Abstract
Introduction
In humans, approximately 5% of all cancers are attributable to HPV infection. Prophylactic vaccines can inhibit viral migration and persistence. However, further studies are still required to develop such treatments. To achieve this goal, we designed a therapeutic HPV DNA vaccine encoding a construct of E6/E7/L1 and used NSP4 antigen as an adjuvant to assess the efficiency of this construct in generating antigen-specific antitumor immune responses.
Materials and methods
Sixty female C57BL/6 mice (6–8 weeks old) were purchased from the Institute Pasteur of Iran. Through a subcutaneous (s.c) injection of a suspension of 100 µl PBS containing 106 TC-1 cells/mouse in the back side, 30 of them became cancerous, while 30 of them were healthy control mice. To amplify E6/E7/L1-pcDNA3 and NSP4-pcDNA3, the competent cells of DH5α and to generate a tumor, TC-1 cell line was used. Mice were then immunized with the HPV DNA vaccine. Cell proliferation was assessed by MTT assay. Finally, cytokine responses (IL-4, IL-12, IFN- γ) were measured in the supernatant of mice spleen cells.
Result
Mice receiving the NSP4/E6-E7-L1 vaccine had the highest stimulatory index compared to other groups, although it was not statistically significant. Interleukin 4/12 and IFN-γ production were significantly higher in E6-E7-L1 / NSP4 group and E6-E7-L1 group compared to other groups (P < 0.05). Among different groups, E6/E7/L1 + NSP4 group was able to slow down the tumor growth process, but it was not significant (p > 0.05). Among the aforementioned cytokines, IFN-γ and IL-12 are among the cytokines that stimulate the Th1 pathway and IL-4 cytokine stimulates the Th2 pathway and B lymphocytes.
Conclusion
Our data revealed that the present vaccine can reduce tumor size, and cytokine measurement showed that it stimulates innate and acquired immune responses, thus it can be a therapeutic vaccine in the tumor-bearing mice model.
Similar content being viewed by others
Introduction
Human papillomavirus (HPV) is a major etiological pathogen in cancers, such as cervical, vulvar, vaginal, anal, penile, and head and neck tumors. Cervical cancer immunotherapy must target high-risk HPV16, 18 that cause 50% and 20% of cervical cancers, respectively [1, 2]. Prophylactic vaccines can inhibit HPV migration and persistence, but HPV-induced cancer is still the reason for more than 200,000 deaths in populations annually. Therefore, the need to develop such therapeutic strategies still prevails [3, 4].
The best therapeutic HPV vaccine should be capable of both eliminating virions and transforming cells expressing viral proteins. The viral oncoproteins (E6/E7) are appropriate antigen targets for immunotherapy because they are key factors in cell transformation, and they are constantly expressed by HPV-related neoplastic epithelial and tumor cells (4). The cellular immune response is responsible for removing HPV-transformed cells. Thus, optimal therapeutic vaccines should be designed in a way to raise adaptive response. Several experimental therapeutic vaccines for HPV-related oncogenesis have been introduced in trials but the majority of them had only partial signs of progress. These models were adjuvanted proteins, DNA vaccines, live vaccines, or dendritic cell-related vaccines [3].
Nevertheless, prophylactic HPV vaccines are designed to avoid the spread of HPV infection by triggering a considerable antiviral antibody response toward viral L proteins. Today two approved preventive HPV vaccines are available in the market (Cervarix and Gardasil), and their efficacy in decreasing the incidence of high-level cervical abnormalities has been confirmed [5]. Noticeably, however, due to the integration of the HPV genome in the host genome, the L1 and L2 proteins cannot be expressed in transformed cells in some stages of the disease, rendering the current vaccines useless in that phase of infection [2, 6].
On the other hand, DNA vaccines are capable of triggering the immune response by multiple mechanisms at the same time, instantly through designing several target antigens or combining immune-modulatory factors [7]. Furthermore, using these vaccines is highly beneficial, as they are resistant to temperature changes and are also cost-effective, making them a preferred vaccine choice worldwide. Although DNA vaccines have shown to be weak immunogenic vaccines in clinical trials, researchers have established a DNA vaccine strategy that uses a combination of codon-optimized constructs to target both humoral and cellular immunity [8, 9].
Using an adjuvant is an approach to increase the immunogenicity of these vaccines. Recently, researchers showed that Rotavirus nonstructural protein 4 (NSP4) has immune response stimulation functions [10]. Due to the NSP4 adjuvant properties for increasing the efficiency of the vaccine, we used the NSP4 coding plasmid together with E6/E7/L1 coding plasmid. Moreover, codon optimization, which was primarily applied to enhance protein expression, should also limit the possibility of recombination of the vaccine sequences with wild-type HPV viruses [8]. In this study, we designed a therapeutic HPV DNA vaccine encoding a construct of E6/E7/L1 and used NSP4 antigen as an adjuvant in C57BL/6 mice. Furthermore, we assessed the efficiency of this construct in generating antigen-specific antitumor immune responses to protect mice from tumor challenges and to treat preexisting tumors in mice. Finally, we determined the immunity type that is responsible for the observed antitumor responses in vaccinated, tumor-challenged mice.
Material & methods
Bacterial strain and cell line
The DH5α strain of Escherichia coli competent cells was used as a bacterial host for transformation in the presence of calcium chloride and heat shock treatment, based on the method of Cohen et al [11]. Briefly, E. coli strain DH5α was cultured at 37 °C in Luria Bertani (LB) medium, supplemented with 50 µg/mL ampicillin. TC-1 cell line (primary epithelial cell of the lung of C57BL / 6 mice) was cultured in Roswell Park Memorial Institute medium (RPMI) 1640 (Gibco BRL, Paisley, UK) supplemented with 2 mM/L L-glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin, 5 × 10− 5 M β-mercaptoethanol, and 10% fetal bovine serum (FBS) at 70% confluency 20 h before transfection.
DNA vaccine construction
We constructed a pcDNA3.1 plasmid containing codon optimized E6/E7/L1 gene expression cassette under human cytomegalovirus (HCMV) immediate-early promoter and provided NSP4-pcDNA3.1 expression vector from Tarbiat Modares University (Fig. 1). Purified polymerase chain reaction (PCR) products were cloned into a pcDNA3.1 cloning vector and were confirmed by sequencing. The digested gene was sub-cloned into a pcDNA3.1 eukaryotic expression vector (Invitrogen, Canada). E. coli DH5α strain bacteria were transformed with the plasmids and plated on LB plates containing 100 µg/mL ampicillin. The selected colonies were extracted using a Gene All kit according to the manufacturer’s instructions. Cloning confirmation was done by restriction enzyme digestion and PCR.
Tumor challenge
For the therapeutic experiments, C57BL/6 mice were challenged by a subcutaneous (s.c) injection of a suspension of 100 µl PBS containing 106 TC-1 cells/mouse in the back side and then, were grouped into six cages. The flow diagram of the experiment was as Fig. 2.
Vaccination
In this study, sixty female C57BL/6 mice (6–8 weeks old, weighing 17–20 g) were bought from the Institute Pasteur of Iran (Karaj, Iran). All animal experimental protocols were used according to WHO and Animal Ethics Committee of Golestan University of medical sciences approved protocols of appropriate mice care (No. Ir.goums.rec.1397.226) and the mice were acclimated with the laboratory conditions (light–dark cycles, temperatures of 22 °C ± 2 °C) for 1 week before the experiment. Mice were divided into two groups of 30, cancerous and healthy. Both groups were distributed in six sub-groups as described in Table 1.
Immunizations
For DNA vaccine immunizations, mice were immunized subcutaneously in the abdominal area with a total of 30 µg HPV DNA vaccine (HPV16-E6/E7/L1 and Rotavirus NSP4, secreted and ubiquitinated construct pooled 1:1 by weight). Mice were injected intraperitoneally with 200 µg of monoclonal antibodies targeting PD-L1 (clone B7-H1, BioXcell) one dose weekly for 3 weeks following the first vaccination.
MTT
Two weeks after the final immunization, to euthanize mice, they were first anesthetized with ketamine (75 mg/kg) injected subcutaneously. The spleen of each mouse was removed, and lymphocyte proliferation was evaluated using the MTT method according to the manufacturer´s recommendations (Sigma) by Phytohemagglutinin (PHA) and TC1 lysate. In this way, we prepared the TC1 cell by sonicating and removing the cell wall and releasing the protein.
ELISA
One week after the last immunization, freshly isolated splenocytes of immunized mice in the different treated groups were cultured and stimulated with PHA and TC1. The cell supernatants were collected and assayed for the presence of IFN-γ, IL-4, and IL-12 using commercially available sandwich-based enzyme-linked immunosorbent assay (ELISA) kits (in comparison to unstimulated controls) (Peprotech, New Jersey, US), following the manufacturer’s instructions. All tests were performed in triplicate for each mouse. Given the importance of vaccine immunogenicity, we chose cytokines which play the key role in defining the type of immune response. Among the cytokines mentioned, IFN-γ and IL-12 are among the cytokines that stimulate the Th1 pathway and innate immunity, and IL-4 cytokine stimulates the adaptive immunity including Th2 pathway and B lymphocytes.
Statistical analysis
Statistical analysis was performed by Graph Pad Prism version 8.0.1. The levels of statistical significance for differences between experimental groups were determined using T-test. Survival curves were compared by a log-rank (Mantel Cox) test. Kruskal–Wallis was performed and differences were considered significant (P-value < 0.05).
Result
Effectiveness of vaccination on tumor size
Seven days after the injection of TC-1 cells, a tumor was observed in C57BL/6 mice. Tumor size was measured at 6 different time intervals during the vaccination program. The results of the measurement showed that the tumor size was lower in the vaccinated groups compared to the control groups. Interestingly, the E6/ E7/L1 + NSP4 group was able to slow down the tumor growth process compared to the other groups, but it was not statistically significant (p > 0.05). (Fig. 3)
Lymphocyte proliferation assay
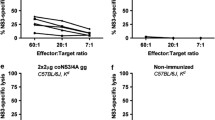
The results revealed that the stimulation index of vaccinated groups is significantly higher than the control groups (p < 0.05). The mice receiving the NSP4 / E6-E7-L1 vaccine had the highest stimulatory index compared to other groups. The comparison of the results of lymphocyte proliferation assay in different groups and the control group was not significant (p > 0.05) (Fig. 4).
IFN- γ, IL-4 and IL-12 secretion levels
Co-administration of the NSP4 adjuvant with a E6/E/L1 vaccine induced significantly higher amounts of IFN-γ, IL-4 and IL-12 in vivo. The supernatants of splenocytes, stimulated with PHA and TC-1, from each group were collected one week after the third immunization. ELISA was performed to detect the level of IFN-γ (A), IL-4 (B), IL-12 (C) in splenocyte cultures. All of cytokines were significantly higher in E6-E7-L1 / NSP4 group and E6-E7-L1 group compared to other groups
To investigate the level of IL-4/12 and IFN-γ production, spleen cells of immunized mice in different groups were cultured and also stimulated with specific antigens. Results of nonparametric post-hoc test showed that interleukin-4/12 and IFN- γ production was significantly higher in E6-E7-L1 / NSP4 group and E6-E7-L1 group compared to control and other groups (p < 0.01) (Fig. 5). Although, the differences between these two groups (tumor-bearing and healthy) was not significant.
Discussion
Cancer immunotherapy using DNA vaccines has emerged as a potentially promising approach for the control of tumors. This study showed that the pcDNA3.1- E6-E7-L1/NSP4 vaccine could elicit a stronger specific CD8 + T cell-mediated immune response than other groups. Our results showed that vaccination by pcDNA3.1-E6-E7-L1/NSP4 can decrease tumor size and could elicit potentially protective and therapeutic antitumor responses against HPV16 expressing TC-1 tumor model in mice. Moreover, we showed that the pcDNA3.1-E6-E7-L1/NSP4-induced antitumor immune response may be CD8 + T cell-dependent. In the current study, numerous important mechanisms contributed to the enhanced immunogenicity of pcDNA3.1- E6-E7-L1/NSP4 DNA vaccine were observed.
Previous studies reported that coadministration of DNA encoding BPVL1 enhances the immunogenicity of the E7 DNA vaccine by increasing CD4 + T cell responses that are known to assist the generation of CD8 + T cell response [12]. The CD8 + T cells served as the basis for the design of a fusion BPVL1-E7 DNA vaccine, and now we used that data for the design of an E6-E7-L1/NSP4 DNA vaccine. Additionally, several studies have tested the conjugation of E7 to L1 VLPs as a method to enhance the E7 immune response [13,14,15]. It has been shown that intact HPV-L1 VLPs can interact with DCs to directly induce potent adaptive immune responses in the absence of adjuvants [16]. Potentially, it occurs in a TLR-MyD88 pathway-dependent manner [17]. In contrast to our results, Tahmatan et al. and Sajjadian et al. studies showed that HPV16 DNA-E7 inoculation did not increase IL-4 expression compared to the simple vector control group [18, 19]. In Rahimi et al. study, there was a significant difference between using the E7 DNA vaccine and PBS. In their study, due to the simultaneous use of both Hitchner B1 and LaSota strains with the HPV-16 E7 DNA vaccine, the expression level of IL-4 was much higher than in other groups [20]. In the present study, the vaccine could also stimulate humoral immunity, which can serve as a basis for preventing future infections or treating HPV infections. Many researchers in the field of antitumor vaccines developed various vaccines for HPVs, but our study is one of the researches designing a preventive and therapeutic vaccine on mouse models which revealed the efficacy of a vaccine in cellular and humoral immunity.
The HPV E6 and E7 proteins have been demonstrated to be constantly expressed in cervical cancer cells [21]; thus they are rational targets for HPV-16 therapeutic vaccination in cases with cervical carcinoma. Studies revealed that endothelial cells are capable to take up the E7 viral protein added to the culture medium [22]. Moreover, the HPV-16 E6/E7 + cancerous cells can be genetically modulated with DNA coding for immune regulatory cytokines or adjuvants utilized for vaccination. Immunological studies have shown a key role for CD8 + T lymphocytes in the suppression of an aggressively growing carcinoma [23].
Recent studies have revealed that HPV-16 L1 protein has stimulatory effects on cellular immunity; therefore, it could be applied for the manufacture of therapeutic vaccines. Cheung et al. (2004) engineered the L1E7hpSCA1 vector expressing the L1 and E7 proteins with the codon optimization for mammalian cell expression. Once their vaccine was injected into the muscle, they found that this vector had strongly induced T cell cytotoxicity and also is capable of lysing the TC-1 cells expressing E7. In animal models, tumor growth was suppressed in vaccinated mice [24]. The protection toward challenge by TC-1 tumor was also revealed by vaccination with chimeric vector harboring the HPV-16 L1/L2 viral capsid proteins and the E7 oncoprotein [14]. In our study, we used E6/E7 coding genes engineered by mutating six known oncogenic regions within E6 and E7. These mutations should disable E6 to mediate the degradation of p53 and also bind to the PDZ motif inactivating the tumor suppressor protein PTPN13. Mutations in E7 (H2P, C24G, E46G, and L67R) can prevent its capability to inactivate pRb and binding to Mi2β that enhances cellular growth [25].
Since the CD8 + T cells have a key immunological role in tumor elimination, we compared in vitro cytotoxic effect of the splenic cells isolated from the different vaccinated mice. Our findings showed a rise in CTL activity of the animal vaccinated with L1, E6, and E7 as a DNA vaccine plus NSP4. For clarification of this study, we considered that improvement of E6 and E7 oncoproteins as inducers of cellular immunity accompanied by L1 and NSP4 adjuvant could be due to its specific epitopes. Bellone et al. (2009) found that the HPV-16 L1 DNA vaccine strongly increases the activity of vaginal CD8 + T cells, which are critical for the elimination of virus-infected cells [26].
In comparison with the Bellone et al. study, the findings of this study revealed that CTL response was stronger than in some previous studies, possibly because of the stage of the tumor, the cell line, the animal model, and the method of CTL assay [26]. Also, pro-inflammatory cytokines may stimulate the natural killer (NK) cells, and these NK cells have a key role in tumor growth suppression [27]. Although we didn’t evaluate NK cell activity in our animal models, the role of NK cells in tumor elimination and growth cannot be ignored. The regulation of T helper cells as effector cells is undeniable for effective immunity; hence, enhancing all immune responses is required for considerable protection [28].
Conclusion
In summary, we developed a novel therapeutic DNA-based HPV16 vaccine, encoding an E6/E7/L1 protein and NSP4 adjuvant, which was codon-optimized for enhanced expression, and combines sequences that are secreted and ubiquitinated to induce a balanced humoral and cell-mediated immune response. We showed that this vaccine can be immunogenic and protective in the TC-1 tumor-free model and tumor-bearing mice. This approach would be one of the promising therapeutic vaccines in HPV-related cancer cases.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Smalley Rumfield C, Roller N, Pellom ST, Schlom J, Jochems C. Therapeutic vaccines for HPV-associated malignancies. ImmunoTargets Ther. 2020;7:167–200.
Behboudi E, Mokhtari-Azad T, Yavarian J, Ghavami N, Seyed Khorrami SM, Rezaei F, Charostad J, Shatizadeh Malekshahi S, Shafiei-Jandaghi NZ. Molecular detection of HHV1-5, AAV and HPV in semen specimens and their impact on male fertility. Hum Fertil. 2019;22(2):133–8.
Chandra J, Dutton JL, Li B, Woo W-P, Xu Y, Tolley LK, et al. DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection. J immunotherapy (Hagerstown Md: 1997). 2017;40:62.
Flatley JE, McNeir K, Balasubramani L, Tidy J, Stuart EL, Young TA et al. Folate status and aberrant DNA methylation are associated with HPV infection and cervical pathogenesis. Cancer Epidemiol Biomarkers Prev. 2009:1055–9965. EPI-09-0493.
Castle PE, Maza M, Prophylactic. HPV vaccination: past, present, and future–CORRIGENDUM. Epidemiol Infect. 2016;144:2472.
Klaes R, Woerner SM, Ridder R, Wentzensen N, Duerst M, Schneider A, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59:6132–6.
Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012;162:171–82.
Liu WJ, Zhao K-N, Gao FG, Leggatt GR, Fernando GJ, Frazer IH. Polynucleotide viral vaccines: codon optimisation and ubiquitin conjugation enhances prophylactic and therapeutic efficacy. Vaccine. 2001;20:862–9.
Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu T-C, et al. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 2016;15:989–1007.
Kavanagh OV, Ajami NJ, Cheng E, Ciarlet M, Guerrero RA, Zeng CQ-Y, et al. Rotavirus enterotoxin NSP4 has mucosal adjuvant properties. Vaccine. 2010;28:3106–11.
Cohen SN, Chang AC, Boyer HW, Helling RB. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci. 1973;70:3240–4.
Yang B, Yang A, Peng S, Pang X, Roden RB, Wu T-C, et al. Co-administration with DNA encoding papillomavirus capsid proteins enhances the antitumor effects generated by therapeutic HPV DNA vaccination. Cell Biosci. 2015;5:35.
Keshavarz M, Miri SM, Behboudi E, Arjeini Y, Dianat-Moghadam H, Ghaemi A. Oncolytic virus delivery modulated immune responses toward cancer therapy: Challenges and perspectives. Int Immunopharmacol. 2022;108:108882.
Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, Verma Y, Esmy PO, Poli UR, Shah A, Zomawia E. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. 2021;22(11):1518–29.
Freyschmidt E-J, Alonso A, Hartmann G, Gissmann L. Activation of dendritic cells and induction of T cell responses by HPV 16 L1/E7 chimeric virus-like particles are enhanced by CpG ODN or sorbitol. Antivir Ther. 2004;9:479–90.
Lenz P, Day PM, Pang Y-YS, Frye SA, Jensen PN, Lowy DR, et al. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–55.
Yang R, Murillo FM, Cui H, Blosser R, Uematsu S, Takeda K, et al. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J Virol. 2004;78:11152–60.
Tahamtan A, Ghaemi A, Gorji A, Kalhor HR, Sajadian A, Tabarraei A, et al. Antitumor effect of therapeutic HPV DNA vaccines with chitosan-based nanodelivery systems. J Biomed Sci. 2014;21:69.
Sajadian A, Tabarraei A, Soleimanjahi H, Fotouhi F, Gorji A, Ghaemi A. Comparing the effect of toll-like receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch Virol. 2014;159:1951–60.
BaghbanRahimi S, Soleimanjahi H, Mohebbi A, Ebrahimzadeh MS, Alizade L, Ghaemi A. Evaluation of DNA vaccine encoding HPV-16 E7 on lymphocyte proliferation induction in human papilloma virus-associated tumor animal models. Jorjani Biomed J. 2017;5:14–24.
DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77:1551–63.
Zhou JZ, Jou J, Cohen E. Vaccine strategies for Human Papillomavirus-Associated Head and Neck cancers. Cancers. 2022;14(1):33.
Razavi-Nikoo H, Behboudi E, Aghcheli B, Hashemi SM, Moradi A. Bac to Bac System Efficiency for preparing HPV type 16 Virus-Like particle vaccine. Arch Razi Inst. 2023;78(3):997–1003.
Cheung Y-K, Cheng SC-S, Sin FW-Y, Xie Y. Plasmid encoding papillomavirus type 16 (HPV16) DNA constructed with codon optimization improved the immunogenicity against HPV infection. Vaccine. 2004;23:629–38.
Wieking BG, Vermeer DW, Spanos WC, Lee KM, Vermeer P, Lee WT, et al. A non-oncogenic HPV 16 E6/E7 vaccine enhances treatment of HPV expressing tumors. Cancer Gene Ther. 2012;19:667–74.
Bellone S, El-Sahwi K, Cocco E, Casagrande F, Cargnelutti M, Palmieri M, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8 + cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8 + CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779–89.
Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–80.
Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–36.
Acknowledgements
The authors would like to thank Golestan University of Medical Sciences and the laboratory.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceive and design of the experiments: A.M; data analysis: M.A, E.B; writing of the paper: E.B, S.S; performance of the experiments: S.S, M.A; Read and confirm of final version of article: A.M, E.B, M.N, S.S, M.A, A.O; Revise: E.B, A.M, M.N.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was carried out according to the principles of the declaration of Helsinki. All animal experimental protocols were used according to WHO and Animal Ethics Committee of Golestan University of medical sciences approved protocols of appropriate mice care (Code: Ir.goums.rec.1397.226).
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sadr-Momtaz, S., Aftabi, M., Behboudi, E. et al. NSP4 as adjuvant for immunogenicity and design of effective therapeutic HPV16 E6/E7/L1 DNA vaccine in tumor-bearing and healthy C57BL/6 mice. BMC Res Notes 16, 164 (2023). https://doi.org/10.1186/s13104-023-06445-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06445-5