Abstract
Purpose
With the present work, we aimed to assess the occurrence of ochratoxigenic fungi and Ochratoxin A (OTA) in dried grapes from Tunisia.
Methods
Dried grapes samples (n = 90) were investigated for the presence of ochratoxigenic fungi, which were further characterized at the species level through amplification of the internal transcribed spacer (ITS) region and polymerase chain reaction (PCR) product sequencing. Fungal isolates were tested for their ochratoxigenic potential by high-performance liquid chromatography with fluorescence detection (HPLC-FLD), as well as dried grapes samples after an immunoaffinity column (IAC) clean-up procedure.
Results
Black Aspergilli isolates were the dominant genre among the filamentous fungi found in dried grapes samples and were the only OTA-producing fungi encountered. Aspergillus niger aggregate were the most frequently found isolates reaching 70%, 80%, and 85% in dried grapes samples from regions of Kelibia, Sfax, and Rafraf, respectively, while covered 100% of the relevant mycobiota found in imported samples. Aspergillus carbonarius isolates were found only in Sfax’s and Kelibia’s samples, while uniseriate Aspergilli were found between 7 and 20% in dried grapes from Kelibia, Sfax, and the imported samples. The in vitro OTA production test showed that 88.9% of OTA-producing isolates belonged to A. carbonarius with OTA levels varying from 0.06 to 1.32 μg/g of Czapek Yeast Agar (CYA). The remaining OTA-producing fungi (11.1 %) belonged to A. niger aggregate group having a maximum OTA potential of 2.88 μg/g CYA, and no uniseriate Aspergilli isolate was able to produce OTA. All dried grapes samples were free of OTA presence.
Conclusion
According to the present study’s findings, no OTA contamination was recorded in the investigated samples from Tunisian market. Nevertheless, the presence of strong OTA producers A. carbonarius in samples originated from the two out of three studied Tunisian regions, as well the high incidences of Aspergillus niger aggregate group with an attested potential for OTA production in all samples, necessitates further research on Tunisian dried grapes. Additionally, a continuous analysis of staple food of the Mediterranean diet is imperative to insure the best quality for the consumers and prevent potential health problems.
Similar content being viewed by others
Introduction
Vitis vinifera L. (Vitaceae) is a species of Vitis, native to the Mediterranean region (Fernandes et al. 2013). Its production is widespread worldwide for fresh consumption and industrial processing of grapes. In 2014, world production of grapes exceeded 74 million tons (FAOSTAT 2014), of which more than 1.5 million tons correspond to dried grapes, having an increase of 10% compared with 2010 data (FAO-OIV 2016). Grape is a very sensitive fruit to various pathogens such as bacteria and molds. Indeed, the effects of fungal growth in grapes lead to numerous effects such as the production of metabolites that may affect the organoleptic properties of grapes and derived products, but also the production of toxic compounds namely Ochratoxin A (OTA) (Dachery et al. 2019), which may be produced in vineyards by several fungal species such as Penicillium verrucosum in temperate climates and Aspergillus ochraceus and Aspergillus carbonarius in tropical and warm ones (Amézqueta et al. 2012). Such fungi can contaminate crops prior to harvest and more commonly during storage (EFSA 2006) and lead therefore to the contamination of dried grapes, grape juices, and several types of wine (Welke 2019).
After several toxicological studies, OTA have shown various health implications to human and animals’ health that make it to be considered as one of the most important mycotoxins with a worldwide concern. OTA was implicated in renal toxicity, mutagenicity, genotoxicity, teratogenicity, immunotoxicity, and possibly neurotoxicity (JECFA 2001; Pfohl-Leszkowicz and Manderville 2007). OTA has also been classified as a possible human carcinogen (group 2B) by the International Agency for Research on Cancer (IARC 1993). The Food and Agricultural Organization, the World Health Organization, and the Joint Expert Committee on Food Additives established a provisional OTA tolerable daily intake of 14 ng/kg body weight (JECFA 1995). Several countries have established regulations on OTA occurrence in food, and the European Community established maximum permitted OTA levels are 10 and 2 ng/g for dried vine fruits and for grape juices, respectively (European Commission 2006).
Another related to OTA emerging risk, but still under investigation by the scientific community, is the transformation of parent mycotoxin to several modified forms, defined as “modified mycotoxins” due to physical, chemical, or biological phenomena taking place on the field, during processing, and storage of products (Freire and Sant’Ana 2018; Welke 2019). Modified mycotoxins could either perform toxic effects when consumed or even converted again into the parent mycotoxin in the human digestive system (Berthiller et al. 2013).
The Tunisian population consumes high amounts of dried fruits directly, mixed to special salted or sweet dishes such as couscous (a semolina main typical dish from Northern Africa) which are very appreciated by consumers and especially children. It is important to note that this consumption becomes very high in special celebrations such as Ramadan month and weddings.
OTA and its modified forms could potentially contaminate grapes and derived products like raisins all over the production cycle and especially at a post-harvest stage—in cases of processing under conditions promoting the growth of ochratoxigenic fungi (Freire and Sant’Ana 2018; Merlela et al. 2015; Palumbo et al. 2015).
Several studies have investigated the contribution of dried vine fruits (raisins, sultanas, and currants) to the total dietary exposure to OTA (Akdeniz et al. 2013; Hajok et al. 2019; Galvis-Sanchez et al. 2008; Lombaert et al. 2004; Miraglia and Brera 2002; Wei et al. 2018; Yurdakul et al. 2019). Numerous surveys have been carried out in Mediterranean countries on the occurrence of ochratoxigenic mycoflora in grapes from setting and/or veraison to harvest time (Abrunhosa et al. 2001; Battilani et al. 2003, 2004, 2006a, b; Bau et al. 2005; Bejaoui 2005; Belli et al. 2006; Covarelli et al. 2012; Lasram et al. 2007, 2013; Oueslati et al. 2010; Serra, et al. 2003; Stefanaki et al. 2003). However, very scarce data from surveys are available on the contamination with ochratoxigenic fungi and the OTA levels in dried grapes marketed in Tunisia up to now (Azaiez et al. 2015). The aim of this work was to determine the occurrence of ochratoxigenic fungi and OTA in dried grapes and assess the OTA contamination risk in this type of products.
Material and methods
Dried grapes sampling
Samples of dried grape (n = 90) were purchased from local markets of three traditional grape-producing regions in Tunisia during the production period of August 2017. Thirty samples of local dried grapes were purchased from each one. The three regions were Sfax, Rafraf, and Kelibia located in the South, in the North, and in the North East of Tunisia, respectively, and are characterized by different climatic conditions. The most common traditional grape dehydration procedure relates pre-treatment and drying in the sun as follows: fresh grapes are washed with water and then soaked 15 s in 5% NaOH solution at 95 °C, drained and rinsed again with cool water, soaked in an emulsion of 7% K2CO3 and 0.4% olive oil for 2–3 min, drained again, and distributed on drying racks in a single layer. Finally, sulfur fumigation (4 g/kg of grapes) was performed for 10 h before a direct sun drying process.
Mycobiota isolation and molecular identification of ochratoxigenic fungi
Dried grapes were randomly chosen from each sample and were surface-sanitized with 8% sodium hypochlorite, followed by 10% alcoholic solution (Covarelli et al. 2012). Grapes were rinsed thoroughly with sterilized distilled water and dried aseptically on sterilized filter paper. Five grapes per sample were placed with triplicate repetitions on Petri dishes containing Malt Extract Agar medium (MEA) supplemented with chloramphenicol (100 mg/L). Plates were incubated at 25 °C for 7 days. Colonies of potential OTA-producing fungi (Aspergillus and Penicillium spp.) were classified by genera according to the appropriate keys for the identification of Pitt and Hocking (2009). Purified and single-spore isolates of Aspergillus and Penicillium spp. were inoculated on Czapek Yeast Agar medium (CYA) and incubated at 25 °C for 7 days in order to evaluate their OTA production in vitro. Black Aspergilli were identified at species level microscopically, based on the spores and conidial heads morphology using appropriate identification keys as described by Abarca et al. (2004). Molecular identification of fungi was done after DNA extraction from fresh mycelia as described by Lecellier and Silar (1994). DNA was purified with classical phenol-chloroform mixture, followed by ethanol precipitation. DNA pellet was resuspended in 100 μL of sterile water before further PCR and sequencing steps.
PCR and sequencing analysis
The amplification of the ITS region was performed using ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers as described by Lasram et al. (2013). PCR products were directly sequenced in both directions with the ITS primers. The resulting DNA sequences were analyzed using CLUSTAL X 2.1. Isolates identification was determined based on the best score obtained by the comparison of the obtained DNA sequences with those available in the data of the National Center for Biotechnology and Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
OTA production
Pure and single-spore cultured strains were tested for their OTA production on CYA medium according to Bragulat et al. (2001). Briefly, plates were inoculated at three different points and incubated for 7 days at 25 °C. From each plate, three agar plugs (∅ = 6 mm) were removed, placed into an amber vial, and extracted with 1 mL of analytical grade methanol for 1 h. Then, the extract was filtered through a membrane filter (MillexR SLHV 013NK; Millipore, USA) before chromatographic analysis.
OTA quantification in dried grapes samples
OTA content was quantified in the analyzed samples according to Stroka et al. (2000) with slight modifications. All chemicals used for the analysis were HPLC grade, and briefly, 10 g of dried grapes were blended with 40 mL of methanol-water (80:20, v/v) for 3 min. Extracts were filtrated on Whatman filter paper No. 4. An aliquot of 10 mL was diluted with 60 mL of PBS buffer (pH 7.4). OTA clean-up was performed using an immunoaffinity column (IAC OchraStar™, Romer Labs Diagnostic GmbH, Austria). The column was conditioned with 10 mL of PBS at a flow rate of 5 mL/min. Then, the diluted filtrate was applied to the IAC column with a rate of 1–2 drops per second. The column was washed with 20 mL of water and then dried with gentle air stream. OTA was eluted using 2 mL of methanol into a glass amber vial. The eluent was evaporated to dryness under a gentle stream of nitrogen and reconstituted with 500 μL of the mobile phase before HPLC analysis.
ΗPLC determination
HPLC analysis was performed using a Smartline liquid chromatography system (Knauer, Germany) equipped with an online vacuum degasser (Smartline 5000), an auto-sampler (Smartline 3900), a quaternary pump (Smartline 1000), and Shimadzu RF-10AXL fluorescence detector (IET, USA). The analytical column was a Eurospher RP C18 (5 μm, ODS2, 4.6 × 150 mm, Milford, MA, USA) with a guard column packed with the same phase. OTA detection was carried out using 330 nm and 460 nm as fluorescence excitation and emission wavelengths, respectively. ClarityChrom chromatography station software (Knauer, Germany) was used to control the system and the signal process. Quantification of OTA was performed by measuring its peak area with the help of a calibration curve calculated from standard solutions. The mobile phase was a mixture of acetonitrile to water to acetic acid, (57:41:2, v/v/v). The OTA retention time was ca. 4 min. Detection and quantification limits (LOD and LOQ) for the analysis of ochratoxigenic potential of isolated species were 0.02 and 0.04 μg OTA/g of CYA medium with a signal-to-noise ratio of 3:1, while for dried grapes samples were 0.03 μg/L and 0.05 μg/L, with a signal-to-noise ratio of 10:1. The OTA recovery was 89 ± 4% (mean ± SD, n = 3).
Statistical analysis
The distribution of the ochratoxigenic isolates found was statistically analyzed with SAS software (version 8.02, SAS Institute, Inc., Cary, NC, USA) to assess the effect of the sample origin by analysis of variance followed by LSMEAN. Statistical significance was judged at both p < 0.05 and p < 0.0001.
Results
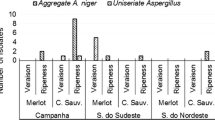
The occurrence of the identified fungi from dried grapes samples is presented in Fig. 1 and showed the presence of Aspergillus niger aggregate (73%), Aspergillus carbonarius (10%), Aspergillus japonicus (10%), Aspergillus flavus (8%), and Aspergillus ochraceus (1%). Aspergillus niger aggregate were the most frequently found isolates reaching 70%, 80%, and 85% in dried grapes samples from the regions of Kelibia, Sfax, and Rafraf, respectively, while covered 100% of the relevant mycobiota found in imported dried grapes samples. Aspergillus carbonarius isolates were found only in Sfax’s and Kelibia’s samples, while the uniseriate Aspergilli were found between 7 and 20% in dried grapes from Kelibia, Sfax, and the imported samples. Furthermore, Aspergillus niger distribution in the Sfax and Kelibia regions was significantly higher than in the imported and Rafraf samples. Similarly, Aspergillus japonicus distribution was significantly higher in the Sfax and Kélibia regions. However, both Aspergillus carbonarius and Aspergillus ochraceus distributions were not affected significantly by the sample origin (Table 1). Identification procedure also revealed the presence of the genera Penicillium, Alternaria, Botrytis, and Rhizopus. Among the potential OTA-producing fungi, only black Aspergilli represented by Aspergillus section Nigri group and Aspergillus ochraceus were isolated from the analyzed samples, while no isolates of Penicillium verrucosum were identified.
PCR amplification and DNA sequencing were used to confirm the macroscopic and microscopic identification of the observed genera. The electrophoresis profile (Fig. 2) and DNA sequencing resulted in eight strains of Aspergillus carbonarius, one strain of Aspergillus niger, and one strain of Aspergillus ochraceus, with high scores reaching 100% and permitting the confirmation of microscopic identification made previously.
A total of 139 black Aspergilli isolates were tested for their OTA production on CYA. Chromatograms of an OTA-positive isolate and OTA standard are presented in Fig. 3. The OTA quantification revealed that only nine isolates were able to produce OTA. Eight out of thirteen identified A. carbonarius isolates (88.88%) and only one from 111 A. niger aggregate isolates (11.11%) were confirmed for OTA production (Table 2). No OTA production was detected for the uniseriate Aspergillus japonicus and the Aspergillus ochraceus isolates. The OTA production by Aspergillus carbonarius isolates varied between 0.09 and 1.32 μg/g CYA. However, the OTA production by the Aspergillus niger isolate was higher and reached 2.88 μg/g CYA.
Despite the isolation of highly OTA-producing strains, all the analyzed dried grapes samples have not shown quantifiable OTA concentrations after their analysis with IAC for clean-up and HPLC-FLD for quantification.
Discussion
In warmer climate countries, such as Tunisia, Alternaria, Cladosporium, Botrytis, and Rhizopus spp. are the most abundant fungi at the beginning of grapes ripening, while the genres Aspergillus and Penicillium are the most common fungi contaminating grapes during the late stages of ripening, harvest, and grapes’ solar drying, with the predominance of Aspergillus species which are capable of mycotoxin production like OTA (Abarca et al. 2003; Belli et al. 2005; García-Cela et al. 2015; Oliveri and Catara 2011; Valero et al. 2005). During this post-harvest treatment, grapes are dried until their sugar level is extremely high, but at the same time, this process can produce adverse effects in the fruit mycoflora like an increase of fungal development and OTA production (Bau et al. 2005; Merlela et al., 2015; Palumbo et al. 2015; Pardo et al. 2005).
Several studies conducted in southern European countries had shown that other ochratoxigenic spp. like Aspergillus ochraceus was detected at very low incidences while Penicillium verrucosum was not isolated from grapes grown in warm climates (Pitt et al. 2000; Covarelli et al. 2012). Therefore, the fungal population’s diversity depended on several factors such as the grapes variety, maturity stage, climatic conditions, and agricultural practices, but OTA in warm climates was linked only with black Aspergilli (Battilani et al. 2004; Cabanés and Bragulat 2018; Esteban et al. 2004; Lopez de Cerain et al. 2002; Mitchell et al. 2004; Oliveri and Catara, 2011; Serratosa et al. 2010).
As it is shown in Fig. 1, black Aspergilli were the most abundant mycobiota in our study and relevant studies have shown these isolates are very resistant to sunlight exposure and to hot and dry environments (Akdeniz et al. 2013; Serra et al. 2003). Indeed, grapes are subjected to a drying process until they acquire the desired sugar content, and aw decrease quietly from 0.95 to 0.75. Within these conditions, only a few microorganisms such as black Aspergilli are still able to develop (Hocking et al. 2007). Even if in the case of solar dehydration, the direct sunrays reduce the viability of A. carbonarius spores (Leong et al. 2006); in general, black Aspergilli are less susceptible than other species to the germicidal UV rays and the strong sunlight heating due to their thick, heavily melanized walled pigmented spores (García-Cela et al. 2016). In comparison with the other species, the dried grape substrate provides a competitive advantage for black Aspergilli (Pitt and Hocking 2009).
Moreover, it is noticed that in Tunisia, a caustic soaking with K2CO3 is implemented as a bleaching solution to accelerate the drying process by making the grape skin weaker (Covarelli et al. 2012; Simate and Ahrné 2005). This procedure may enhance the fungal development especially the OTA-producing isolates. In addition, the use of SO2 as an antioxidant to fresh grapes can also be the origin of the variability in the level of contamination since it is showing an antifungal activity (Dilip 2003). These pretreatments aim to control fungal growth and their potential OTA production.
Regarding the fact that OTA production by the Aspergillus niger isolate was higher of A. carbonarius isolates, culture substrate used for the fungal growth may play an important role in the enhancement of OTA production. In fact, it was reported by Esteban et al. (2004) that mean OTA concentration produced by the A. niger aggregate and A. carbonarius isolates on YES and CYA medium was different.
Lasram et al. (2007) reported that Aspergillus carbonarius was the stronger OTA-producing fungi isolated from Tunisian grapes with a production level reaching 10 μg/g CYA, while several studies reported also that A. carbonarius was the most important source of OTA in dried grapes (Covarelli et al. 2012; Somma et al. 2012; Merlera et al. 2015).
It may be concluded that the occurrence of OTA producers in some dried grapes samples may occur regardless of the grapes varieties or the resveratrol content which may decrease the OTA concentration (De Rossi et al. 2012). However, environmental conditions (mainly temperature and Rh) in the vineyards offer the most important stimulations to the fungal contamination and their mycotoxin production (Oueslati et al. 2010; Zhang et al. 2014). In addition, in a Tunisian survey on table and wine grapes, Lasram and co-workers (2012) found out that most contaminated grapes, presenting also considerable levels of OTA (up to 5.85 μg/L), were those originating from Rafraf region which is characterized by a humid climate in contrast with those from the Regueb region, characterized by an arid climate, where furthermore A. carbonarius was rarely isolated. Also, mycobiota interactions may be a cause for the OTA production, as for example, the presence of Eurotium amstelodami can help Aspergillus niger to colonize grapes, through its enzymatic activity which offers to Aspergillus isolates nutrients release and entrance ability to grapes (Valero et al. 2007), while filamentous fungi of grapevine may trigger either stimulation or decrease in OTA production by A. carbonarius depending to the species (Kogkaki et al. 2015). In contrast, other interactions, as for example with yeasts or bacteria, may cause an inhibition or a decrease in OTA production (Kapetanakou et al. 2012).
The absence of quantifiable concentrations of OTA in the tested dried grapes may demonstrate that the toxin-producing fungi may exist in a sample but no Ochratoxin A is synthesized within as it depends on several environmental factors, mainly temperature, rainfall, and humidity. Nevertheless, correlation between the incidence of OTA-producing strains and OTA contamination is frequently reported in relevant studies (Somma et al. 2012; Lasram et al. 2012). Regarding reports on dried grapes, Zinedine et al. (2007) detected OTA levels in samples from Morocco varying between 0.05 and 4.95 ng/g; Zhang et al. (2014) reported a high number of contaminated dried grapes from the Chinese market, with almost 60% of tested samples being positive, and OTA range between 0.07 and 12.83 μg/kg. Palumbo et al. (2015) found that 54% of conventionally cultured and 34% of organic cultivation raisins from the US markets were contaminated with OTA in ranges between 0.34 and 15.34 ng/g. In another recent study of Yurdakul et al. (2019) on Turkish dried grapes, none of the samples out of 17 tested presented detectable OTA concentration, although in previous surveys, for Turkey raisins, small incidence (8%) and mean concentration (1.15 μg/Kg) were reported (Akdeniz et al. 2013) and Sultanas were found highly contaminated (Aksoy et al. 2007; Meyvaci et al. 2005).
Drying process and pretreatments are also important factors influencing the OTA contamination of grapes (Somma et al. 2012; Valero et al. 2008; Zhang et al. 2014) but also its modified forms (Freire et al. 2018a). For example, Rafraf is a Tunisian region that is known for using a soaking solution containing CaO, ash, and Pistacia lentiscus branches, which has an astringent oleoresin having therapeutic role, within fumigation processes (Harbi Ben Slimen 2005). Chemical pretreatments are also used to help drying procedure such as K2CO3 solution. These pretreatments may cause the OTA decrease or inhibition of its production by the contaminating fungi (Valero et al. 2005). In fact, grapes composition will be modified quickly resulting in reduced nutrients not only for Aspergillus spp., but also for antagonistic mycoflora (Kogkaki et al. 2015), which may use the previously produced OTA as a source of carbon and energy for its metabolism, but also may transform the toxic OTA into modified mycotoxins (Bragulat et al. 2017; Freire et al. 2018b).
Conclusion
We currently identified the potential ochratoxigenic fungi and studied the OTA contamination in dried grapes samples marketed in Tunisia. Aspergillus is the most frequent fungi genre contaminating the studied samples. Aspergillus section Nigri group represented by Aspergillus niger aggregate, Aspergillus carbonarius, and Aspergillus japonicus was frequently found. Among all isolates, only 6.47% were OTA producers and the only Aspergillus ochraceus strain found was not ochratoxigenic. All the analyzed samples were free of OTA presence, although they included OTA-producing strains within their mycoflora.
Nevertheless, if we account also the fact that a part of the produced OTA could undergo a transformation to its modified forms, normally remaining undetected during the testing for parent mycotoxin (Freire and Sant’Ana 2018), and acknowledging that their potential production could be triggered in dried grapes either by chemical treatments (soaking solution), as well from biological (antagonist microorganisms) or even environmental (traditional dehydration, origin, and type of grape) conditions, a more rigorous investigation is necessary to ensure to the level of safety needed (Freire et al. 2017, 2018a, 2018b, 2019).
As there is not enough information on OTA occurrence in dried grapes from Tunisia, further investigations should be carried out to further evaluate the impact of climatic conditions of the production regions, the effect of different chemical treatments, and the drying process of grapes on the ochratoxigenic fungi contamination and their OTA production potential. In addition, in order to guarantee food product safety, inspections should be legislated not only for those mycotoxins linked with a certain product but also for other or modified mycotoxins (Freire and Sant’Ana, 2018; Welke et al. 2019). It also remains important to conduct more toxicological studies to elucidate not only OTA but also its modified form intake from consumption of dried grape products and to ensure consumer safety.
Change history
30 September 2020
An amendment to this paper has been published and can be accessed via the original article.
References
Abarca ML, Accensi F, Bragulat MR, Castella G, Cabanes FJ (2003) Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. Journal of Food Protection 66:504–506
Abarca ML, Accensi F, Cano J, Cabanes JF (2004) Taxonomy of black Aspergilli. Applied and Environmental Microbiology 86:33–49
Abrunhosa L, Paterson RRM, Kozakiewicz Z, Lima N, Venancio A (2001) Mycotoxin production from fungi isolated from grapes. Letters in Applied Microbiology 32:240–242
Akdeniz AS, Ozden S, Alpertunga B (2013) Ochratoxin A in dried grapes and grape-derived products in Turkey. Food Additives and Contaminants: Part B: Surveillance 6(4):265–269
Aksoy U, Eltem R, Meyvaci KB, Altindisli A, Karabat S (2007) Five-year survey of ochratoxin A in processed sultanas from Turkey. Food Additives and Contaminants 24:292–296
Amézqueta S, Schorr-Galindo S, Murillo-Arbizu M, González-Peñas E, López de Cerain A, Guiraud JP (2012) OTA-producing fungi in food staffs. A review. Food Control 26:259–268
Azaiez I, Font G, Mañes J, Fernández-Franzón M (2015) Survey of mycotoxins in dates and dried fruits from Tunisian and Spanish markets. Food Control 51:340–346
Battilani P, Barbano C, Marin S, Sanchis V, Kozakiewicz Z, Magan N (2006a) Mapping Aspergillus section Nigri in southern Europe and Israel based on geostatistical analysis. International Journal of Food Microbiology 111:72–82
Battilani P, Giorni P, Bertuzzi T, Formenti S, Pietri A (2006b) Black Aspergilli and ochratoxin A in grapes in Italy. International Journal of Food Microbiology 111:53–56
Battilani P, Logrieco A, Giorni P, Cozzi G, Bertuzzi T, Pietri A (2004) Ochratoxin A production by Aspergillus carbonarius on some grape varieties grown in Italy. Journal of the Science of Food and Agriculture 84:1736–1740
Battilani P, Pietri A, Bertuzzi AT, Languasco L, Giorni P, Kozakiewicz Z (2003) Occurrence of ochratoxin A-producing fungi in grapes grown in Italy. Journal of Food Protection 66:633–636
Bau M, Castella G, Bragulat MR, Cabanes FJ (2005) DNA-based characterization of ochratoxin-A producing and non-producing Aspergillus carbonarius strains from grapes. Research in Microbiology 156:375–381
Bejaoui H., 2005. Champignons ochratoxinogènes et ochratoxine A (OTA) dans des vignobles Français et procédés biologiques de décontamination de l'OTA dans les moûts de raisin (PhD thesis). Toulouse, France: Institut National Polytechnique de Toulouse.
Belli N, Marin S, Sanchis V, Ramos AJ (2006) Impact of fungicides on A. carbonarius growth and ochratoxin A production on synthetic grape-like medium and on grapes. Food Additives and Contaminants 63:1021–1029
Belli N, Mitchell D, Marin S, Alegre I, Ramos AJ, Magan N, Sanchis V (2005) Ochratoxin A-producing fungi in Spanish wine grapes and their relationship with meteorological conditions. European Journal of Plant Pathology 113:233–239
Berthiller F, Crews C, Dall’Asta C, De Saeger S, Haesaert G, Karlovsky P, Oswald IP, Seefelder W, Speijers G, Stroka J (2013) Masked mycotoxins: a review. Molecular Nutrition & Food Research 57:165–186
Bragulat MR, Abarca ML, Cabañes FJ (2001) An easy screening method for fungi producing ochratoxin A in pure culture. International Journal of Food Microbiology 71:139–144
Bragulat MR, Eustaquio A, Cabañes FJ (2017) Study on the presence of ochratoxin α in cultures of ochratoxigenic and non-ochratoxigenic strains of Aspergillus carbonarius. PLoS ONE 12(10):e0185986
Cabanés FJ, Bragulat MR (2018) Black aspergilli and ochratoxin A-producing species in foods. Current Opinion in Food Science 23:1–10
Covarelli L, Beccari G, Marini A, Tosi L (2012) A review on the occurrence and control of ochratoxigenic fungal species and ochratoxin A in dehydrated grapes, non-fortified dessert wines and dried vine fruit in the Mediterranean area. Food Control 26:347–356
Dachery B, Hernandes KC, Veras FF, Schmidt L, Augusti PR, Manfroi V, Zini CA, Welke JE (2019) Effect of Aspergillus carbonarius on ochratoxin a levels, volatile profile and antioxidant activity of the grapes and respective wines. Food Research International 126:1–9
De Rossi A, Ricelli A, Reverberi M, Bello C, Fabbri AA, Fanelli C, Corradini D, Nicoletti I (2012) Grape variety related trans-resveratrol induction affects Aspergillus carbonarius growth and ochratoxin A biosynthesis. International Journal of Food Microbiology 156:127–132
Dilip KA (2003) Fungal biotechnology in agricultural, food, and environmental applications. Mycology 21:295–296
Esteban A, Abarca ML, Bragulat MR, Cabanes FV (2004) Effects of temperature and incubation time on production of ochratoxin A by black aspergilla. Research in Microbiology 155:861–866
European Commission (2006) Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union L365:6–24
Fernandes F, Ramalhosa E, Pires P, Verdial J, Valentao P, Andrade P, Bento A, Pereira JA (2013) Vitis vinifera leaves towards bioactivity. Industrial Crops and Products 43:434–440
Food and Agriculture Organisation Statistics-FAOSTAT (2014) FAOSTAT Division Database. Available at: http://faostat3.fao.org/, Accessed 2018.
Food and Agriculture Organization of the United Nations - International Organisation of Vine and Wine-FAO-OIV (2016) FAO-OIV Focus 2016 Table and Dried Grapes: Non-alcoholic products of the vitivinicultural sector intended for human consumption. FAO-OIV Publications, p 64 Available at: www.fao.org/publications
Freire L, Furtado MM, Guerreiro TM, da Graca JS, da Silva BS, Oliveira DN, Catharino RR, Sant’Ana AS (2019) The presence of ochratoxin A does not influence Saccharomyces cerevisiae growth kinetics but leads to the formation of modified ochratoxins. Food and Chemical Toxicology 133:1–8
Freire L, Guerreiro TM, Carames ETS, Lopes LS, Orlando EA, Pereira GE, Pallone JAL, Catharino RR, Sant’Ana AS (2018a) Influence of maturation stages in different varieties of wine grapes (Vitis vinifera) on the production of ochratoxin A and its modified forms by Aspergillus carbonarius and Aspergillus niger. Journal of Agriculture and Food Chemistry 66:8824–8831
Freire L, Guerreiro TM, Pia AKR, Lima EO, Oliveira DN, Melo CFOR, Catharino RR, Sant'Ana AS (2018b) A quantitative study on growth variability and production of ochratoxin A and its derivatives by A. carbonarius and A. niger in grape-based medium. Scientific Reports 8:14573.
Freire L, Passamani FRF, Thomas AB, Nassur RCMR, Silva LM, Paschoal FN, Pereira GE, Prado G, Batista LR (2017) Influence of physical and chemical characteristics of wine grapes on the incidence of Penicillium and Aspergillus fungi in grapes and ochratoxin A in wines. International Journal of Food Microbiology 241:181–190
Freire L, Sant’Ana AS (2018) Modified mycotoxins: an updated review on their formation, detection, occurrence, and toxic effects. Food and Chemical Toxicology 111:189–205
Galvis-Sanchez AC, Barros AS, Delgadillo I (2008) Method for analysis dried vine fruits contaminated with ochratoxin A. Analytica Chimica Acta. 617:59–63
García-Cela E, Crespo-Sempere A, Gil-Serna J, Porqueresa A, Marin S (2015) Fungal diversity, incidence and mycotoxin contamination in grapes from two agro-climatic Spanish regions with emphasis on Aspergillus species. Journal of the Science of Food and Agriculture 95:1716–1729
García-Cela ME, Marín S, Reyes M, Sanchis V, Ramos AJ (2016) Conidia survival of Aspergillus section Nigri, Flavi and Circumdati under UV-A and UV-B radiation with cycling temperature/light regime. Journal of the Science of Food and Agriculture 96:2249–2256
Hajok I, Kowalska A, Piekut A, Ćwieląg-Drabek M (2019) A risk assessment of dietary exposure to ochratoxin A for the Polish population. Food Chemistry 284:264–269
Harbi Ben Slimen M (2005) Patrimoine et traditions viticoles à Rafraf. IRESA Press, Tunis
Hocking AD, Leong SL, Kazi BA, Emmett RW and Scott ES (2007) Fungi and mycotoxins in vineyards and grape products. International Journal of Food and Microbiology 119:84-88.
International Agency Research of Cancer – IARC (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. In: IARC monographs on the evaluation of carcinogenic risks to humans, Vol. 56 (pp. 26-32). Lyon: IARC Press.
Joint FAO/WHO Expert Committee on Food Additives – JECFA (1995) Evaluation of certain food additives and contaminants. World Health Organisation (WHO) Technical Report Series, Report 44, Series 859.
Joint FAO/WHO Expert Committee on Food Additives – JECFA (2001). Evaluation of certain food additives and contaminants. WHO Technical Report Series, Report 55, Series 901.
Kapetanakou AE, Kollias JN, Drosinos EH, Skandamis PN (2012) Inhibition of A. carbonarius growth and reduction of ochratoxin A by bacteria and yeast composites of technological importance in culture media and beverages. International Journal of Food Microbiology 152:91–99
Kogkaki EA, Natskoulis PI, Magan N, Panagou EZ (2015) Effect of interaction between Aspergillus carbonarius and non-ochratoxigenic grape-associated fungal isolates on growth and ochratoxin A production at different water activities and temperatures. Food Microbiology 46:521–527
Lasram S, Belli N, Chebil S, Zghonda N, Mliki A, Vicente S, Ghorbel A (2007) Occurrence of ochratoxigenic fungi and ochratoxin A in grapes from a Tunisian vineyard. International Journal of Food Microbiology 114:376–379
Lasram S, Oueslati S, Ben Jouira H, Chebil S, Mliki A, Ghorbel A (2013) Identification of ochratoxigenic Aspergillus section Nigri isolated from grapes by ITS-5.8S rDNA sequencing analysis and in silico RFLP. Journal of Phytopathology 161:280–283
Lasram S, Oueslati S, Mliki A, Ghorbel A, Silar P, Chebil S (2012) Ochratoxin A and ochratoxigenic black Aspergillus species in Tunisian grapes cultivated in different geographic areas. Food Control 25:75–80
Lecellier G, Silar P (1994) Rapid method for nucleic acids extraction from Petri dish-grown mycelia. Current Genetics 25:122–123
Leong SL, Hocking AD, Scott ES (2006) Survival and growth of Aspergillus carbonarius on wine grapes before harvest. International Journal of Food Microbiology 111:83–87
Lombaert GA, Pellaers P, Neumann G, Kitchen D, Huzel V, Trelka R, Kotello S, Scott PM (2004) Ochratoxin A in dried vine fruits on the Canadian retail market. Food Additives and Contaminants 21:578–585
Lopez de Cerain A, Gonzalez-Penas E, Jiménez AM, Bello J (2002) Contribution to the study of ochratoxin A in Spanish wines. Food Additives and Contaminants 19:1058–1064
Merlera GG, Muñoz S, Coelho I, Cavaglieri LR, Torres AM, Reynoso MM (2015) Diversity of black Aspergilli isolated from raisins in Argentina: polyphasic approach to species identification and development of SCAR markers for Aspergillus ibericus. International Journal of Food Microbiology 210:92–101
Meyvaci KB, Altindisli A, Aksoy U, Eltem R, Turgut H, Arasiler Z, Kartal N (2005) Ochratoxin A in sultanas from Turkey I: Survey of unprocessed sultanas from vineyards and packing-houses. Food Additives and Contaminants 22:1138–1143
Miraglia M, Brera C (2002) Assessment of dietary intake of ochratoxin A by the population of EU member states. In: Reports of Experts Participating in Task 3.2.7. Directorate-General Health and Consumer Protection, Rome, Italy.
Mitchell D, Parra R, Aldred D, Magan N (2004) Water and temperature relations of growth and ochratoxin A production by Aspergillus carbonarius strains from grapes in Europe and Israel. Journal of Applied Microbiology 97:439–445
Oliveri C, Catara V (2011) Mycoflora and biodiversity of black Aspergilli in vineyard eco-systems. In: Grillo O (ed) The Dynamical Processes of Biodiversity - Case Studies of Evolution and Spatial Distribution. IntechOpen, Rijeka, Croatia, pp 259–279
Oueslati S, Lasram S, Ramos A, Marin S, Mliki A, Sanchez V, Ghorbel A (2010) Alternating temperatures and photoperiods effect on growth and ochratoxin A by Aspergillus carbonarius isolated from Tunisian grapes. International Journal of food microbiology 139:210–213
Palumbo JD, O’Keeffe TL, Ho YS, Santillan CJ (2015) Occurrence of ochratoxin A contamination and detection of ochratoxigenic Aspergillus species in retail samples of dried fruits and nuts. Journal of Food Protection 78:836–842
Pardo E, Marín S, Sanchis V, Ramos AJ (2005) Impact of relative humidity and temperature on visible fungal growth and OTA production of ochratoxigenic Aspergillus ochraceus isolates on grapes. Food Microbiology 22:383–389
Pfohl-Leszkowicz A, Manderville RA (2007) Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Molecular Nutrition and Food Research 51:61–99
Pitt JI, Basilico JC, Abarca ML, Lopez C (2000) Mycotoxins and toxigenic fungi. Medical Mycology 38:41–46
Pitt JI, Hocking AD (2009) Fungi and food spoilage. Blackie Academic and Profesional, NewYork, USA
Serra R, Abrunhosa L, Kozakiewicz Z, Venancio A (2003) Black Aspergillus species as ochratoxin A producers in Portuguese wine grapes. International Journal of Food Microbiology 88:63–68
Serratosa MP, Lopez-Toledano A, Millan C, Medina M, Merida J (2010) Changes of ochratoxin A in grapes inoculated with Aspergillus carbonarius and subjected to chamber-drying under controlled conditions. Journal of Agricultural and Food Chemistry 58:11907–11912
Simate IN, Ahrné LM (2005) Dehydration of tropical fruits. In: Hui YH (ed) Handbook of Food Science, Technology, and Engineering, CRC Press, vol 4. Taylor and Francis Group, New York, USA, pp 104.9–104.11
Somma S, Perrone G, Logrieco AF (2012) Diversity of black Aspergilli and mycotoxin risks in grape, wine and dried vine fruits. Phytopathologia Mediterranea 51:131–147
Stefanaki I, Foufa E, Tsatsou-Dritsa A, Dais P (2003) Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Additives and Contaminants 20:74–83
Stroka J, Anklam E, Jorissen U, Gilbert J (2000) Immunoaffinity column cleanup with liquid chromatography using post-column bromination for determination of aflatoxins in peanut butter, pistachio paste, fig paste, and paprika powder: collaborative study. Journal of AOAC International 83:320–334
Valero A, Marin S, Ramos AJ, Sanchez V (2005) Ochratoxin A-producing species in grapes and sun-dried grapes and their relation to ecophysiological factors. Letters in Applied Microbiology 41:196–201
Valero A, Marín S, Ramos AJ, Sanchis V (2008) Survey: ochratoxin A in European special wines. Food Chemistry 108:593–599
Valero A, Olivan S, Marin S, Sanchis V, Ramos AJ (2007) Effect of intra and interspecific interaction on OTA production by A. section Nigri in grapes during dehydration. Food Microbiology 24:254–259
Wei D, Wu X, Xu J, Dong F, Liu X, Zheng Y, Ji M (2018) Determination of Ochratoxin A contamination in grapes, processed grape products and animal-derived products using ultra-performance liquid chromatography-tandem mass spectroscopy system. Scientific Reports 8:2051
Welke JE (2019) Fungal and mycotoxin problems in grape juice and wine industries. Current Opinion in Food Science 29:7–13
Yurdakul OK, Sahindokuyucu Kocasari F, Yalcin H, Keyvan E (2019) Survey of Ochratoxin A in coffee, dried grapes and grape pekmez samples in Burdur, Turkey. Journal of Research in Veterinary Medicine 38:46–51
Zhang X, Li J, Zong N, Zhou Z, Ma L (2014) Ochratoxin A in dried vine fruits from Chinese markets. Food Additives & Contaminants: Part B: Surveillance 7:157–161
Zinedine A, Soriano JM, Juan C, Mojemmi B, Molto JC, Bouklouze A, Cherrah Y, Idrissi L, El Aouad R, Manes J (2007) Incidence of ochratoxin A in rice and dried fruits from Rabat and Salé area, Morocco. Food Additives and Contaminants 24:285–291
.
Funding
N/A
Author information
Authors and Affiliations
Contributions
SC and HBI designed the study, AB-A and WR-B designed the molecular biology work, SO and WR-B have conducted the laboratory work and drafted the article, PN helped collating the experimental data and assessed the presented data, all authors helped editing the research paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the original article (Chebil et al. 2020), two authors were missing from the author’s list. Dr. Wafa Rjiba - Bahri and Dr. Hanen Ben Ismail have been added as co-authors for their contributions in designing the molecular biology work, conducting the laboratory work and drafting the article, and designing the study, respectively. Dr. Wafa Rjiba-Bahri and Dr. Hanen Ben Ismail declare no conflict of interest. The author’s list in the original article has been updated, as well as the list of affiliations and the statement in the Authors’ contributions..
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chebil, S., Rjiba-Bahri, W., Oueslati, S. et al. Ochratoxigenic fungi and Ochratoxin A determination in dried grapes marketed in Tunisia. Ann Microbiol 70, 38 (2020). https://doi.org/10.1186/s13213-020-01584-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-020-01584-7