Abstract
Background
Neuropsychiatric symptoms (NPS) are common in older people, may occur early in the development of dementia disorders, and have been associated with faster cognitive decline. Here, our objectives were to investigate whether plasma levels of neurofilament light chain (NfL), glial fibrillary acid protein (GFAP), and tau phosphorylated at threonine 181 (pTau181) are associated with current NPS and predict future NPS in non-demented older people. Furthermore, we tested whether the presence of NPS combined with plasma biomarkers are useful to predict Alzheimer’s disease (AD) pathology and cognitive decline.
Methods
One hundred and fifty-one participants with normal cognition (n = 76) or mild cognitive impairment (n = 75) were examined in a longitudinal brain aging study at the Memory Centers, University Hospital of Lausanne, Switzerland. Plasma levels of NfL, GFAP, and pTau181 along with CSF biomarkers of AD pathology were measured at baseline. NPS were assessed through the Neuropsychiatric Inventory Questionnaire (NPI-Q), along with the cognitive and functional performance at baseline and follow-up (mean: 20 months). Different regression and ROC analyses were used to address the associations of interest.
Results
None of the three plasma biomarker was associated with NPS at baseline. Higher GFAP levels were associated with the presence of NPS at follow-up (OR = 2.8, p = .002) and both, higher NfL and higher GFAP with an increase in the NPI-Q severity score over time (β = 0.25, p = .034 and β = 0.30, p = .013, respectively). Adding NPS and the plasma biomarkers to a reference model improved the prediction of future NPS (AUC 0.72 to 0.88, p = .002) and AD pathology (AUC 0.78 to 0.87, p = .010), but not of cognitive decline (AUC 0.79 to 0.85, p = .081).
Conclusion
Plasma NfL and GFAP are both associated with future NPS and NPS severity change. Considering the presence of NPS along with blood-based AD-biomarkers may improve the prediction of clinical progression of NPS over time and inform clinical decision-making in non-demented older people.
Similar content being viewed by others
Introduction
Neuropsychiatric symptoms (NPS) such as apathy, depression, agitation, and sleep disturbances, are common in older people, with rates of 80% or more in patients with cognitive impairment and dementia [1]. NPS frequently occur already at preclinical or prodromal stages of Alzheimer’s disease (AD) [2, 3] and therefore can be seen as a risk factor for the progression to dementia [4,5,6]. NPS have been associated with lower quality of life, more frequent hospitalizations, and earlier death [7,8,9]. Early detection and evaluation of NPS is therefore important for a more accurate prognosis and more specific treatment interventions to lower disease burden and potentially improve long-term outcomes.
NPS are generally assessed by clinical examination and by interviewing the patients or their caregivers about single symptoms like apathy, depression, irritability, or agitation [10]. There is an important inter-individual heterogeneity regarding the causes and factors contributing to the manifestation and duration of NPS, and it is generally difficult to estimate whether the observed symptoms are mainly related to acute factors like psychological stress, and/or pathophysiological changes. Easily available biomarkers indicating NPS-related pathology and the associated risk for long-term consequences could enable a more accurate etiological diagnosis and prognosis in patients having NPS.
To date, little is known about the underlying pathophysiology of NPS. Some studies investigated associations of NPS with AD using cerebrospinal fluid (CSF) biomarkers or brain PET-imaging. One study found that apathy correlated with increased levels of phosphorylated and total tau in CSF [11], another one reported increased aggressive behavior in AD patients in association with lower beta-amyloid 1–42 peptide (Aβ42) levels [12]. However, a meta-analysis of 21 studies addressing the association of NPS with CSF biomarkers of AD pathology showed inconsistent findings [13]. A study using tau- and amyloid PET analysis found that NPS correlate with tau but not with amyloid pathology [14]. In a study in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort some symptoms such as apathy, anxiety, and delusions were associated with amyloid pathology in participants with mild cognitive impairment (MCI), but not in cognitively unimpaired subjects or patients with dementia [15].
PET imaging and CSF analysis are based on expensive and/or invasive procedures. Thus, there is a need for biomarkers easier to obtain such as blood-based markers, to detect pathology and to predict or track disease progression in clinical settings. Neurofilament light chain (NfL) has been proposed as a biomarker for axonal damage and neuronal injury of different etiologies, including AD [16]. Glial fibrillary acid protein (GFAP) is a marker of astrocytosis and astroglial activation and has also been related to AD and early stages of AD [17, 18]. Another blood-based biomarker candidate is tau phosphorylated at threonine 181 (pTau181), which indicates cerebral tau pathology specific for AD [19]. All three blood markers have been shown to be useful for detecting and monitoring neurodegeneration [20,21,22,23,24]. Some studies also evaluated NfL and GFAP in the blood as potential markers for primarily psychiatric disorders such as depression or schizophrenia. Higher NfL levels were found to be associated with major depression [25], while higher GFAP levels were associated with schizophrenia [26, 27]. Nevertheless, little or inconsistent evidence of the associations between those plasma biomarkers with NPS in the context of cognitive decline and AD is available [28,29,30,31,32].
Here, our aim was to investigate whether plasma NfL, GFAP, and pTau181 levels are associated with NPS and related long-term clinical outcomes, including the presence and the severity of NPS, and cognitive decline at follow-up visits. Furthermore, we evaluated the usefulness of the single plasma biomarkers or combinations of them to predict future NPS and cognitive decline. Additionally, we assessed whether the presence of NPS combined with measures of plasma NfL, GFAP and pTau181 improves the prediction of cerebral AD pathology as indicated by CSF biomarkers.
Methods
Study population
We included 151 subjects from a brain aging study performed at the memory centers of the Department of Psychiatry and the Department of Clinical Neurosciences, University Hospital of Lausanne in Switzerland [33]. All participants were community-dwelling older people with and without cognitive impairment recruited among memory clinic patients or through journal announcements and word of mouth. Cognitively impaired participants met the diagnostic criteria for MCI [34] and had a Clinical Dementia Rating (CDR) score of 0.5. Cognitively unimpaired individuals had a CDR score of 0. Exclusion criteria for all participants were current major psychiatric or neurological disorders, severe or instable physical diseases or substance use disorder, unstable medication and all medical conditions that may significantly contribute to cognitive impairment or interfere with cognitive performance in the administered tasks.
Clinical and neuropsychological assessment
Comprehensive clinical and neuropsychological assessments were performed at baseline and at follow-up visits with the participants and their proxies. Cognitive performance was assessed using a comprehensive neuropsychological test battery as previously described [33]. This battery included the domains memory, language, attention, as well as, executive and visuospatial functions. The instrumental activities of daily living (IADL) questionnaire assessed the functional impairment of each participant [35, 36]. The clinical assessment, including physical, neurological, and psychogeriatric examination, and all described neuropsychological assessments were used to determine the CDR and CDR sum of boxes (CDR-SB) scores [37, 38]. Cognitive impairment was defined as CDR = 0.5. Because the time to follow up varied in this cohort, cognitive decline was defined as ∆CDR-SB/time to follow-up in months > 0.5 [39], whereas the baseline CDR-SB score was subtracted from the CDR-SB score at follow-up. All tests and scales are validated and widely used in the field.
NPS at baseline and the follow-up visits were assessed using the Neuropsychiatric Inventory-Questionnaire (NPI-Q) [10]. This is a self-administered questionnaire completed by informants with regular contact with the individual. It includes twelve domains (delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, aberrant motor activity, nighttime behavioral disturbances, and appetite/eating changes). Each domain was scored separately based on its severity, rated with values from one to three. The sum of the twelve scores yielded the total NPI-Q severity score, whereas the maximum score is 36.
The presence of NPS was defined as NPI-Q > 0 at baseline or follow-up (i.e., future NPS). Based on the presence of any NPS at baseline, participants were divided into two subgroups: NPS positive (NPI-Q > 0) and NPS negative (NPI-Q = 0). The change in the NPS severity was investigated using the ∆NPI-Q severity score between baseline and follow-up divided by the time to follow-up in months, whereas the baseline NPI-Q score was subtracted from the NPI-Q score at follow-up.
Biological assessments
Venous and lumbar punctures were performed between 8:30 and 9:30 am after overnight fasting at the recruiting centers. Ten to twelve milliliters of CSF were collected for analysis in a polypropylene tube, using a standardized technique with a 22G atraumatic spinal needle. Routine cell count and protein quantification were performed. CSF and plasma samples were centrifuged at 4 °C, immediately aliquoted, and frozen at − 80 °C until assayed.
Plasma NfL concentrations were measured using the NF-light™ kit on a Single molecule array (Simoa) HD-X Analyzer (Quanterix, Billerica, MA, USA), following the recommendations by the manufacturer. Plasma pTau181 levels were measured using an inhouse Simoa assay [21]. Briefly, an AT270 mouse monoclonal antibody (MN1050; Invitrogen, Waltham, MA, USA) was coupled to paramagnetic beads (103,207; Quanterix) and used for capture. As the detector, we used the anti-tau mouse monoclonal antibody Tau12 (806,502; BioLegend, San Diego, CA, USA), conjugated to biotin (A3959; Thermo Fisher Scientific, Waltham, MA, USA), while GSK-3β phosphorylated full-length recombinant tau441 (TO8–50FN; SignalChem, Vancouver, BC, Canada) was used as calibrator. Fluorescent signals were converted to average enzyme per bead numbers, and specimen concentrations extrapolated from four-parametric logistic curves generated with known calibrator concentrations. Plasma GFAP was measured using a second generation simple plex GFAP assay (ProteinSimple, CA, USA) on an ultrasensitive microfluidic platform (Ella, Bio-Techne, Minneapolis, USA), according to the manufacturers’ instructions [39]. Intra-assay coefficients of variation were less than 10%. Inter-assay imprecision was evaluated through repeating the measurement in six random samples with a mean variance of 11.2% (lowest 2.2% and highest 19.3%).
CSF Aβ42, total tau (tTau), and pTau181 concentrations were measured using commercially available ELISA kits (Fujirebio Europe, Gent, Belgium). The presence of CSF AD pathology was defined as a pTau181/Aβ42 ratio > 0.078, reflecting the concomitant presence of amyloid and tau pathology. This center cut-off was previously defined using study site data and is in line with previous publications [40]. It was further confirmed by using longitudinal clinical follow-up data and comparing it to the literature as previously described [41].
For the genotyping of the apolipoprotein E (APOE) ε2/ε3/ε4 polymorphism, DNA was extracted from whole blood using the QIA symphony DSP DNA Kit (Qiagen, Hombrechtikon, Switzerland). Single nucleotide variation rs429358 and rs7412 were genotyped using the TaqMan assays C___3084793_20 and C____904973_10, respectively (Thermo Fischer Scientific, Waltham, MA). Participants with one or more ε4 allele were classified as carriers.
Data preparation
Outliers (mean ± 3 standard deviations, n = 2 for each of the three biomarkers) were kept in the dataset as post-hoc sensitivity analysis did not change results for any of the performed analysis when removing the outliers. No missing value imputation was performed to prevent bias (n = 0 for NfL n = 2 for pTau181, and n = 23 for GFAP). GFAP concentrations were measured after the NfL and pTau181 assessments in the remaining plasma samples which resulted in a lower n for the GFAP results. Plasma NfL, GFAP and pTau181 were ln-transformed and normalized.
Statistical analysis
Descriptive statistics and regression analysis were performed using SPSS (IBM Corporation, Version 29.0, Armonk, NY, USA). For categorical variables, we calculated absolute and relative frequencies. For continuous variables, we calculated means and standard deviations. For cohort characteristics, two-tailed t-test and Mann-Whitney-U-test were applied for continuous and Pearson’s Chi-squared test for categorical variables. Normality of each variable was tested using the Shapiro-Wilk test to determine the appropriate statistical test. All statistical models were verified for possible overfitting using the Hosmer-Lemeshow test for goodness-of-fit. Models with a Hosmer-Lemeshow chi-squared value yielding a p-value > 0.05 were rejected and the previous iteration was considered instead. The alpha value was set at 0.05 for all statistical tests.
Associations of plasma biomarkers with current NPS, future NPS, and NPS severity change
To assess the relationship between the plasma biomarkers and the presence of NPS at baseline and follow-up we used binary logistic regression analysis with the presence of NPS (NPI-Q > 0) as dependent variable. To assess associations of the plasma biomarkers with NPS severity change over time we used linear regression analysis with the change in the NPI-Q severity score as the dependent variable. Plasma NfL, GFAP, and pTau181 were set as independent variables. Age and sex were added to the models as covariates. Furthermore, the same approach was used stratifying based on cognitive status (cognitively impaired with CDR = 0.5 vs. cognitively unimpaired with CDR = 0 at baseline). In a further exploratory step, we also added AD status as a variable to determine possible confounding effects.
Associations of plasma biomarkers with cognitive and functional decline and ad cerebral pathology
Cognitive and functional decline was used as the dependent variable within linear regression analysis, while the binary definition of cerebral AD pathology was used as the dependent variable within binary logistic regression analysis. NfL, GFAP, and pTau181 were set as independent variables, while age and sex were added as covariates.
Prediction of future NPS
To assess the predictive performance of single markers, a combination of them and considering the presence of baseline NPS, we computed the area under the curve (AUC) of receiver operating characteristics (ROC) curves using the pROC package in R [42]. Predictive models of future NPS (defined as NPI-Q > 0 at follow-up visit, indicating the presence of any NPS at follow-up [1, 43]), were built.
A convenience reference model including easily available clinical data, such as sex, age and baseline cognitive status, was built. The presence of baseline NPS and plasma NfL, GFAP and pTau181 - first all of them separately, then a combination of them - were added to the reference model. All models were then compared using the DeLong method [44]. Additionally, sensitivity and specificity along with accuracy were calculated for the different models.
Prediction of cognitive and functional decline and ad status with plasma biomarkers
To evaluate the performance of baseline NPS, single biomarkers, and combinations thereof to predict cognitive and functional decline (defined as worsening of the CDRSB > 0.5/year [45]) or the presence of cerebral AD pathology, the same approach using ROC analysis as mentioned above was used.
Results
Study participants
Of the included 151 participants, 76 had normal cognition (CDR = 0) and 75 had mild cognitive impairment (CDR = 0.5). Clinical, demographic, and biological characteristics of the participants grouped by the presence/absence of NPS are shown in Table 1.
Participants with NPS were older and had more marked impairment in global cognition (i.e., lower mini mental status examination (MMSE) scores) and higher disease severity (i.e., higher CDR and CDR-SB scores). In addition, the presence of cerebral AD pathology as indicated by a CSF AD biomarker profile was more frequent in participants with NPS. The distributions of the CSF core AD biomarkers between the two groups are shown in supplementary Table S1. The plasma biomarker levels did not differ between the participants with and those without NPS at baseline (see Fig. 1). In those having NPS, the mean NPI-Q severity score at baseline was 5.0 ± 4.6 and at follow-up 4.9 ± 4.2. The distribution of the NPI-Q scores at baseline and follow up was right-skewed and leptokurtic (at baseline skewedness of 2.8 and kurtosis of 10.6, at follow-up skewedness of 2.0 and kurtosis of 4.2). This reflected the known floor-effect of the NPI-Q with overrepresentation of zero values and is shown in Figure S1. In those having NPS the most common symptoms were anxiety (51.4%), apathy (41.7%), sleeping disorders (38.9%), irritability (36.1%), depression (31.9%) and eating disorders (31.9%) (for all frequencies see supplementary Table S2). Follow-up data on NPS was available in 108 participants and on cognitive impairment in 137 participants.
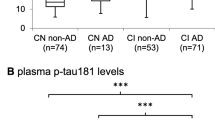
Plasma levels of GFAP, NfL and pTau181 at baseline in participants with and without NPS
Description: Violin plot showing the concentration (in pg/ml, y-axis) of plasma GFAP, NfL and pTau181 (x-axis) grouped by the presence (orange) or absence (blue) of NPS defined as NPI-Q > 0. The dashed lines indicate the mean, and the dotted lines indicate the standard deviation. P-values from group comparison are shown above each plot. GFAP, glial fibrillary acid protein; NfL, neurofilament light chain; NPI-Q, neuropsychiatric inventory questionnaire; NPS, neuropsychiatric symptoms; pTau181, tau phosphorylated at threonine 181
Associations of plasma biomarkers with current NPS, future NPS, and NPS severity change over time
Results of the associations between plasma biomarkers and NPS at baseline, with future NPS and with NPS severity change are shown in Table 2.
No plasma marker was associated with the NPS at baseline. Higher GFAP levels were associated with future NPS at follow-up. Higher NfL and higher GFAP levels were associated with an increase of the NPI-Q severity score (see Figure S1) over time, while lower NfL and GFAP levels were associated with a decrease of the NPI-Q severity score over time. These associations of GFAP with future NPS and NPS severity change remained significant after considering AD pathology (Table S3). Results stratified based on cognitive impairment are shown in Table S4.
Associations of plasma biomarkers with cognitive and functional decline and ad pathology
Higher levels of NfL were associated with cognitive and functional decline and with the presence of cerebral AD pathology, but both associations lost significance after controlling for age. Higher levels of GFAP were associated with cognitive and functional decline and with AD pathology and remained significant after controlling for age and sex (β = 0.24, 95% CI 0.04–0.43, p = .019; OR 3.3, 95% CI 1.9–5.6, p < .001).
While pTau181 showed no association with cognitive and functional decline, higher levels of pTau181 were associated with AD pathology after controlling for age and sex (OR 1.6, 95% CI 1.0–2.4, p = .041).
Prediction of future NPS
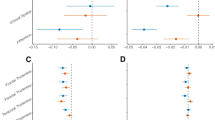
To evaluate the utility of the plasma biomarkers alone, in combination, and considering the presence of baseline NPS we performed ROC analysis. When added to the reference model, baseline NPS alone and in combination with GFAP improved the prediction of future NPS. The combination of baseline NPS and the three plasma biomarkers together in addition to the reference model was the best model to predict future NPS (from AUC 0.72 to 0.88, p = .002), as shown in Fig. 2A). Adding plasma biomarkers resulted in a higher AUC as compared to adding baseline NPS only, but the difference was not significant (from AUC 0.84 to 0.88, p = .132).
For the prediction of future NPS, the reference model had a specificity of 55%, a sensitivity of 83% and an accuracy of 70%, while the best model including NPS and the three plasma biomarkers improved to 78%, 81% and 79%, respectively.
Predictive models for future NPS (A) and AD status (B)
Description: Results from the ROC-analysis, whereas each bar corresponds to the AUC value (y-axis) of a prediction model. Different single markers and combinations of them, compared with a reference model based on clinical data only, are shown. The legend on the right shows’ which markers are included in each model. Future NPS was defined as NPI-Q severity score > 0, cognitive status as ∆CDR-SB > 0.5/time to follow-up in months and AD status as center cut-off pTau181/Aβ42 ratio > 0.078. Asterisks are indicating if a model was significantly better compared to the reference model. Aβ42, beta-amyloid 1–42 peptide; GFAP, glial fibrillary acid protein; NfL, neurofilament light chain; NPI-Q, neuropsychiatric inventory questionnaire; NPS, neuropsychiatric symptoms; pTau181, tau phosphorylated at threonine 181.
Prediction of cognitive and functional decline and ad pathology
No single marker or combination was able to improve the prediction of cognitive and functional decline at follow-up (from AUC 0.79 (reference model) to 0.85 (model including age, sex, cognitive status, the presence of NPS at baseline and plasma NfL, GFAP, and pTau181), p = .081), when compared to the reference model. However, the combination of the three plasma biomarkers with the presence of NPS at baseline was the best model to predict cerebral AD pathology and improved prediction when added to the reference model (from AUC 0.78 to 0.87, p = .010). While the reference model had a specificity of 75%, a sensitivity of 61% and an accuracy of 70%, the best model improved to 88%, 69% and 81%, respectively (see Fig. 2B).
Discussion
None of the three plasma biomarkers was associated with NPS at baseline. Higher plasma GFAP levels were associated with NPS at follow-up and with an increase in NPS severity over time, while higher plasma NfL levels were associated with an increase in NPS severity. Considering NPS along with the three plasma biomarkers improved the prediction of future NPS, but not with cognitive and functional decline, as compared to a reference model based on clinical features. In addition, considering the presence of NPS along with the plasma biomarkers improved the prediction of cerebral AD pathology as defined using CSF biomarkers.
Plasma GFAP was not associated with the presence of NPS at baseline. However, higher plasma GFAP was associated with the presence of NPS at follow-up and with an increase of the NPI-Q severity score over time. These associations seem to be driven by the results in the group of participants with cognitive impairment suggesting that plasma GFAP may be useful as a marker for the prediction of future or more severe NPS in particular in patients presenting with cognitive impairment. A few previous studies investigated the relation between GFAP levels and psychiatric symptoms so far [46, 47], but only one study in the context of AD [28]. In the latter study the association between glial markers (plasma GFAP and microglial activation measured by TSPO-PET) and the NPI-Q score were investigated in a longitudinal research cohort including individuals with normal cognition, MCI, AD dementia, or other types of dementia. While plasma GFAP showed no association with NPS in this study, microglial activation in different brain regions as measured by TSPO-PET was associated with NPS. GFAP is considered a marker of astrocytic cell activation which is related to neuroinflammation in AD [48]. Also, neuroinflammation as measured by CSF or blood proteins such as cytokines, or imaging of microglial activation has been associated with NPS before [28, 49, 50]. In this context our results suggest that GFAP represents a potential biomarker for detecting neuroinflammation associated with NPS persistence over time. Our results suggest that GFAP in combination with clinical data may be a useful marker to detect patients at risk for the presence of future or more severe NPS over time. Additional studies are needed to further address the relationships between neuroinflammation, increased GFAP levels, and NPS, especially in the context of neurocognitive disorders.
Plasma NfL was not associated with the presence of NPS at baseline in our cohort. This is in line with the few existing studies investigating associations of NPS with NfL concentrations in plasma or CSF [30, 51, 52]. However, a recent study in a cohort of individuals at clinical stages from cognitively unimpaired to dementia reported associations of plasma NfL with some single symptoms, such as aberrant motor behavior, anxiety, sleep disturbance, disinhibition, and euphoria [29]. Of note, only amyloid positive individuals were included, which could have biased the results. A possible explanation for the lack of a clear association of NfL with NPS is that axonal degeneration as indicated by increased NfL levels may be not specifically related to NPS and also occur in the absence of NPS. However, higher NfL was associated with with increasing NPS severity scores at follow-up, indicating a possible value as prognostic marker. To our knowledge, previous studies have not investigated NfL in relation to the evolution of NPS over time. Higher plasma NfL at baseline may indicate higher intensity of neurodegenerative processes which may more likely lead to the development of NPS. Our results suggest that higher plasma NfL indicates a higher risk of having (more severe) NPS in the future.
Plasma pTau181 was not associated with baseline NPS. This finding is in line with a recent study in a similar cohort [29]. In our study, plasma pTau181 was also not related to NPS at follow-up. In a previous study using data from the ADNI cohort NPS were only associated with plasma pTau181 in participants having NPS at two different visits within one year (defined as mild behavioral impairment [5]), but not in those having NPS at only one visit [31]. A few studies addressed the relationship between CSF pTau181 and NPS. While in a longitudinal study in cognitively unimpared older people, CSF pTau181 was correlated with higher NPI-Q scores after one-year [53], other studies did not find an association [32, 54, 55]. There are also few studies investigating the relationship of tau pathology with depression. While a recent meta-analysis found no association between plasma pTau181 with depressive symptoms [56], another study found elevated tau-PET levels beeing associated with a clinical depression diagnosis in cognitively unimpaired older people [57]. Comparing these findings is difficult due to the different methods and populations considered (e.g., memory-clinic setting vs. research cohorts). Overall, our results, along with the previous ones, suggest that plasma pTau181 is not closely related to NPS and it may not be an useful marker for NPS and NPS evolution over time.
We further showed that considering the presence of baseline NPS along with plasma NfL, GFAP, and pTau181 improved the prediction of future NPS at follow-up visit. The presence of NPS at baseline showed the strongest contribution to the prediction model. To our knowledge, no previous study has investigated the potential of plasma biomarkers combined with NPS to predict the evolution of NPS over time.
Our findings suggest that plasma NfL, GFAP, and pTau181, together with easily accessible clinical assessments such as the NPI-Q may be helpful to predict future manifestation of NPS.
No single marker or combination with NPS was able to predict cognitive and functional decline. This may be related to the strong reference model in our study including age and baseline cognitive impairment, which both are known to be related to more rapid cognitive decline.
The combination of plasma NfL, GFAP, and pTau181 with NPS was the best model to predict cerebral AD pathology. These results show the importance of capturing NPS in memory-clinic settings to improve diagnosis and inform decision-making on further diagnostics and treatment. If confirmed, NPS along with cognitive measurements and easily accessible blood biomarkers could be of particular importance for decision-making on additional diagnostic steps. However, studies investigating whether the presence of NPS may predict the developmemt of AD pathology are still needed and could be helpful to disentangle the complex relation of NPS and AD pathology.
There are some limitations to our study. We considered the presence of overall NPS, without differentiating between single symptoms. However, considering single symptoms would result in too small subgroups in our sample. Furthermore, we have not included participants with dementia or more severe NPS that may strongly interfere with cognitive performance. Accordingly, our findings may not be fully representative for the older people presenting with NPS. Plasma NfL, GFAP, and pTau181 were solely measured at baseline, wherefore no longitudinal changes in their levels were available. However, our study is to our best knowledge the first one investigating the associations between plasma NfL, GFAP, and pTau181 levels and the longitudinal evolution of NPS in older people with and without MCI. Including and following up participants that were cognitively unimpaired or at mild stages of cognitive impairment represents a strength of this study. This allows for assessing the potential contribution of NPS in relation to plasma biomarkers for early diagnosis and prognosis, which is of particular interest in the memory clinic patients.
Conclusion
Our findings indicate that capturing NPS along with plasma NfL, GFAP, and pTau181 in memory clinic patients may be helpful to predict the evolution of NPS. Considering these findings could also help to improve personalized decision-making on further diagnostics and treatment while using non-invasive assessment methods. Further work is needed to confirm and extend these observations, considering specific NPS and additional blood-based biomarker candidates, to improve the prediction of the NPS manifestation and evolution over time.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Aβ42:
-
Beta-amyloid 1–42 peptide
- AD:
-
Alzheimer’s disease
- ADNI:
-
Alzheimer’s Disease Neuroimaging Initiative
- APOEe4:
-
Apolipoprotein E epsilon 4
- AUC:
-
Area under the curve
- CDR-SB:
-
Clinical dementia rating sum-of-boxes
- CDR:
-
Clinical dementia rating
- CI:
-
Confidence interval
- CSF:
-
Cerebrospinal fluid
- GFAP:
-
Glial fibrillary acid protein
- IADL:
-
Instrumental activities of daily living
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini mental status examination
- NfL:
-
Neurofilamet light chain
- NPI-Q:
-
Neuropsychiatric Inventory Questionnaire
- NPS:
-
Neuropsychiatric symptoms
- pTau181:
-
Tau phosphorylated at threonine 181
- ROC:
-
Receiver operating characteristics
- Simoa:
-
Single molecule array
- tTau:
-
Total tau
References
Eikelboom WS, van den Berg E, Singleton EH, Baart SJ, Coesmans M, Leeuwis AE, et al. Neuropsychiatric and cognitive symptoms across the Alzheimer disease clinical spectrum: cross-sectional and longitudinal associations. Neurology. 2021;97(13):e1276–87.
Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the cache county study. Int J Geriatr Psychiatry. 2008;23(2):170–7.
Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Xu W, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–71.
Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532–9.
Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195–202.
Peters ME, Rosenberg PB, Steinberg M, Norton MC, Welsh-Bohmer KA, Hayden KM, et al. Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: the cache county study. Am J Geriatr Psychiatry. 2013;21(11):1116–24.
Dietlin S, Soto M, Kiyasova V, Pueyo M, de Mauleon A, Delrieu J, et al. Neuropsychiatric symptoms and risk of progression to Alzheimer’s disease among mild cognitive impairment subjects. J Alzheimers Dis. 2019;70(1):25–34.
Spiegl K, Luttenberger K, Graessel E, Becker L, Scheel J, Pendergrass A. Predictors of institutionalization in users of day care facilities with mild cognitive impairment to moderate dementia. BMC Health Serv Res. 2021;21(1):1009.
Peters ME, Schwartz S, Han D, Rabins PV, Steinberg M, Tschanz JT, et al. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: the cache county dementia progression study. Am J Psychiatry. 2015;172(5):460–5.
Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9.
Skogseth R, Mulugeta E, Jones E, Ballard C, Rongve A, Nore S, et al. Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):559–63.
Engelborghs S, Maertens K, Vloeberghs E, Aerts T, Somers N, Mariën P, et al. Neuropsychological and behavioural correlates of CSF biomarkers in dementia. Neurochem Int. 2006;48(4):286–95.
Showraki A, Murari G, Ismail Z, Barfett JJ, Fornazzari L, Munoz DG, et al. Cerebrospinal fluid correlates of neuropsychiatric symptoms in patients with Alzheimer’s disease/mild cognitive impairment: a systematic review. J Alzheimers Dis. 2019;71(2):477–501.
Tissot C, Therriault J, Pascoal TA, Chamoun M, Lussier FZ, Savard M, et al. Association between regional tau pathology and neuropsychiatric symptoms in aging and dementia due to Alzheimer’s disease. Alzheimers Dement (N Y). 2021;7(1):e12154.
Goukasian N, Hwang KS, Romero T, Grotts J, Do TM, Groh JR, et al. Association of brain amyloidosis with the incidence and frequency of neuropsychiatric symptoms in ADNI: a multisite observational cohort study. BMJ Open. 2019;9(12):e031947.
Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther. 2018;10(1):71.
Chatterjee P, Pedrini S, Stoops E, Goozee K, Villemagne VL, Asih PR, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):27.
Kim KY, Shin KY, Chang KA. GFAP as a potential biomarker for Alzheimer’s disease: a systematic review and meta-analysis. Cells. 2023;12(9).
Clark C, Lewczuk P, Kornhuber J, Richiardi J, Maréchal B, Karikari TK, et al. Plasma neurofilament light and phosphorylated tau 181 as biomarkers of Alzheimer’s disease pathology and clinical disease progression. Alzheimers Res Ther. 2021;13(1):65.
Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791–9.
Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422–33.
Verberk IMW, Laarhuis MB, van den Bosch KA, Ebenau JL, van Leeuwenstijn M, Prins ND, et al. Serum markers glial fibrillary acidic protein and neurofilament light for prognosis and monitoring in cognitively normal older people: a prospective memory clinic-based cohort study. Lancet Healthy Longev. 2021;2(2):e87–95.
Mattsson N, Andreasson U, Zetterberg H, Blennow K, Initiative ADN. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557–66.
Karikari TK, Benedet AL, Ashton NJ, Lantero Rodriguez J, Snellman A, Suárez-Calvet M, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s disease neuroimaging initiative. Mol Psychiatry. 2021;26(2):429–42.
Bavato F, Cathomas F, Klaus F, Gütter K, Barro C, Maceski A, et al. Altered neuroaxonal integrity in schizophrenia and major depressive disorder assessed with neurofilament light chain in serum. J Psychiatr Res. 2021;140:141–8.
Tarasov VV, Svistunov AA, Chubarev VN, Sologova SS, Mukhortova P, Levushkin D, et al. Alterations of astrocytes in the context of schizophrenic dementia. Front Pharmacol. 2019;10:1612.
Desai P, Krueger KR, Mendes de Leon C, Wilson RS, Evans DA, Rajan KB. Depressive symptoms, glial fibrillary acid protein concentrations, and cognitive decline in a cohort study. J Gerontol Biol Sci Med Sci. 2024;79(2).
Schaffer Aguzzoli C, Ferreira PCL, Povala G, Ferrari-Souza JP, Bellaver B, Soares Katz C, et al. Neuropsychiatric symptoms and microglial activation in patients with Alzheimer disease. JAMA Netw Open. 2023;6(11):e2345175.
Huang L, Huang Q, Xie F, Guo Q. Neuropsychiatric symptoms in Alzheimer’s continuum and their association with plasma biomarkers. J Affect Disord. 2024;348:200–6.
Naude JP, Gill S, Hu S, McGirr A, Forkert ND, Monchi O, et al. Plasma neurofilament light: a marker of neurodegeneration in mild behavioral impairment. J Alzheimers Dis. 2020;76(3):1017–27.
Ghahremani M, Wang M, Chen HY, Zetterberg H, Smith E, Ismail Z et al. Plasma P-Tau181 and neuropsychiatric symptoms in preclinical and prodromal Alzheimer disease. Neurology. 2022.
Krell-Roesch J, Rakusa M, Syrjanen JA, van Harten AC, Lowe VJ, Jack CR, et al. Association between CSF biomarkers of Alzheimer’s disease and neuropsychiatric symptoms: Mayo Clinic study of aging. Alzheimers Dement. 2023;19(10):4498–506.
Popp J, Oikonomidi A, Tautvydaitė D, Dayon L, Bacher M, Migliavacca E, et al. Markers of neuroinflammation associated with Alzheimer’s disease pathology in older adults. Brain Behav Immun. 2017;62:203–11.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256(3):240–6.
Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–7.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4.
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72.
Fazeli B, Huss A, Gómez de San José N, Otto M, Tumani H, Halbgebauer S. Development of an ultrasensitive microfluidic assay for the analysis of glial fibrillary acidic protein (GFAP) in blood. Front Mol Biosci. 2023;10:1175230.
Duits FH, Teunissen CE, Bouwman FH, Visser PJ, Mattsson N, Zetterberg H, et al. The cerebrospinal fluid Alzheimer profile: easily said, but what does it mean? Alzheimers Dement. 2014;10(6):713–e232.
Mathys J, Gholamrezaee M, Henry H, von Gunten A, Popp J. Decreasing body mass index is associated with cerebrospinal fluid markers of Alzheimer’s pathology in MCI and mild dementia. Exp Gerontol. 2017;100:45–53.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77.
Rabl M, Clark C, Dayon L, Bowman GL, Popp J. Blood plasma protein profiles of neuropsychiatric symptoms and related cognitive decline in older people. J Neurochem. 2023;164(2):242–54.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Borland E, Edgar C, Stomrud E, Cullen N, Hansson O, Palmqvist S. Clinically relevant changes for cognitive outcomes in preclinical and prodromal cognitive stages: implications for clinical Alzheimer trials. Neurology. 2022.
Bark L, Larsson IM, Wallin E, Simrén J, Zetterberg H, Lipcsey M, et al. Central nervous system biomarkers GFAp and NfL associate with post-acute cognitive impairment and fatigue following critical COVID-19. Sci Rep. 2023;13(1):13144.
Edwards KA, Lange RT, Lippa SM, Brickell TA, Gill JM, French LM. Serum GFAP, NfL, and tau concentrations are associated with worse neurobehavioral functioning following mild, moderate, and severe TBI: a cross-sectional multiple-cohort study. Front Neurol. 2023;14:1223960.
Singh D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J Neuroinflammation. 2022;19(1):206.
Clark C, Richiardi J, Maréchal B, Bowman GL, Dayon L, Popp J. Systemic and central nervous system neuroinflammatory signatures of neuropsychiatric symptoms and related cognitive decline in older people. J Neuroinflammation. 2022;19(1):127.
Capuron L, Castanon N. Role of inflammation in the development of neuropsychiatric symptom domains: evidence and mechanisms. Curr Top Behav Neurosci. 2017;31:31–44.
Bloniecki V, Zetterberg H, Aarsland D, Vannini P, Kvartsberg H, Winblad B, et al. Are neuropsychiatric symptoms in dementia linked to CSF biomarkers of synaptic and axonal degeneration? Alzheimers Res Ther. 2020;12(1):153.
Aktas O, Renner A, Huss A, Filser M, Baetge S, Stute N et al. Serum neurofilament light chain: no clear relation to cognition and neuropsychiatric symptoms in stable MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(6).
Babulal GM, Stout SH, Head D, Holtzman DM, Fagan AM, Morris JC, et al. Neuropsychiatric symptoms and Alzheimer’s disease biomarkers predict driving decline: brief report. J Alzheimers Dis. 2017;58(3):675–80.
Babulal GM, Ghoshal N, Head D, Vernon EK, Holtzman DM, Benzinger TLS, et al. Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am J Geriatr Psychiatry. 2016;24(11):1095–104.
Johansson M, Stomrud E, Insel PS, Leuzy A, Johansson PM, Smith R, et al. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer’s disease. Transl Psychiatry. 2021;11(1):76.
Twait EL, Kamarioti M, Verberk IMW, Teunissen CE, Nooyens ACJ, Monique Verschuren WM et al. Depressive symptoms and plasma markers of Alzheimer’s disease and neurodegeneration: a coordinated meta-analysis of 8 cohort studies. Am J Geriatr Psychiatry. 2024.
Babulal GM, Roe CM, Stout SH, Rajasekar G, Wisch JK, Benzinger TLS, et al. Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. J Alzheimers Dis. 2020;74(4):1045–55.
Dixon JR. The international conference on harmonization good clinical practice guideline. Qual Assur. 1998;6(2):65–74.
Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Acknowledgements
MR was funded by the “Filling the Gap” grant from the University of Zurich. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), and the UK Dementia Research Institute at UCL (UKDRI-1003). KB is supported by the Swedish Research Council (#2017-00915 and #2022-00732), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the National Institute of Health (NIH), USA, (grant #1R01AG068398-01), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), and the Alzheimer’s Association 2022-2025 Grant (SG-23-1038904 QC). TKK was funded by the Swedish Research Council (Vetenskapsrådet #2021-03244), the Alzheimer’s Association Research Fellowship (#AARF-21-850325), the Aina (Ann) Wallströms and Mary-Ann Sjöbloms stiftelsen, and the Emil och Wera Cornells stiftelsen. FB was supported by the EMDO Stiftung, Switzerland, and by the Fonds für wissenschaftliche Zwecke im Interesse der Heilung von psychischen Krankheiten, Switzerland.
Funding
This work was supported by grants from the Swiss National Science Foundation (grant number 320030_141179; 320030_204886) and from the Synapsis Foundation - Dementia Research Switzerland (grant number 2017-PI01).
Author information
Authors and Affiliations
Contributions
MR wrote the initial draft of the manuscript, conducted data modelling, statistical analyses, interpreted the data, and prepared the figures. FB, CC, PL, JK, TK, KB, and HZ contributed to the assessment of plasma GFAP, NfL and pTau181, including assessments, data preparation and quality control. CC contributed to data modelling, statistical analyses, and writing. JP designed the study, supervised the work, and contributed to data interpretation and manuscript writing. All authors read, provided critical feedback, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was conducted in accordance with applicable laws and regulations, including the International Conference on Harmonization, Guideline for Good Clinical Practice [58], and the ethical principles from the Declaration of Helsinki [59]. The study was approved by the local ethics committee of canton Vaud, Switzerland (No. 171/2013). Prior to inclusion, written informed consent was obtained from all participants.
Competing interests
PL received consultation and/or lecture honoraria from IBL International, Fujirebio Europe, AJ Roboscreen, Biogen, and Roche. KB has served as a consultant, at advisory boards, or at data monitoring committees for Acumen, ALZPath, BioArctic, Biogen, Eisai, Julius Clinical, Lilly, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). CC received consultation and speaker honoraria from OM Pharma Suisse. MR received speaker honoraria from OM Pharma Suisse. JP received consultation and speaker honoraria from Nestle Institute of Health Sciences, Innovation Campus EPFL, Ono Pharma, Lilly, Roche, Eisai, Schwabe Pharma, OM Pharma Suisse and from Fujirebio Europe. The other authors declare no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rabl, M., Zullo, L., Lewczuk, P. et al. Plasma neurofilament light, glial fibrillary acid protein, and phosphorylated tau 181 as biomarkers for neuropsychiatric symptoms and related clinical disease progression. Alz Res Therapy 16, 165 (2024). https://doi.org/10.1186/s13195-024-01526-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01526-4