Abstract
Purpose
Immunoglobulin replacement therapy is a standard treatment for patients with antibody production deficiencies, which is of interest in patients with chronic obstructive pulmonary disease (COPD). This systematic review, registered with PROSPERO (CRD42021281118), assessed the current literature regarding immunoglobulin replacement therapy on COPD clinical outcomes in patients with low immunoglobulin G (IgG) serum concentrations.
Methods
Literature searches conducted from inception to August 23, 2021, in databases including MEDLINE, EMBASE, and CINAHL. Population (sex, age, comorbidities), baseline clinical characteristics (pulmonary function testing results, IgG levels), and outcome (hospitalizations, emergency department visits) were extracted after title/abstract and full text screening. The Cochrane risk of bias assessment form was used for risk of bias assessment of randomized controlled trials and the National Heart, Lung, and Blood Institute (NHLBI) assessment was used for pre and post studies.
Results
A total of 1381 studies were identified in the preliminary search, and 874 records were screened after duplicates were removed. Screening 77 full texts yielded four studies that were included in the review.
Conclusion
It is unclear whether immune globulin replacement therapy reduces acute exacerbation frequency and severity in COPD. Current evidence suggests that it is worth considering, but better developed protocols for administration of immune globulin supplementation is required for future randomized controlled trials.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory condition characterized by airflow limitation, and it is one of the leading causes of death worldwide [1, 2]. Approximately 212.3 million people globally were reported to have COPD in 2019, and the prevalence is increasing [3]. COPD is marked by progressive chronic symptoms and episodic acute exacerbations (AECOPD) that further impair quality of life, worsen lung function [4], and increase the risk of mortality [5].
The most common aetiologies of AECOPD are respiratory tract infections followed by eosinophilic airway inflammation, and both occur simultaneously in some patients [6,7,8,9]. The most frequently implicated pathogens in AECOPD are bacteria including Haemophilus influenzae, Streptococcus pneumoniae, and Moraexlla catarrhalis, and respiratory viruses including influenza and parainfluenza viruses, rhinoviruses, and coronaviruses. Strategies to reduce the burden of respiratory infections are thus of significant interest to prevent AECOPD [10].
Studies have shown that approximately 25% of COPD patients have reduced immunoglobulin G (IgG) serum concentrations [11], and that this finding is independently associated with AECOPD and hospitalization risk [12,13,14]. IgG serum concentrations and AECOPD severity also appear to be inversely related [15, 16]. Given the well-established role of IgG in host defense against respiratory infections, these data have raised the question of whether treatment of low IgG in this patient population may reduce AECOPD risk [17].
Immune globulin is a plasma-derived therapeutic product consisting of IgG obtained from thousands of blood donors [18]. Immune globulin therapy (IGT) is a well-established treatment modality to prevent infections in patients with primary and secondary antibody deficiencies [19]. Both intravenous (IVIG) and subcutaneous (SCIG) treatment significantly reduce the frequency and severity of bacterial respiratory infections in this patient population [15, 20]. However, IGT is expensive and adverse reactions are common among those receiving IVIG [21]. Therefore, it is imperative that the effectiveness of IGT is well validated prior to its regular usage as adjunct therapy in COPD.
We conducted a systematic review to understand whether IgG replacement therapy for patients with COPD and decreased serum IgG levels decreases AECOPD frequency.
Methods
The review was registered with PROSPERO (CRD42021281118) prior to initiation and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22].
Search strategy
Comprehensive searches were completed in the following databases from inception to August 23, 2021, to identify relevant publications: MEDLINE, Embase (Ovid interface), CINAHL Plus with Full Text (EBSCOhost interface), Cochrane Library Trials database (Wiley Interface), Scopus, Web of Science Core Collection, and Google Scholar (on August 27, 2021). The search used a combination of subject headings and relevant keywords related to COPD and IGT and was conducted by a health sciences librarian (LD) experienced in systemic review methodology (as detailed in Table 1). The following grey literature formats were included: conference abstracts (from Embase and Web of Science), clinical trial registry records (from Cochrane Trials), and publications from the Google Scholar search. The reference lists of relevant articles were screened, and authors of the articles of interest were contacted to ask for any relevant raw data. No language or publication types were restricted during the search.
Study selection
Two reviewers (JK and HV) independently screened the titles and abstracts retrieved from the searches for relevance. Studies were eligible for inclusion if the included COPD patient populations have documented decreased serum IgG levels (serum IgG levels < 7/gL). Studies that include immunoglobulin replacement therapy (IVIG or SCIG) in addition to usual medical treatment for COPD (i.e. bronchodilators, inhaled steroids, antibiotics, oral steroids) were included. Studies with no outcome measure of COPD severity and symptoms and patient populations with asthma-COPD overlap syndrome (ACOS) were excluded. The primary outcomes included AECOPD frequency, hospitalizations and emergency department (ED) visits for AECOPD, and mortality from AECOPD events. The screening process was completed in Covidence. The full texts of relevant studies were then independently reviewed, and study authors were contacted for additional information on patient populations and outcomes, as required. A consensus was reached by discussion for to resolve all conflicts, which was managed by Covidence (Additional file 1).
Risk of bias
Two reviewers (JK and AA) independently assessed the risk of bias of all included studies. The Cochrane risk of bias assessment was used for randomized controlled trials (RCT) [23]. Risk of bias categories included low risk, high risk, or unclear risk of bias for participant random allocation, patient selection, and outcome assessment. For before and after studies, the risk of bias assessment tools from the NHLBI was used [24]. Sources of bias were graded, and the differences in bias assessment were settled through a discussion until consensus was reached.
Data extraction and synthesis of results
Data was independently extracted by both reviewers (JK and HV), and the items to be reported were finalized by consensus. Data extraction tables were created from the extracted raw data in Microsoft Excel. The data tables include specific study characteristics (study design, enrollment dates for treatment, location, funding, conflicts of interest), patient demographics (sample size, method of recruitment, sex, mean age, smoking history, and comorbidities), intervention (IgG route of administration and dosage), and clinical baseline characteristics and outcomes (pulmonary function testing (PFT) results, Ig levels). Authors of two studies with incomplete or unclear data were contacted but did not respond. Studies with incomplete or unclear data were not included in the final data extraction.
Due to the heterogeneity of the population characteristics, comparisons made, and reported outcomes, and the lack of homogeneity in quantitative outcomes, the systematic review without meta-analysis (SWIM) guidelines were used for reporting [25].
Results
Study selection and individual study characteristics
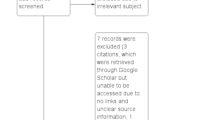
The PRISMA flow diagram summarizes the results of the study selection process (Fig. 1). A total of 1381 references were identified after a comprehensive literature search. Removing the duplicates resulted in 874 unique abstracts being screened for relevance, and 77 articles were assessed for full-text eligibility. We excluded 73 records based on the following exclusion criteria: wrong patient population (n = 15), wrong study design (n = 47), wrong intervention (n = 2), wrong comparator (n = 2), wrong outcomes (n = 2), no translation of the abstract or article in English (n = 2), and studies not reporting that the patients had hypogammaglobulinemia (n = 3).
Studies with COPD patients or patients with hypogammaglobulinemia as a subpopulation of a larger target population that did not report individual outcomes specific to the subpopulation above were excluded. Authors of these studies were contacted but did not respond. Initially, ten studies and abstracts were included. Three studies were excluded as the patients were reported to have specific antibody deficiency (SAD), or insufficient data was provided for whether the patients had hypogammaglobulinemia before starting IgG replacement therapy [26,27,28]. One study reported on outcomes of chronic pulmonary disease which presents itself phenotypically different from COPD due to causal factors such as common variable immunodeficiency (CVID) [29]. Another two studies were conducted with a subpopulation of patients with COPD and hypogammaglobulinemia, but the specific outcomes were not reported [30, 31]. Authors for these studies were contacted to obtain raw data, but the data was not provided.
Four studies were included after full text review. Two studies were retrospective case series, two were retrospective chart reviews (one of which was available in conference abstract form only) and one was a RCT [32,33,34,35]. The studies were published between 2010 and 2021, two studies published in Canada [33, 35], and two in the United States [32, 34]. The patient population all had COPD and had hypogammaglobulinemia with a significant proportion of patients from two studies had CVID [32, 34]. The excluded studies and the reasons for their exclusion are available upon request. A detailed description of the included studies is provided in Table 2, and the specific outcomes measured for each study are found in Table 3.
In three of the four included studies, baseline clinical characteristics consisting of PFT results, Ig levels, and other specific AECOPD parameters (hospitalizations for AECOPD events, ED visits, total exacerbations) showed poor lung health, as expected. The main outcomes of interest varied extensively between studies as recorded in Table 2. Baleeiro and Mull qualitatively explained an improvement in symptoms and decrease in frequency of exacerbations [32]. The three other studies quantified changes in AECOPD frequency associated with IRT by assessing hospitalizations, ED visits, and/or systemic glucocorticosteroid prescriptions [33,34,35].
Risk of bias results
The risk of bias for the RCT was high and it was deemed as poor quality using the Cochrane risk of bias assessment tool due to its poor treatment adherence [23, 35]. One study, which was available only in abstract form, also had a high risk of bias as assessed using the NHLBI assessment tool as the study design and outcomes data provided were limited [24, 32]. Two studies had a low risk of bias and were assessed as good quality studies using the NHLBI tool [24, 33, 34]. Figure 2a, b and Table 4 outline the assessment of risk of bias for each study in more depth.
Discussion
Using a rigorous systematic review process, we identified four studies that evaluated COPD outcomes after IGT. The main objective was to determine if COPD patients with low IgG serum concentration would benefit from IGT to prevent AECOPD.
One pilot placebo controlled RCT designed to address this question was identified [35]. Seventy patients were randomized to receive IVIG or placebo (1:1) for 48 weeks. The patients had very severe baseline airflow limitation with an average FEV1 < 1L. The authors found no difference in the frequency of all AECOPD or AECOPD needing hospitalization between the placebo and treatment groups. There was a trend of improvement in the time-to-first AECOPD event. No difference in pulmonary function measures was seen between the treatment and placebo groups.
These findings controverted prior observational studies that found a significant reduction in the frequency of moderate and severe AECOPD [32,33,34]. However, these observational studies were limited by their retrospective design, small sample sizes, and possible referral bias as they were conducted in conjunction with immunodeficiency clinics. The observational studies also included a higher proportion of patients with moderate versus severe airflow obstruction compared to the RCT, two included patients with comorbid post-infectious bronchiectasis [32, 33] (a possible independent marker of an antibody deficiency syndrome), and one only included patients with demonstrable impairment in specific antibody production [34].
There was significant heterogeneity in reported outcomes across the studies. This issue is well known in the COPD research community and has spurred movements such as the DisEntangling Chronic Obstructive Pulmonary Disease Exacerbations clinical trials NETwork (DECODE-NET) [36], which has proposed a core outcome set to improve standardization [37]. Hospitalizations and ED visits were more consistently reported in the studies identified, but these may also not be the most informative metrics. A systematic review found that time-to-event may be a more suitable outcome parameter than length or frequency of hospitalization stays due to extraneous factors like social circumstances or comorbidities [38]. Additionally, patient reported outcomes reflecting symptom burden and quality of life, should also be included [37].
Given the paucity of data in this area, further studies are greatly needed. An important finding of the RCT was that treatment adherence for both the IVIG and control groups was low—68.8 ± 5.7% and 59.4 ± 6.3%, respectively [35]. This observation indicates that additional trials with this design may be challenging to conduct. Future studies should thus consider using SCIG instead of IVIG as it is better tolerated, does not require venous access, and is more cost effective [21].
Improved patient selection may also be critical for the successful use of IRT in patients with COPD and reduced serum IgG. First, given the issues with treatment adherence and cost, IRT studies should particularly target COPD patients who have recurrent AECOPD despite maximal therapy including combined glucocorticosteroids long-acting muscarinic antagonists, and long-acting beta-agonist inhaler [39]. Second, biomarkers that predict the type of AECOPD for which an individual is at risk may be important. For example, patients with type 2 airway inflammation evidenced by blood eosinophils > 300/µL may represent a distinct pathophysiological subgroup [6] that is better managed with anti-type 2 biologicals such as dupilumab [40]. COPD patients with recurrent infective exacerbations due to Haemophilus influenzae and Moraxella catarrhalis may derive benefit from chronic azithromycin [41], and a trial of azithromycin may be warranted prior to trying IRT. Third, assessment of specific antibody production by measuring vaccine response to peptide and polysaccharide vaccines may better identify COPD patients with defective humoral immunity. Memory B cell enumeration by flow cytometry may also be useful, particularly to differentiate between low IgG from systemic glucocorticosteroid use and impaired IgG production [42]. However, these measures will increase the cost and complexity of patient evaluation. Fourth, it is possible that patients with moderate rather than severe airflow obstruction would benefit most from IRT. Structural changes of the lungs, including advanced airway remodelling and emphysematous destruction of the pulmonary parenchyma rather than defective adaptive immunity, may be the main driver of increased infection susceptibility in patients with advanced COPD.
Conclusion
There is currently insufficient evidence to support the routine use of IGT in COPD patients with low IgG to prevent acute exacerbations. The quality of currently available data is low due to poor IGT adherence, small sample sizes, heterogeneity of the populations studied, and incomplete outcome reporting. Additional well-designed, prospective studies are needed to address this important clinical question. Future studies should select COPD patients with recurrent exacerbations despite optimized therapy and use SCIG instead of IVIG given its better tolerability.
References
Romiti GF, Corica B, Pipitone E, et al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-analysis of 4,200,000 patients. Eur Heart J. 2021;42(35):3541–54. https://doi.org/10.1093/eurheartj/ehab453.
Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–32. https://doi.org/10.1183/09031936.06.00124605.
Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the global burden of disease study 2019. BMJ. 2022. https://doi.org/10.1136/bmj-2021-069679.
Adatia A, Wahab M, Shahid I, et al. Effects of cigarette smoke exposure on pulmonary physiology, muscle strength and exercise capacity in a retrospective cohort with 30,000 subjects. PLOS ONE. 2021. https://doi.org/10.1371/journal.pone.0250957.
2023 Gold Report—Global initiative for chronic obstructive lung disease. 2023. GOLD. https://goldcopd.org/2023-gold-report-2/. Accessed 1 Aug 2023.
Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(6):662–71. https://doi.org/10.1164/rccm.201104-0597oc.
Gutiérrez Villegas C, Paz-Zulueta M, Herrero-Montes M, Parás-Bravo P, Madrazo Pérez M. Cost analysis of chronic obstructive pulmonary disease (COPD): a systematic review. Health Econ Rev. 2021. https://doi.org/10.1186/s13561-021-00329-9.
Johnson KM, Bryan S, Ghanbarian S, Sin DD, Sadatsafavi M. Characterizing undiagnosed chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2018. https://doi.org/10.1186/s12931-018-0731-1.
Kjarsgaard M, Adatia A, Bhalla A, et al. Underestimation of airway luminal eosinophilia by quantitative sputum cytometry. Allergy Asthma Clin Immunol. 2021. https://doi.org/10.1186/s13223-021-00567-w.
Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–21. https://doi.org/10.1164/rccm.200506-859oc.
Palikhe NS, Niven M, Fuhr D, et al. Low immunoglobulin levels affect the course of COPD in hospitalized patients. Allergy Asthma Clin Immunol. 2023. https://doi.org/10.1186/s13223-023-00762-x.
Holm AM, Andreassen SL, Christensen VL, et al. Hypogammaglobulinemia and risk of exacerbation and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;15:799–807. https://doi.org/10.2147/copd.s236656.
Leitao Filho FS, Ra SW, Mattman A, et al. Serum IGG subclass levels and risk of exacerbations and hospitalizations in patients with COPD. Respir Res. 2018. https://doi.org/10.1186/s12931-018-0733-z.
Leitao Filho FS, Ra SW, Mattman A, et al. Serum IGG and risk of exacerbations and hospitalizations in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2017. https://doi.org/10.1016/j.jaci.2017.01.046.
Jolles S, Orange JS, Gardulf A, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179(2):146–60. https://doi.org/10.1111/cei.12485.
Cowan J, Mulpuru S, Alvarez G, Corrales-Medina V, Cameron DW. Chronic obstructive pulmonary disease exacerbation frequency and serum IGG Levels. J Allergy Clin Immunol. 2018;141(2):830–1. https://doi.org/10.1016/j.jaci.2017.09.036.
Moore BB, Moore TA, Toews GB. Role of T- and B-lymphocytes in pulmonary host defences. Eur Respir J. 2001;18(5):846–56. https://doi.org/10.1183/09031936.01.00229001.
Prevot J, Jolles S. Global immunoglobulin supply: Steaming towards the iceberg? Curr Opin Allergy Clin Immunol. 2020;20(6):557–64. https://doi.org/10.1097/aci.0000000000000696.
Compagno N, Malipiero G, Cinetto F, Agostini C. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 2014. https://doi.org/10.3389/fimmu.2014.00626.
Mouthon L, Lortholary O. Intravenous immunoglobulins in infectious diseases: where do we stand? Clin Microbiol Infect. 2003;9(5):333–8. https://doi.org/10.1046/j.1469-0691.2003.00694.x.
Ritchie B, Martins KJB, Tran DT, et al. Economic impact of self-administered subcutaneous versus clinic-administered intravenous immunoglobulin G therapy in Alberta, Canada: a population-based cohort study. Allergy Asthma Clin Immunol. 2022. https://doi.org/10.1186/s13223-022-00735-6.
Page MJ, McKenzie JE, Bossuyt PM, et al. The Prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/bmj.n71.
Julian HP, Savović J, Page MJ et al. 2023. Chapter 8: assessing risk of bias in a randomized trial. Cochrane Training. https://training.cochrane.org/handbook/current/chapter-08. Accessed 1 Aug 2023.
Study Quality Assessment Tools. 2021. NHLBI. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 1 Aug 2023.
Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWIM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. https://doi.org/10.1136/bmj.l6890.
Alachkar H, Anwar MA. The clinical effectiveness of immunoglobulin infusion therapy in specific antibody deficiency (SAD). J Clin Immunol. 2014. https://doi.org/10.1007/s10875-014-0101-9.
Barth JM, Jaeger D, Broder M. Is there a benefit of an additive standard immunoglobulin therapy for the clinical course of COPD patients under chronic immunosuppressive therapy? Eur Resp J. 2001;16(Suppl. 31). https://www.ers-education.org/lr/show-details/?idP=28766
Zhu J, Wan X. Role of immunoglobulin in treating chronic obstructive pulmonary disease with fungal infection. Chinese J Infect Control. 2014. https://doi.org/10.3969/j.issn.1671-9638.2014.05.007.
Gracia J, Vendrell M, Alvarez A, et al. Immunoglobulin therapy to control lung damage in patients with common variable immunodeficiency. Int Immunopharmacol. 2004;4(6):745–53. https://doi.org/10.1016/j.intimp.2004.02.011.
van Kessel DA, Hoffman TW, Velzen-Blad H, et al. Long-term clinical outcome of antibody replacement therapy in humoral immunodeficient adults with respiratory tract infections. EBioMedicine. 2017;18:254–60. https://doi.org/10.1016/j.ebiom.2017.03.025.
Stein MR, Koterba A, Rodden L, Berger M. Safety and efficacy of home-based subcutaneous immunoglobulin G in elderly patients with primary immunodeficiency diseases. Postgrad M J. 2011;123(5):186–93. https://doi.org/10.3810/pgm.2011.09.2474.
Baleeiro C, Mull N. Prevalence of common variable immunodeficiency (CVID) among patients with recurrent respiratory tract infections. Am J Respir Crit Care Med. 2010. https://doi.org/10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A3187.
Cowan J, Gaudet L, Mulpuru S, et al. A retrospective longitudinal within-subject risk interval analysis of immunoglobulin treatment for recurrent acute exacerbation of chronic obstructive pulmonary disease. PloS ONE. 2015. https://doi.org/10.1371/journal.pone.0142205.
McCullagh BN, Comellas AP, Ballas ZK, Newell JD, Zimmerman MB, Azar AE. Antibody deficiency in patients with frequent exacerbations of chronic obstructive pulmonary disease (COPD). PloS ONE. 2017. https://doi.org/10.1371/journal.pone.0172437.
Cowan J, Mulpuru S, Abdallah SJ, et al. A randomized double-blind placebo-control feasibility trial of immunoglobulin treatment for prevention of recurrent acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:3275–84. https://doi.org/10.2147/copd.s338849.
Mathioudakis AG, Sivapalan P, Papi A, Vestbo J. The disentangling chronic obstructive pulmonary disease exacerbations clinical trials network (DECODE-net): Rationale and vision. Eur Respir J. 2020;56(1):2000627. https://doi.org/10.1183/13993003.00627-2020.
Alexander GM, Fekri A, Alvar A, et al. ERS statement: a core outcome set for clinical trials evaluating the management of COPD exacerbations. ERJ. 2022;59(5):2102006.
Mathioudakis AG, Moberg M, Janner J, Alonso-Coello P, Vestbo J. Outcomes reported on the management fo COPD exacerbations: a systematic survey of randomised controlled trials. ERJ Open Res. 2019;5(2):00072–2019. https://doi.org/10.1183/23120541.00072-2019.
Ferguson GT and Make B. 2023. UpToDate. https://www.uptodate.com/contents/management-of-refractory-chronic-obstructive-pulmonary-disease. Accessed 1 Aug 2023.
Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. NEJM. 2023;389(3):205–14. https://doi.org/10.1056/nejmoa2303951.
Cuevas E, Huertas D, Monton C, et al. Systemic and functional effects of continuous azithromycin treatment in patients with severe chronic obstructive pulmonary disease and frequent exacerbations. Front Med. 2023. https://doi.org/10.3389/fmed.2023.1229463.
Petrov AA, Adatia A, Jolles S, et al. Antibody deficiency, chronic lung disease, and comorbid conditions: a case-based approach. J Allergy Clin Immunol Prac. 2021;9(11):3899–2908. https://doi.org/10.1016/j.jaip.2021.09.031.
Acknowledgements
None
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature search was performed by Liz Dennett. Material preparation and data collection were performed by Justin Kim and Harrisios Vliagoftis. Data analysis was performed by Justin Kim, Harrisios Vliagoftis, and Adil Adatia. The first draft of the manuscript was written by Justin Kim and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study relied exclusively on publicly available information that is legally accessible to the public, and thus ethics board approval was not required as per our institutional policy: (https://www.ualberta.ca/research/services/research-ethics/do-i-need-research-ethics-approval.html).
Consent for publication
Not applicable.
Competing interests
AA has received honoraria and travel support from Takeda Pharmaceuticals and Biocryst; honoraria from CSL Behring and Covis Pharma; and research support from Ionis Pharmaceuticals and Astria Therapeutics, outside of the submitted work. JK, LD, MO, AH, and HV reports no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA 2020 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, J.J.Y., Dennett, L., Ospina, M.B. et al. Effectiveness of immunoglobulin replacement therapy in preventing infections in patients with chronic obstructive pulmonary disease: a systematic review. Allergy Asthma Clin Immunol 20, 30 (2024). https://doi.org/10.1186/s13223-024-00886-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-024-00886-8