Abstract
Background
Non-invasive brain stimulation (NIBS) combined with cognitive training (CT) may have shown some prospects on improving cognitive function in patients with Alzheimer’s disease (AD) and mild cognitive impairment (MCI). However, data from clinical trials or meta-analysis involving NIBS combined with CT have shown controversial results. The aim of this systematic review and meta-analysis was to evaluate short-term and long-term effects of NIBS combined with CT on improving global cognition and other specific cognitive domains in patients with AD and MCI.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Five electronic databases including PubMed, Web of Science, EBSCO, Cochrane Library and Embase were searched up from inception to 20 November 2023. The PEDro scale and the Cochrane’s risk of bias assessment were used to evaluate risk of bias and methodological quality of included studies. All statistical analyses were conducted with Review Manager 5.3.
Results
We included 15 studies with 685 patients. The PEDro scale was used to assess methodological quality with a mean score of 7.9. The results of meta-analysis showed that NIBS combined with CT was effective on improving global cognition in AD and MCI (SMD = 0.52, 95% CI (0.18, 0.87), p = 0.003), especially for patients accepting repetitive transcranial magnetic stimulation (rTMS) combined with CT (SMD = 0.46, 95% CI (0.14, 0.78), p = 0.005). AD could achieve global cognition improvement from NIBS combined with CT group (SMD = 0.77, 95% CI (0.19, 1.35), p = 0.01). Transcranial direct current stimulation (tDCS) combined with CT could improve language function in AD and MCI (SMD = 0.29, 95% CI (0.03, 0.55), p = 0.03). At evaluation follow-up, rTMS combined with CT exhibited larger therapeutic responses to AD and MCI in global cognition (SMD = 0.55, 95% CI (0.09, 1.02), p = 0.02). AD could achieve global cognition (SMD = 0.40, 95% CI (0.03, 0.77), p = 0.03) and attention/working memory (SMD = 0.72, 95% CI (0.23, 1.20), p = 0.004) improvement after evaluation follow-up from NIBS combined with CT group.
Conclusions
Overall, NIBS combined with CT, particularly rTMS combined with CT, has both short-term and follow-up effects on improving global cognition, mainly in patients with AD. tDCS combined with CT has advantages on improving language function in AD and MCI. Future more studies need evaluate cognitive effects of NIBS combined with CT on other specific cognitive domain in patients with cognitive deterioration.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease with severe deterioration of cognitive function and activity of daily living [1]. Mild cognitive impairment (MCI) is the preclinical stage of AD and every patient who develops AD would first experiences this stage [2]. In China, epidemiological investigations show that the estimated prevalence of MCI is 15.5% among adults aged over 60 years [3]. Among those with MCI, about 15% would develop dementia after 2 years, and 33% progress to AD within 5 years [4, 5]. Progressive cognitive deterioration imposes a heavy burden on patients and their families. The economic value of care to be provided by families and other unpaid caregivers of patients with dementia has reached $339.5 billion in the United States in 2022 [1], meanwhile, the cost of social care for AD is higher than the global average in China [6]. While some pharmacological interventions, such as monoclonal antibodies targeting Aβ (e.g., Lecanemab) [7], have demonstrated potential benefits in mitigating cognitive decline and preserving function in early AD, the overall effectiveness of these treatments remains limited and warrants further investigation [8]. In recent years, there is growing interest in exploring the benefits of non-pharmacological interventions.
Non-invasive brain stimulation (NIBS), typically including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), is a class of cost-effective, safe, and easy-to-administer techniques which can modulate brain excitability and plasticity to improve cognition function in AD and MCI [9, 10]. However, a meta-analysis by Inagawa et al. [11] thought NIBS showed limited effects on improving cognitive function in AD and MCI. Cognitive training (CT) is defined as treatment focusing on guided practice on tasks for specific cognitive functions. Plenty of evidences indicated that CT could improve cognitive functions in AD and MCI [12,13,14,15], possibly due to the reciprocity between cognitive mental activity stimulated by CT and cerebral biochemistry [16]. NIBS modulates neural plasticity directly in targeted regions and networks of brain, while CT may improve cognitive function in AD and MCI by indirectly modulate brain plasticity. A randomized controlled trials by Lee et al. [17] found a significant effect of rTMS combined with CT on improving memory and language domains in AD. Similarly, another clinical trial by Andrade et al. [18] showed tDCS combined with CT modulated cortical activity and improved global cognition in AD. NIBS combined with CT for AD and MCI seems to achieve better cognitive improvement, however, there is still a lack of high-level evidence at present.
Current research on the effects of NIBS combined with CT on improving cognitive function has shown controversial results. Two meta-analyses results found NIBS combined with CT had no conclusive advantage on improving cognitive function in MCI or AD [9, 19]. Those meta-analyses included few studies to qualitative synthesis, and the overall certainty of evidence was very low. Another meta-analysis including patients with Parkinson’s disease, MCI, AD and other multiple neuropsychiatric disorders [20], but the result did not find the effects of NIBS combined with CT. That meta-analysis might result in high heterogeneity due to different types of patients included. Consequently, we completed a systematic review and meta-analysis to re-evaluate the effect of NIBS combined with CT on cognitive function in AD and MCI from all available clinical studies when compared to only NIBS, CT or placebo. This will help us better understand the potential of NIBS combined with CT to provide solutions for cognitive deterioration, with the aim of outlining more robust interventions for patients with AD and MCI in the future.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21]. The protocol of this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO): CRD42023417926.
Search strategy
The search from the earliest available to 20 November 2023 was identified in following databases: PubMed, Web of Science, EBSCO, Cochrane Library and Embase. The selected keywords and search strategy were shown in supplementary material 1. Hand searching was also conducted to identify potentially relevant studies.
Eligibility criteria
The inclusion criteria were determined according to the PICOS approach: (1) patients were diagnosed with MCI or AD according to Peterson`s criteria of MCI [22], DSM-5 [23] or NIA-AA`s criteria of AD [24]; (2) the interventions were combination of NIBS(e.g., tDCS or rTMS) with CT; (3) the control group could be either a combination of CT with sham NIBS, a combination of NIBS with sham CT, only CT, only NIBS, or a placebo group; (4) study design was randomized controlled trial (RCT) or randomized cross-over design published; (5) articles were published in English. The exclusion criteria were as follow: (1) other intervention than NIBS or CT; (2) participants aged < 60 years; (3) studies were published as conference proceedings or dissertations.
Data extraction and quality assessment
The included studies were independently reviewed and selected based on the eligibility criteria by two reviewers (WL and CG). Titles and abstracts of all potentially relevant studies were screened, and full texts of the possible included studies were then screened for final inclusion. Another two reviewers (TY and JH) extracted required data of all included studies independently into a predesigned sheet. The data extracted from those studies included first author, year of publication, study characteristics (study design, population, intervention time, group design, NIBS parameters and follow-up time) and outcome measures. Corresponding authors of included records were contacted for missing data. Primary articles with missing data/variables that could not be used for all outcomes analyses were not included in this review. Any disagreements during data extraction were discussed and adjudicated by a third reviewer (LM).
Methodological quality assessment for each study was assessed using items adapted from the PEDro scale [25]. Two experienced reviewers (TY and WL) independently rated the included studies using the PEDro scale. Risk of bias assessments for each study were conducted by two experienced reviewers (TY and WL) according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions [26]. These items were designed to assess whether the study contained methodological bias that could affect meta-analysis results. When any disagreements during the assessments were discussed, a third reviewer (LX) participated in negotiation to jointly decide the quality of the included studies.
Data analysis
The results of all included RCTs and cross-over designs studies were used standard meta-analytic methods to evaluate the effects of NIBS combined with CT in AD and MCI. The means and standard deviations (SDs) of the change were used to calculate the absolute magnitude of change of outcome measures after interventions for experiment and control groups. The standardised mean differences (SMDs) with 95% confidence intervals (CIs) were calculated for continuous variables. Significant difference was set as P-value ≤ 0.05, and 95% CIs were also presented. Statistical heterogeneity was evaluated using chi-square test and I2 statistic. The values of I2 > 40% was considered to represent high statistical heterogeneity [27]. All meta-analysis results were performed using a random effects model, because there could be variability between studies due to different diagnostic types or applications of NIBS interventions. In this review, we chose to conduct separate meta-analysis for any cognitive domain that were investigated in at least 3 included studies. All statistical analysis was conducted using Review Manager 5.3.
Results
Search results
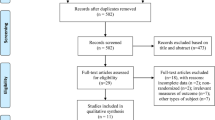
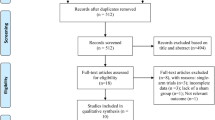
According to before mentioned search strategy, 1148 published studies were identifies from the selected database. Fifty-nine studies were retrieved after screening titles and abstracts. Forty-one studies were excluded due to study design (n = 37; 1 review, 9 study protocols, 22 conference abstracts, 1 participants aged < 60 years, 4 non-randomized controlled studies), full texts not available (n = 4). Three additional studies were excluded as complete data was not obtained from the articles or authors. Finally, 15 studies with 685 patients met the eligibility criteria (Fig. 1). Patients demographic characteristics were found in Table 1. Mean age of patients included studies ranged between 69.0 and 76.6 years old, and education years of most patients had mean over 6 years except 2 studies [18, 28]. For pre-treatment cognitive assessment, Lu et al. [29] used ADAS-Cog, Gonzalez et al. [30] used MoCA, and the others used MMSE.
Study characteristics
Details of 15 included studies were summarized in Table 2. Studies included in this meta-analysis were published between 2013 and 2022. Among those studies, 9 used tDCS as intervention of NIBS [18, 28,29,30,31,32,33,34,35], another 6 used rTMS [17, 36,37,38,39,40]. Two studies used randomized cross-over design [34, 35], the others used randomized controlled design. For target patients, 4 studies included MCI [29, 30, 32, 35], 9 studies included AD or other dementia [17, 18, 28, 33, 34, 36,37,38, 40], and 2 studies included both AD and MCI [31, 39]. For tDCS stimulation montage, anodal tDCS F3 montage [28, 30,31,32,33] was utilized in half studies, while other studies utilized anodal tDCS T3 montage [29], P3 montage [34], and T6 montage [35], respectively. Only 1 study chose multisite anodal tDCS montages including F3, F4, F5, P4, P5 and CP5 [18]. For stimulation montage of rTMS, 5 studies utilized multisite montages [17, 36,37,38, 40], except 1 study used F3 montage [39]. Most of studeis administered NIBS stimulation and CT simultaneously, except 1 studies administered tDCS earlier than CT [32] and 1 study administered rTMS earlier than CT [40]. We obtained follow-up data from 11 studies, while 2 studies were unable to be included in results analysis due to missing follow-up data [37, 38]. Two studies did not include follow-up assessments in their methodology [18, 35].
Risk of bias assessment
The PEDro scores ranged from 6 to 9, with a median of 7.9, indicating that the methodological quality of included studies was relatively high. All included studies were classified with “Excellent” or “Good” quality, reporting adequately with regard to their “random allocation” and “blind subjects”. However, no studies satisfied the “blind therapists” criteria. A detailed evaluation of PEDro scores was shown in Table 3. In risk of bias assessments, 4 studies were found to have high potential risk of bias because of insufficient concealing group allocation for patients or no fully reporting primary outcomes [28, 33,34,35]. Risk of bias assessments with included studies in this review were shown in Figs. 2 and 3.
Meta-analysis results
Due to the limited or absent data available of rTMS combined with CT studies on specific cognitive domains, we conducted separate meta-analysis for specific cognitive domain in tDCS combined with CT studies. Only subgroup analysis was performed exploring both tDCS and rTMS on global cognition. In this review, cognitive domains were analyzed including global cognition, executive function, attention/working memory, memory, and language. Cognitive domains and outcome measures for each study were shown in Table 4.
Effects of NIBS combined with CT on different cognitive domains
Total of 12 studies with 591 patients reported global cognition scores including 6 studies performing tDCS combined with CT (n = 375) and 6 studies performing rTMS combined with CT (n = 216). The result of meta-analysis showed that NIBS combined with CT significantly improved global cognition scores in AD and MCI (SMD = 0.52, 95% CI (0.18, 0.87), p = 0.003; Fig. 4A). In subgroup data analyses, rTMS combined with CT significantly improved global cognition scores in AD and MCI (SMD = 0.46, 95% CI (0.14, 0.78), p = 0.005; Fig. 4A), while tDCS combined with CT showed no statistically significant effect on global cognition in AD and MCI (SMD = 0.58, 95% CI (-0.06, 1.21), p = 0.08; Fig. 4A).
For meta-analysis of specific cognitive domains, only studies involving tDCS combined with CT reported the results of specific cognitive domains scores. Three studies with 245 patients showed that tDCS combined with CT improved language scores compare to the control group (SMD = 0.29, 95% CI (0.03, 0.55), p = 0.03; Fig. 4E). However, the pooled results of 4 studies with 138 patients on execution function (SMD = 0.02, 95% CI (-0.35, 0.39), p = 0.92, Fig. 4B), 6 studies with 407 patients on attention/working memory (SMD = -0.02, 95% CI (-0.2, 0.18), p = 0.81, Fig. 4C), 6 studies with 418 patients on memory (SMD = 0.13, 95% CI (-0.07, 0.33), p = 0.21, Fig. 4D) all showed no statistically improvement.
Effects of NIBS combined with CT in patients with different diagnosis
Three studies with 315 patients and 5 studies with 382 patients reported attention/working memory and memory scores in MCI, respectively. However, there was no statistically effect of NIBS combined with CT on attention/working memory (SMD = 0.13, 95% CI (-0.51, 0.24), p = 0.50; Fig. 5A) or memory scores (SMD = 0.11, 95% CI (-0.10, 0.32), p = 0.31; Fig. 5B).
Eight studies with 246 patients reported global cognition scores in AD. The result showed that NIBS combined with CT was statistically significant improvement on global cognition scores in AD (SMD = 0.77, 95% CI (0.19, 1.35), p = 0.01; Fig. 5C). However, the pooled results of 3 studies with 72 patients did not identify a statistically significant improve attention/working memory (SMD = 0.63, 95% CI (-0.31, 1.57), p = 0.19; Fig. 5D) or language scores (SMD = 0.27, 95% CI (-0.19, 0.74), p = 0.25; Fig. 5E) in AD.
Effects of NIBS combined with CT on follow-up
A total of 9 studies with 477 patients reported follow-up global cognition including 5 studies performing tDCS combined with CT (n = 339) and 4 studies performing rTMS combined with CT (n = 138). The result showed that there were no statistically global cognition improvement on follow-upin AD and MCI (SMD = 0.24, 95% CI (-0.02, 0.49), p = 0.07, Fig. 6A). While the result of subgroup analysis showed AD and MCI achieved signifcant follow-upglobal cognition improvement in rTMS combined with CT group (SMD = 0.55, 95% CI (0.09, 1.02), p = 0.02, Fig. 6A).
Furthermore, there were no statistically executive function improvement on follow-up in 4 studies with 138 patients (SMD = -0.30, 95% CI (-0.47, 0.24), p = 0.54, Fig. 6B), follow-up attention/working memory in 6 studies with 407 patients (SMD = -0.03, 95% CI (-0.24, 0.18), p = 0.78, Fig. 6C), follow-up memory in 5 studies with 387 patients (SMD = 0.13, 95% CI (-0.11, 0.37), p = 0.29, Fig. 6D) or follow-up language in 3 studies with 245 patients (SMD = 0.02, 95% CI (-0.27, 0.32), p = 0.88; Fig. 6E) either.
Effects of NIBS combined with CT in patients with different diagnosis on follow-up
Three studies with 335 patients reported follow-up attention/working memory and follow-up memory scores in MCI. The pooled results showed that MCI did not achieved signifcant follow-up attention/working memory (SMD = -0.21, 95% CI (-0.44, 0.01), p = 0.06; Fig. 7A) or follow-up memory scores (SMD = 0.18, 95% CI (-0.04, 0.41), p = 0.11; Fig. 7B) improvement in NIBS combined with CT group.
Six studies with 182 patients and 3 studies with 72 patients reported follow-up global cognition and follow-up attention/working memory in AD, respectively. The pooled results showed NIBS combined with CT signifcantly improved follow-up global cognition (SMD = 0.40, 95% CI (0.03, 0.77), p = 0.03; Fig. 7C) and follow-up attention/working memory (SMD = 0.72, 95% CI (0.23, 1.20), p = 0.004; Fig. 7D) in AD. However, 3 studies with 72 patients did not achieve signifcant follow-up language improvement in AD (SMD = 0.12, 95% CI (-0.37, 0.61), p = 0.63; Fig. 7E).
Discussion
This systematic review and meta-analysis aimed to evaluate the effects of NIBS combined with CT on cognitive function in AD and MCI including 15 studies with patients. The results of meta-analysis provided the following clear evidence: (1) rTMS combined with CT could improve short-term and follow-up global cognition in AD; (2) only AD could achieve short-term and follow-up global cognition improvement from NIBS combined with CT; (3) the benefits of NIBS combined with CT on follow-up attention/working memory were observed in AD; (4) tDCS combined with CT could improve short-term language in AD and MCI.
In this meta-analysis, we provided clear evidence that NIBS combined with CT could improve global cognition in AD and MCI as compared with only NIBS, CT or placebo. In addition, patients with AD achieved global cognition improvement from NIBS combined with CT group. Study outcomes from Chu et al. [41] and Wang et al. [42] were inconsistent with our results. There was a possible reason that the results by Chu et al. might be due to the limited number studies using NIBS combined with CT. Although AD have limited benefits derived from CT [43], NIBS seemed to help them maximize the benefits from CT as much as possible. It is currently thought that NIBS is able to induces and acquires brain's capacity for neuroenhancement [44], which may improve cognitive performance of patients. As a treatment approach to activate brain, CT could enhance functional network connectivity and functional efficiency of brain regions [45], and improved neuroplasticity of brain. When NIBS combined with CT, two treatments showed a synergistic effect presenting with greater neuroenhancement and neuroplasticity of brain, thereby strengthening cognitive performance in AD and MCI. It was noteworthy that the effects of individualised CT might only benefit in one specific cognitive domain, making it difficult to generalize to other specific cognitive domains [28]. Given the limited data available of included studies, we couldn't draw conclusions about the effect of NIBS combined with CT on improving global cognition in MCI. A meta-analysis by Xu et al. [46] found that NIBS could improve global cognition in MCI. If future more studies could obtain supports of sufficient data, a reciprocal synergistic effect of NIBS combined with CT in MCI maybe support causal hypothesis.
The result of subgroup analysis showed that rTMS combined with CT could improve global cognition in AD and MCI, while tDCS combined with CT not. Due to the absence of significantly effective pharmacotherapy or non-drug therapy on cognitive rehabilitation, patients and their families often struggle to choose which intervention would be more beneficial. Comparative efficacy of rTMS and tDCS in AD and MCI from previous studies was not clear [9, 47]. A meta-analysis by Wang et al. [42] did not compare the effects of rTMS combined with CT and tDCS combined with CT in AD and MCI. Our result contributed to providing recommendations for patients with cognitive impairment to choose more effective treatment of cognitive rehabilitation. Generally, rTMS produces more focused and deeper stimulations on brain regions and directly induces action potentials, whereas tDCS modulates the resting membrane potential of neurons and stimulates a more superficial and broader part of the cerebral cortex [48]. In addition, the current intensity of tDCS is more affected by skull and skin, resulting in some resistance to the current reaching the cerebral cortex. These influences weaken reciprocal synergistic effect between tDCS and CT, increasing treatment variability for patients. In studies involving rTMS combined with CT, only study by Bagattini et al. [39] included a small number of patients with MCI, therefore the meta-analysis results related to rTMS combined with CT might mainly reflect performances for patients with AD, not for patients with AD and MCI.
With regards to specific cognitive domain, tDCS combined with CT could improve language scores in AD and MCI, which is consistent with Chu et al. [41]. Meinzer et al. [49] recorded brain changes in MCI during tDCS stimulation using task-related and resting fMRI, showing that low accuracy of semantic flow tests might be related to hyperactivity of bilateral prefrontal area. The above study results found that Anodal tDCS signifcantly improved the accuracy of language tests in MCI, reduced task-related prefrontal hyperactivity and facilitated normalization of abnormal network structure in resting-state fMRI. The synergistic effects of tDCS combined with CT maybe enhance language improvement in AD and MCI. Nevertheless, as language function was measured only in 3 studies, and the main contribution of this result came from Lu et al. [29] with a risk of publication bias, the improvement of language should be taken with caution.
In follow-up cognition improvement, we found that NIBS combined with CT could improve follow-up global cognition in AD, especially for patients accepting rTMS combined with CT. The results indicated that NIBS combined with CT has a post-treatment sustainable effect in AD. Both NIBS and CT can regulate the excitability of neurons, alter neurotransmitter levels and enhance brain functional connectivity in AD and MCI [15, 50]. The synergistic effects of tDCS combined with CT maybe strengthen those brain excitability which may be related to sustainable effects [40]. Studies in this meta-analysis did not have a fixed follow-up period, with follow-up ranging from 2 weeks to 6 months. Moreover, follow-up effects could be influenced by multiple factors such as stimulation frequency, intensity, dropout rates and CT protocols [51], hence follow-up attention/working memory effects of NIBS combined with CT need to provide more evidences.
The strength of this article included the latest and most comprehensive synthesis of up-to-date evidence on the effects of NIBS combined with CT in AD and MCI. We registered in advance with a prespecified protocol on PROSPERO and strictly adhered to the PRISMA statement. The PEDro scale was used to assess methodological quality of included studies, and the Cochrane Handbook for Systematic Reviews of Interventions was used to evaluate the risk of bias. However, there were several limitations in this systematic review and meta-analysis. The use of different scales to evaluate global cognition and specific cognitive domains in AD and MCI might lead to high heterogeneity of the results. Some authors could not be contacted for raw data of three potentially eligible studies [52,53,54]. Due to the limited data available, cognitive effects of rTMS combined with CT on specific cognitive domain in AD and MCI could not be fully observed. It was also difficult to categorize patients into subgroups based on treatment parameters of NIBS and characteristics of CT, as these characteristics would lead to heterogeneity of some results.
Conclusions
NIBS combined with CT, particularly rTMS combined with CT, has both short-term and follow-up effects on improving global cognition, mainly in patients with AD. tDCS combined with CT has advantages on improving language function in AD and MCI. Future more studies need evaluate cognitive effects of NIBS combined with CT on other specific cognitive domain in patients with cognitive deterioration.
Availability of data and materials
All data generated or analyzed are included in this published article and its supplementary materials.
Abbreviations
- AD:
-
Alzheimer’s disease
- MCI:
-
Mild cognitive impairment
- NIBS:
-
Non-invasive brain stimulation
- rTMS:
-
Repetitive transcranial magnetic stimulation
- tDCS:
-
Transcranial direct current stimulation
- CT:
-
Cognitive training
References
Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19:1598-695. https://doi.org/10.1002/alz.13016.
Petersen RC. Mild cognitive impairment. Continuum (Minneapolis, Minn). 2016;22:404–18. https://doi.org/10.1212/con.0000000000000313.
Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5:e661–71. https://doi.org/10.1016/s2468-2667(20)30185-7.
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology. 2018;90:126–35. https://doi.org/10.1212/wnl.0000000000004826.
Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra. 2013;3:320–32. https://doi.org/10.1159/000354370.
Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19:81–92. https://doi.org/10.1016/s1474-4422(19)30290-x.
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. https://doi.org/10.1056/NEJMoa2212948.
Marasco RA. Current and evolving treatment strategies for the Alzheimer disease continuum. Am J Managed Care. 2020;26:167–76. https://doi.org/10.37765/ajmc.2020.88481.
Teselink J, Bawa KK, Koo GK, Sankhe K, Liu CS, Rapoport M, et al. Efficacy of non-invasive brain stimulation on global cognition and neuropsychiatric symptoms in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review. Ageing Res Rev. 2021;72:101499. https://doi.org/10.1016/j.arr.2021.101499.
Xie Y, Li Y, Nie L, Zhang W, Ke Z, Ku Y. Cognitive enhancement of repetitive transcranial magnetic stimulation in patients with mild cognitive impairment and early Alzheimer’s disease: a systematic review and meta-analysis. Front Cell Dev Biol. 2021;9:734046. https://doi.org/10.3389/fcell.2021.734046.
Inagawa T, Narita Z, Sugawara N, Maruo K, Stickley A, Yokoi Y, et al. A meta-analysis of the effect of multisession transcranial direct current stimulation on cognition in dementia and mild cognitive impairment. Clin EEG Neurosci. 2019;50:273–82. https://doi.org/10.1177/1550059418800889.
Bahar-Fuchs A, Martyr A, Goh AM, Sabates J, Clare L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev. 2019;3:CD013069. https://doi.org/10.1002/14651858.CD013069.pub2.
Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res Rev. 2013;12:263–75. https://doi.org/10.1016/j.arr.2012.07.003.
Hill NT, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. 2017;174:329–40. https://doi.org/10.1176/appi.ajp.2016.16030360.
Wu J, He Y, Liang S, Liu Z, Huang J, Tao J, et al. Computerized cognitive training enhances episodic memory by down-modulating posterior cingulate-precuneus connectivity in older persons with mild cognitive impairment: a randomized controlled trial. Am J Geriatr Psychiatry. 2023;31:820–32. https://doi.org/10.1016/j.jagp.2023.04.008.
Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev. 2010;34:670–7. https://doi.org/10.1016/j.neubiorev.2009.12.008.
Lee J, Choi BH, Oh E, Sohn EH, Lee AY. Treatment of alzheimer’s disease with repetitive transcranial magnetic stimulation combined with cognitive training: a prospective, randomized, double-blind, placebo-controlled study. J Clin Neurol (Seoul, Korea). 2016;12:57–64. https://doi.org/10.3988/jcn.2016.12.1.57.
Andrade SM, Machado D, Silva-Sauerc LD, Regis CT, Mendes C, de Araújo JSS, et al. Effects of multisite anodal transcranial direct current stimulation combined with cognitive stimulation in patients with Alzheimer’s disease and its neurophysiological correlates: A double-blind randomized clinical trial. Neurophysiol Clin. 2022;52:117–27. https://doi.org/10.1016/j.neucli.2022.02.003.
Cruz Gonzalez P, Fong KNK, Chung RCK, Ting KH, Law LLF, Brown T. Can transcranial direct-current stimulation alone or combined with cognitive training be used as a clinical intervention to improve cognitive functioning in persons with mild cognitive impairment and dementia? A systematic review and meta-analysis. Front Hum Neurosci. 2018;12:416. https://doi.org/10.3389/fnhum.2018.00416.
Burton CZ, Garnett EO, Capellari E, Chang SE, Tso IF, Hampstead BM, et al. Combined cognitive training and transcranial direct current stimulation in neuropsychiatric disorders: a systematic review and meta-analysis. Biol Psychiatr Cogn Neurosci Neuroimaging. 2023;8:151–61. https://doi.org/10.1016/j.bpsc.2022.09.014.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clinical research ed). 2021;372:n160. https://doi.org/10.1136/bmj.n160.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. https://doi.org/10.1111/j.1365-2796.2004.01388.x.
Association AP. Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing; 2013.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. https://doi.org/10.1016/j.jalz.2011.03.005.
Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10:1–7.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. https://doi.org/10.1002/14651858.Ed000142.
Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–9. https://doi.org/10.1111/1469-0691.12494.
Cotelli M, Manenti R, Brambilla M, Petesi M, Rosini S, Ferrari C, et al. Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Front Aging Neurosci. 2014;6:38. https://doi.org/10.3389/fnagi.2014.00038.
Lu H, Chan SSM, Chan WC, Lin C, Cheng CPW, Wa LLC. Randomized controlled trial of TDCS on cognition in 201 seniors with mild neurocognitive disorder. Ann Clin Transl Neurol. 2019;6:1938–48. https://doi.org/10.1002/acn3.50823.
Gonzalez PC, Fong KNK, Brown T. Transcranial direct current stimulation as an adjunct to cognitive training for older adults with mild cognitive impairment: a randomized controlled trial. Ann Phys Rehabil Med. 2021;64:101536. https://doi.org/10.1016/j.rehab.2021.101536.
Rodella C, Bernini S, Panzarasa S, Sinforiani E, Picascia M, Quaglini S, et al. A double-blind randomized controlled trial combining cognitive training (CoRe) and neurostimulation (tDCS) in the early stages of cognitive impairment. Aging Clin Exp Res. 2022;34:73–83. https://doi.org/10.1007/s40520-021-01912-0.
Martin DM, Mohan A, Alonzo A, Gates N, Gbadeyan O, Meinzer M, et al. A pilot double-blind randomized controlled trial of cognitive training combined with transcranial direct current stimulation for amnestic mild cognitive impairment. J Alzheimers Dis. 2019;71:503–12. https://doi.org/10.3233/JAD-190306.
Inagawa T, Yokoi Y, Narita Z, Maruo K, Okazaki M, Nakagome K. Safety and feasibility of transcranial direct current stimulation for cognitive rehabilitation in patients with mild or major neurocognitive disorders: a randomized sham-controlled pilot study. Front Hum Neurosci. 2019;13:273. https://doi.org/10.3389/fnhum.2019.00273.
Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimers’ Dement (New York, N Y). 2017;3:247–53. https://doi.org/10.1016/j.trci.2017.03.003.
de Sousa AVC, Grittner U, Rujescu D, Kuelzow N, Floeel A. Impact of 3-day combined anodal transcranial direct current stimulation-visuospatial training on object-location memory in healthy older adults and patients with mild cognitive impairment. J Alzheimers Dis. 2020;75:223–44. https://doi.org/10.3233/JAD-191234.
Brem A-K, Di Iorio R, Fried PJ, Oliveira-Maia AJ, Marra C, Profice P, et al. Corticomotor plasticity predicts clinical efficacy of combined neuromodulation and cognitive training in alzheimer’s disease. Front Aging Neurosci. 2020;12:200. https://doi.org/10.3389/fnagi.2020.00200.
Vecchio F, Quaranta D, Miraglia F, Pappalettera C, Di Iorio R, L’Abbate F, et al. Neuronavigated Magnetic Stimulation combined with cognitive training for Alzheimer’s patients: an EEG graph study. GeroScience. 2022;44:159–72. https://doi.org/10.1007/s11357-021-00508-w.
Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm (Vienna, Austria : 1996). 2013;120:813–9. https://doi.org/10.1007/s00702-012-0902-z.
Bagattini C, Zanni M, Barocco F, Caffarra P, Brignani D, Miniussi C, et al. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul. 2020;13:1655–64. https://doi.org/10.1016/j.brs.2020.09.010.
Zhang F, Qin Y, Xie L, Zheng C, Huang X, Zhang M. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J Neural Transm (Vienna, Austria : 1996). 2019;126:1081–94. https://doi.org/10.1007/s00702-019-02022-y.
Chu CS, Li CT, Brunoni AR, Yang FC, Tseng PT, Tu YK, et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: a component network meta-analysis. J Neurol Neurosurg Psychiatry. 2021;92:195–203. https://doi.org/10.1136/jnnp-2020-323870.
Wang JY, Qin JY, Ye JY, Li WT, Tong MQ, Ouyang H, et al. The Therapeutic effects of noninvasive brain stimulation combined with cognitive training in elders with Alzheimer’s disease or amnesic mild cognitive impairment. J Prev Alzheimers Dis. 2024;11:222–9. https://doi.org/10.14283/jpad.2024.1.
Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013;2013:Cd003260. https://doi.org/10.1002/14651858.CD003260.pub2.
Antal A, Luber B, Brem AK, Bikson M, Brunoni AR, Cohen Kadosh R, et al. Non-invasive brain stimulation and neuroenhancement. Clin Neurophysiol Pract. 2022;7:146–65. https://doi.org/10.1016/j.cnp.2022.05.002.
Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14:317–24. https://doi.org/10.1016/j.tics.2010.05.002.
Xu Y, Qiu Z, Zhu J, Liu J, Wu J, Tao J, et al. The modulation effect of non-invasive brain stimulation on cognitive function in patients with mild cognitive impairment: a systematic review and meta-analysis of randomized controlled trials. BMC Neurosci. 2019;20:2. https://doi.org/10.1186/s12868-018-0484-2.
Šimko P, Kent JA, Rektorova I. Is non-invasive brain stimulation effective for cognitive enhancement in Alzheimer’s disease? An updated meta-analysis. Clin Neurophysiol. 2022;144:23–40. https://doi.org/10.1016/j.clinph.2022.09.010.
Gomes-Osman J, Indahlastari A, Fried PJ, Cabral DLF, Rice J, Nissim NR, et al. Non-invasive brain stimulation: probing intracortical circuits and improving cognition in the aging brain. Front Aging Neurosci. 2018;10:177. https://doi.org/10.3389/fnagi.2018.00177.
Meinzer M, Lindenberg R, Phan MT, Ulm L, Volk C, Flöel A. Transcranial direct current stimulation in mild cognitive impairment: Behavioral effects and neural mechanisms. Alzheimers Dement. 2015;11:1032–40. https://doi.org/10.1016/j.jalz.2014.07.159.
Chou YH, Sundman M, Ton That V, Green J, Trapani C. Cortical excitability and plasticity in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis of transcranial magnetic stimulation studies. Ageing Res Rev. 2022;79:101660. https://doi.org/10.1016/j.arr.2022.101660.
Chen J, Wang Z, Chen Q, Fu Y, Zheng K. Transcranial direct current stimulation enhances cognitive function in patients with mild cognitive impairment and early/mid Alzheimer's disease: a systematic review and meta-analysis. Brain Sci. 2022;12. https://doi.org/10.3390/brainsci12050562.
Qin Y, Zhang F, Zhang M, Zhu W. Effects of repetitive transcranial magnetic stimulation combined with cognitive training on resting-state brain activity in Alzheimer’s disease. Neuroradiol J. 2022;35:566–72. https://doi.org/10.1177/19714009211067409.
Sabbagh M, Sadowsky C, Tousi B, Agronin ME, Alva G, Armon C, et al. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimers Dement. 2020;16:641–50. https://doi.org/10.1016/j.jalz.2019.08.197.
Pallanti S, Grassi E, Knotkova H, Galli G. Transcranial direct current stimulation in combination with cognitive training in individuals with mild cognitive impairment: a controlled 3-parallel-arm study. CNS Spectr. 2022:1–6. https://doi.org/10.1017/s1092852922000979.
Acknowledgements
Not applicable.
Funding
This research was supported by National Key Research and Development Program of China (2022YFC3602603).
Author information
Authors and Affiliations
Contributions
TY and WL contributed to the work equally and should be regarded as co-first authors. All authors read and approved the final manuscript. TY: conceptualization, methodology, writing—original draft preparation, writing—reviewing and editing. WL: methodology, writing—original draft preparation, writing—reviewing and editing. JH: methodology. LX: methodology. CG: writing—original draft preparation. LM: conceptualization, methodology,. CJ: conceptualization, methodology, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, T., Liu, W., He, J. et al. The cognitive effect of non-invasive brain stimulation combined with cognitive training in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Alz Res Therapy 16, 140 (2024). https://doi.org/10.1186/s13195-024-01505-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01505-9