRI since the DL software used in this study does not use dynamic contrast-enhanced images. Though the performance of bi-parametric MRI is on par with multiparametric MRI [31], various guidelines [32,33,34], including the PI-RADS [4], still recommend multiparametric MRI over bi-parametric prostate MRI. Thus, the results of the study might not be applicable to routine practice where the multiparametric MRI is routinely implemented for patient care. Likewise, we put PI-RADS scores 1 and 2 in the same category (i.e., negative scans) as the DL software does not discriminate between them.
Third, some patients with PI-RADS scores 1 and 2 did not have histopathology in our sample. Nevertheless, these patients had at least 18 months of follow-up data, and the same approach was also followed in the recent large-scale prostate cancer detection challenge [13].
Fourth, we only evaluated whether the DL software improves the diagnostic accuracy in detecting csPCa and the consistency of radiologists with varying experience. We admit that this might downplay the role of DL in prostate diagnostics. For instance, DL may help to reduce prostate MRI reading time, lessening the daily workload of radiologists [11, 30].
Conclusions
In contrast to most earlier studies, the commercially available DL software did not improve the PI-RADS scoring or csPCa detection performance of radiologists with varying levels of experience on external single-center data, potentially suggesting a drop in the performance due to a domain shift. Though we suggest that prostate MRI practitioners should consider a potential drop in the performance using the version of the DL software in clinical practice, the potential benefits of the DL, such as improving efficiency, should not be overlooked. In subsequent studies, we plan to investigate the benefits of the DL software in reading efficiency and confidence, along with the accuracy and consistency of a larger pool of radiologists from different centers on large-scale multi-center data. Furthermore, advancements in the DL technology, accompanied by larger and more representative training data, will likely improve the performance of the DL software in future versions, which we plan to investigate in future studies.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AUROC:
-
The area under the receiver operating curve
- csPCa:
-
Clinically significant PCa
- DL:
-
Deep learning
- MRI:
-
Magnetic resonance imaging
- PCa:
-
Prostate cancer
- PI-RADS:
-
Prostate Imaging-Reporting and Data System
References
Ahdoot M, Wilbur AR, Reese SE et al (2020) MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 382:917–928. https://doi.org/10.1056/NEJMoa1910038
Ahmed HU, El-Shater Bosaily A, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822. https://doi.org/10.1016/S0140-6736(16)32401-1
Kasivisvanathan V, Rannikko AS, Borghi M et al (2018) MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 378:1767–1777. https://doi.org/10.1056/NEJMoa1801993
Turkbey B, Rosenkrantz AB, Haider MA et al (2019) Prostate imaging reporting and Data System Version 2.1: 2019 update of prostate imaging reporting and Data System Version 2. Eur Urol 76:340–351. https://doi.org/10.1016/j.eururo.2019.02.033
Westphalen AC, McCulloch CE, Anaokar JM et al (2020) Variability of the positive predictive value of PI-RADS for prostate MRI across 26 centers: experience of the society of abdominal radiology prostate cancer disease-focused panel. Radiology 296:76–84. https://doi.org/10.1148/radiol.2020190646
Smith CP, Harmon SA, Barrett T et al (2019) Intra- and interreader reproducibility of PI-RADSv2: a multireader study. J Magn Reson Imaging 49:1694–1703. https://doi.org/10.1002/jmri.26555
Yang X, Liu C, Wang Z et al (2017) Co-trained convolutional neural networks for automated detection of prostate cancer in multi-parametric MRI. Med Image Anal 42:212–227. https://doi.org/10.1016/j.media.2017.08.006
Wang X, Yang W, Weinreb J et al (2017) Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep 7:15415. https://doi.org/10.1038/s41598-017-15720-y
Ishioka J, Matsuoka Y, Uehara S et al (2018) Computer-aided diagnosis of prostate cancer on magnetic resonance imaging using a convolutional neural network algorithm. BJU Int 122:411–417. https://doi.org/10.1111/bju.14397
Winkel DJ, Wetterauer C, Matthias MO et al (2020) Autonomous detection and classification of pi-rads lesions in an MRI screening population incorporating multicenter-labeled deep learning and biparametric imaging: proof of concept. Diagnostics. https://doi.org/10.3390/diagnostics10110951
Winkel DJ, Tong A, Lou B et al (2021) A novel deep learning based computer-aided diagnosis system improves the accuracy and efficiency of radiologists in reading biparametric magnetic resonance images of the prostate. Invest Radiol 56:605–613. https://doi.org/10.1097/RLI.0000000000000780
Sanford T, Harmon SA, Turkbey EB et al (2020) Deep-learning-based artificial intelligence for PI-RADS classification to assist multiparametric prostate MRI interpretation: a development study. J Magn Reson Imaging 52:1499–1507. https://doi.org/10.1002/jmri.27204
The PI-CAI Challlenge. https://pi-cai.grand-challenge.org. Accessed 8 Aug 2022
Yang D, Xu D, Zhou KS et al (2017) Automatic liver segmentation using an adversarial image-to-image network. In: Franz A, Jannin P et al (eds) Maier-Hein lena. Springer, Quebec City, pp 507–515
Yu X, Lou B, Shi B et al (2020) False positive reduction using multi-scale contextual features for prostate cancer detection in multi-parametric MRI scans. In: 2020 IEEE 17th international symposium on biomedical imaging (ISBI). IEEE, pp 1355–1359
American Urologic Association (2022) Optimal techniques of prostate biopsy and specimen handling. http://www.auanet.org/guidelines/prostate-biopsy-and-specimen-handling
Samaratunga H, Montironi R, True L et al (2011) International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 1: specimen handling. Mod Pathol 24:6–15. https://doi.org/10.1038/modpathol.2010.178
Fleiss JL, Levin B, Paik MC (2013) Statistical methods for rates and proportions. Wiley
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46. https://doi.org/10.1177/001316446002000104
Vanbelle S (2017) Comparing dependent kappa coefficients obtained on multilevel data. Biom J 59:1016–1034. https://doi.org/10.1002/bimj.201600093
Youn SY, Choi MH, Kim DH et al (2021) Detection and PI-RADS classification of focal lesions in prostate MRI: performance comparison between a deep learning-based algorithm (DLA) and radiologists with various levels of experience. Eur J Radiol 142:109894. https://doi.org/10.1016/j.ejrad.2021.109894
Park KJ, Choi SH, Kim M et al (2021) Performance of prostate imaging reporting and Data System Version 2.1 for diagnosis of prostate cancer: a systematic review and meta-analysis. J Magn Reson Imaging 54:103–112. https://doi.org/10.1002/jmri.27546
Cuocolo R, Cipullo MB, Stanzione A et al (2020) Machine learning for the identification of clinically significant prostate cancer on MRI: a meta-analysis. Eur Radiol 30:6877–6887. https://doi.org/10.1007/s00330-020-07027-w
Schelb P, Kohl S, Radtke JP et al (2019) Classification of cancer at prostate MRI: deep learning versus clinical PI-RADS assessment. Radiology 293:607–617. https://doi.org/10.1148/radiol.2019190938
Schelb P, Wang X, Radtke JP et al (2021) Simulated clinical deployment of fully automatic deep learning for clinical prostate MRI assessment. Eur Radiol 31:302–313. https://doi.org/10.1007/s00330-020-07086-z
Aldoj N, Lukas S, Dewey M, Penzkofer T (2020) Semi-automatic classification of prostate cancer on multi-parametric MR imaging using a multi-channel 3D convolutional neural network. Eur Radiol 30:1243–1253. https://doi.org/10.1007/s00330-019-06417-z
Hosseinzadeh M, Saha A, Brand P et al (2022) Deep learning–assisted prostate cancer detection on bi-parametric MRI: minimum training data size requirements and effect of prior knowledge. Eur Radiol 32:2224–2234. https://doi.org/10.1007/s00330-021-08320-y
Ardila D, Kiraly AP, Bharadwaj S et al (2019) End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med 25:954–961. https://doi.org/10.1038/s41591-019-0447-x
McKinney SM, Sieniek M, Godbole V et al (2020) International evaluation of an AI system for breast cancer screening. Nat 577:89–94. https://doi.org/10.1038/s41586-019-1799-6
Padhani AR, Turkbey B (2019) Detecting prostate cancer with deep learning for MRI: a small step forward. Radiol 293:618–619. https://doi.org/10.1148/radiol.2019192012
Choi MH, Kim CK, Lee YJ, Jung SE (2019) Prebiopsy biparametric MRI for clinically significant prostate cancer detection with PI-RADS Version 2: a multicenter study. AJR Am J Roentgenol 212:839–846. https://doi.org/10.2214/AJR.18.20498
London: National Institute for Health and Care Excellence (NICE) (2021) 2021 exceptional surveillance of prostate cancer: diagnosis and management (NICE guideline NG131)
Brown J, Haider MA, Chin JLK et al (2022) Evidence-based guideline recommendations on multiparametric magnetic resonance imaging in the diagnosis of clinically significant prostate cancer: a Cancer Care Ontario updated clinical practice guideline. Can Urol Assoc J 16:16–23. https://doi.org/10.5489/cuaj.7425
de Rooij M, Israël B, Tummers M et al (2020) ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists’ training. Eur Radiol 30:5404–5416. https://doi.org/10.1007/s00330-020-06929-z
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Each author has made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data. Each author has approved the submitted version (and any substantially modified version that involves the author’s contribution to the study). Each author has agreed to be personally accountable for the author’s contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. A.A.: Manuscript editing, Study design; D.A.: Manuscript writing, Study design; S.E.: Manuscript editing; M.E.S.: Statistical analysis, Data curation; G.Z.: Data curation; S.S.: Data curation; S.K.: Critical review; E.K.: Study design, Critical review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants followed the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The reviewer board waived the need for informed consent.
Consent for publication
Written informed consent was not required because it was related to identification imaging only, and patient anonymity was maintained.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arslan, A., Alis, D., Erdemli, S. et al. Does deep learning software improve the consistency and performance of radiologists with various levels of experience in assessing bi-parametric prostate MRI?. Insights Imaging 14, 48 (2023). https://doi.org/10.1186/s13244-023-01386-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-023-01386-w