Abstract
Hypothesis
A healthy gut with normal intestinal microflora is completely disrupted by oral antibiotics. The byproducts of harmful gut bacteria can interfere with brain development and may contribute to autism. Strategies to improve the gut microflora profile through dietary modification may help to alleviate gut disorders in autistic patients.
Method
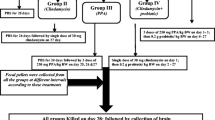
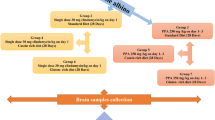
Sixty young male western albino rats were divided into six equal groups. The first group served as the control; the second group was given an oral neurotoxic dose of propionic (PPA) (250 mg/kg body weight/day) for three days. The third group received an orogastric dose of ampicillin (50 mg/kg for three weeks) with a standard diet. Groups 4, 5 and 6 were given an orogastric dose of ampicillin and fed high-carbohydrate, high-protein and high-lipid diets, respectively, for 10 weeks. Biochemical parameters related to oxidative stress were investigated in brain homogenates from each group.
Result
The microbiology results revealed descriptive changes in the fecal microbiota of rats treated with ampicillin either alone or with the three dietary regimens. The results of PPA acid and ampicillin treatment showed significant increases in lipid peroxidation and catalase with decreases in glutathione and potassium compared with levels in the control group. A protein-rich diet was effective at restoring the glutathione level, while the carbohydrate-rich diet recovered lipid peroxidation and catalase activity. In addition, the three dietary regimens significantly increase the potassium level in the brain tissue of the test animals. Lactate dehydrogenase was remarkably elevated in all groups relative to the control. No outstanding effects were observed in glutathione S-transferase and creatine kinase.
Conclusion
The changes observed in the measured parameters reflect the neurotoxic effects of PPA and ampicillin. Lipid peroxide and catalase activity and the levels of glutathione and potassium are satisfactory biomarkers of PPA and ampicillin neurotoxicity. Based on the effects of the three dietary regimens, a balanced diet can protect against PPA or ampicillin-induced neurotoxicity that might induce autistic traits. These outcomes will help efforts directed at controlling the prevalence of autism, a disorder that has recently been associated with PPA neurotoxicity.
Similar content being viewed by others
Background
Human beings and their gut microbiota are in a symbiotic relationship, and a “super organism” that includes both the human organism and microbes has been recently proposed by SommerandBäckhed [1]. It is now clear that bidirectional signaling between the gut and the brain, mainly through the vagus nerve, the so-called “microbiota-gut-vagus-brain axis”, is vital for maintaining homeostasis and may be also involved in the etiology of certain mental disorders [2]. Shaping of the microbiota occurs in parallel with brain development, and the microbiota-gut-brain axis is therefore considered to be a key player during neurodevelopment. Accordingly, early life events during the initial colonization and microbiota development can determine general and mental health in later life [3,4]. Disruptions during these critical periods of dynamic microbiota-host interaction have a great potential to alter brain-gut signaling, affect health throughout life, and increase the risk of neurodevelopmental disorders.
It appears that alterations in the gut microbiota during childhood and adolescence could be susceptible to environmental factors, such as the use of antibiotics, stress, poor diet, and infection, which could result in dysbiosis and have a potentially negative impact on general and mental health, leading to the development of brain disorders later in life. Although antibiotics are common environmental insults with a profound impact on intestinal microbiota, data on antibiotic use as a risk factor for a subsequent neurodevelopmental abnormality are scarce [5-7]. Although the pathophysiology of Autism Spectrum Disorder remains unknown, a number of metabolic pathways appear to be modulated in this disorder and have been highlighted as possible candidate disease mechanisms, which under a given genetic susceptibility, may alter neurological development in early childhood [8,9]. Gastrointestinal symptoms have long indicated that gut bacteria might play a role in the pathophysiology of ASD,and indeed, various studies have shown that the gut microbiota is altered in ASD,although there is little agreement in the literature as to which bacteria might be involved [10,11].
The gut microbiota usually shows a remarkable capacity to recover once a course of antibiotics is stopped. Sandler et al. [12] showed that 8 weeks of treatment with vancomycin, which actively inhibits Clostridium bacteria, improved communication and behavior scores in autistic children, but these scores deteriorated again upon discontinuation of the antibiotic antibiotic which ascertain the role of Clostridium in the etiology of autism.
In addition, gut microbiota provide an important mediator of the bioavailability and toxicity of environmental pollutants (e.g.,heavy metals and propionic acid). These microorganisms and their metabolites have a strong impact on pH, oxidative balance, detoxification enzymes, and xenobiotic-metabolizing and transporting host proteins, all of which may strongly influence the bioavailability of toxins in the gut lumen [13].
Intestinal bacteria are recognized as potentially important factors that contribute to the increased prevalence of diseases associated with antibiotic use, such as hypersensitivity pneumonitis (HP) and autism [14,15]. Finegold [16] proposed that certain antibiotics may play key roles in modifying the gut bacterial flora adversely, allowing the overgrowth of harmful bacteria that are normally suppressed by an intact normal gut flora or probiotics. Antibiotics (such as penicillin, cephalosporin and clindamycin)can also disturb normal flora to allow the overgrowth of Clostridium difficile, which in turn has been associated with the development of autism [17]. These bacteria are spore-formers capable of resisting antibacterial drugs. If the antibiotics are discontinued, the spores germinate and produce toxins and metabolites, including short-chain propionic acid (PPA), which has recently been reported to induce persistent biochemical and behavioral autistic features in rat pups [18-20].
Autistic children with a low level of glutathione usually have amplified levels of toxic metals, such as mercury (Hg) and lead (Pb) [21]. To a certain extent, a low glutathione level decreases the ability of the body to excrete Hg and lead (Pb) [22,23]. The augmented use of certain oral antibiotics, such as ampicillin, also decreases the ability to excrete toxic metals into the feces [24]. Ampicillin has been reported to reduce the excretion of inorganic mercury to 26% and that of total mercury to 60% in the feces compared with the excretion levels of control rats, which can be attributed to altered gut flora. Altered gut flora due to the administration of oral antibiotics has also resulted in the complete inability to excrete mercury in rats [25].
These findings motivate our interest to test and compare the neurotoxic effects of ampicillin to those induced by propionic acid as a metabolite of propionibacteria. Propionic acid producers were induced post-ampicillin treatment of rat pups. The study was also extended to evaluate the effects of high-protein (HP), high-carbohydrate (HC), and high-lipid (HL) diets on the neurotoxic effects of ampicillin. The three dietary regimens used in the present study were not isocaloric but were used to investigate the differences related to their nutritional importance in the presence of ampicillin-induced neurotoxicity regardless of their varied caloric loads.
Because metabolic activity is tightly coupled with neuronal activity, the measured parameters were selected carefully to reflect changes in brain metabolism [26]. These parameters include energy requirements-related parameters (lactate dehydrogenase, and creatine kinase) [26], pro-oxidant/antioxidant status parameters (MDA, GSH, GST, and catalase) [27], and voltage-gated channels-related parameter (K+) [28].
Results
Tables 1 and 2 demonstrate the induced changes in fecal microbiota either in ampicillin-treated or ampicillin treated rats fed various dietary regimens. Six weeks after the antibiotic treatments, the diversity of gastrointestinal flora remained altered, while certain bacterial species disappeared (e.g., Enterobacter cloacae) and certain propionibacteria were introduced (e.g., Klebsiella pneumonia and Proteus vulgaris). In addition, the fungal growth of Candida albicans was observed on ampicillin treatment. Candida tropicalis, Rhizobium radiobacter, and Enterococcus species were observed in rats fed an HLD compared to the other groups except the animals fed an HPD, which demonstrates the induced growth of Rhizobium radiobacter. After 6 weeks of ampicillin treatment (Table 2), it was observed that the fungal growth of both C. albicans and Streptococcus species had ceased, while the rest of the microbial profile was more or less the same.
Table 3 demonstrates the significant depletion of glutathione with PPA acid and ampicillin treatments compared to control. While PPA produced a 20.35% decrease in glutathione (P˂0.033), ampicillin was more effective and produced a 33.82% reduction in GSH levels (P˂0.001). However, PPA induced more lipid peroxidation in the rat pup brains than did ampicillin, with recorded values of 206.48% and 122.94%, respectively. Among the two dietary regimens, the protein-rich diet was effective in restoring GSH levels in the brains of ampicillin-treated rat pups. However, the HPD induced more lipid peroxides (MDA), while the HCD showed ameliorating effect with lipid peroxide levels that were not significantly different (P˂0.204) from the untreated control animals.
Although PPA induced a 16% reduction in the activity of glutathione S-transferase, this effect was not significantly different from that of the control. Ampicillin treatment together with the dietary regimens showed more or less similar effects compared to those of the control. Catalase, another antioxidant enzyme, was induced with both treatments and showed a significant difference with PPA treatment (P˂0.047) but not ampicillin treatment (P˂0.306) compared to control. The catalase activity in rat pups fed an HCD after ampicillin treatment was similar to that of the control (P˂0.306) but significantly different from that of PPA-treated animals (P˂0.006).
Lactate dehydrogenase was remarkably elevated in all groups compared to that of control. Ampicillin induced more or less similar changes compared to those in PPA-treated animals (P˂0.858).
Creatine kinase was the least affected parameter. It exhibited levels similar to those of the control in PPA- and ampicillin-treated animals, as well as those fed an HCD or HPD.
Potassium was significantly lower in PPA-intoxicated rats, while ampicillin did not induce a significant alteration in the K+ level. The three dietary regimens significantly increased the K+ level (P˂0.001).
Table 4 and Figure 1 present Pearson’s correlations between the measured parameters. It is clear that lipid peroxide (MDA) was positively correlated with catalase and potassium and negatively correlated with CK. In addition, potassium was positively correlated with glutathione S-transferase and negatively correlated with CK.
Table 5 shows the ROC analysis with the area under the curve (AUC), specificity and sensitivity of the measured parameters under various experimental conditions. Because AUC is a measure of the overall performance of a diagnostic test, the overall diagnostic performance of the various treatments of the present study can be compared by comparing their AUCs, specificity and sensitivity.
Table 6 presents multiple regression analysis using lipid peroxide as the dependent variable. It is clear that among the measured parameters, CK, catalase and lactate dehydrogenase show significance as independent variables with a P˂0.001 and an R2 value of 0.251.
Discussion
The gut microbiota of mammals play a key role in modulating host physiology. These effects are mostly associated with immunity and nutrient intake. Antibiotic usage strongly affects gut microbial composition and metabolism, thereby impacting human health. Understanding the mechanisms underlying this important interaction remains a major research goal [29].
Tables 1 and 2 demonstrate alterations in the gut microbiota of ampicillin-treated rat pups. Although the microbiology technique applied in this study did not measure the growth of Clostridia species, it was able to effectively detect the overgrowth of Klebsiella pneumonia, which is similar to Clostridia species in that both are propionibacteria and produce propionic acid as a metabolite [30,31].The growth of K. pneumonia in ampicillin-treated rat pups could be supported by the fact that K. pneumonia has β-lactamase enzyme and is thus resistant to ampicillin as a β-lactam antibiotic [32]. Interestingly, the reported overgrowth of Proteus vulgaris in ampicillin-treated rats could be easily related to PPA production. In a study conducted many years ago by Sherman and Shaw [33], Proteus vulgaris was reported to be an opportunistic human pathogen that accelerated the activity of PPA bacteria, which include Clostridia species and K. pneumonia. This phenomenon could explain the role of PPA in inducing biochemical autistic features in ampicillin-treated rat pups.
GSH depletion increases the cellular vulnerability toward oxidative stress, particularly in children due to their lower GSH levels [34,35]. The risk due to impairment of the detoxification capacity in infants is higher because certain toxic environmental factors that induce oxidative stress accumulate in the placenta and are found at higher concentrations in developing infants than in their mothers.
Table 3 demonstrates glutathione depletion as a feature of PPA and ampicillin neurotoxicity, with ampicillin being more toxic than PPA and showing a more significant reduction compared to that of the control. Associated with the glutathione levels, which are reduced in PPA- and ampicillin-stressed animals, Table 3 also shows that in these animals, glutathione levels can be restored by dietary supplementation with a protein-rich diet but not a carbohydrate- or lipid-rich diet. This finding could indicate that rats with GSH deficiency may present a model for realistic toxicological testing, one that is more closely related to the clinical condition of a critically ill patient on drug orantibiotics treatment. The recorded effect of a protein-rich diet in restoring the brain GSH level can be supported by the previous work of Micke et al [36], in which they reported that short-term (14 days) oral supplementation (45 g/day) with whey protein formulas increased the plasma GSH levels in glutathione-deficient patients with advanced HIV infection. However, the inefficiency of HCD and HLD for restoring GSH in ampicillin-treated rats is in good agreement with a recent study of Alzoubi et al. [37] reporting that a high-lipid/high-carbohydrate diet (HLCD) induces oxidative stress that presents as high-lipid peroxides and concomitant low GSH levels and results in neuronal damage and interference with synaptic transmission leading to a decline in cognitive function.
Table 3 also demonstrates lipid peroxides (MDA) elevation as a metabolic marker of PPA and ampicillin neurotoxicity, with ampicillin being less toxic than PPA and showing a reduced but still significant elevation compared to the control. This finding is supported by the recent work of El-Ansary et al. [38], in which GSH depletion and MDA elevation were observed as biochemical autistic features in a rodent model of autism. While an HCD demonstrated a similar change in lipid peroxidation compared to that of the control, the HFD, and unexpectedly, the HPD induced more lipid peroxidation in ampicillin-treated animals. This effect of an HPD could find support in the previous work of CalderónGuzmán et al. [39], which proved that MDA was decreased in several brain areas of malnourished rats fed a low-protein diet (7%). The effect of an HLD on MDA reported in the present study is consistent with the recent work of Amin et al. [40], in which the consumption of an HLD for12 weeks was proven to be adequate to increase the level of MDA, a marker of oxidative stress, in the brains of rats. Induced lipid peroxidation in HLD-fed ampicillin-treated rats can be explained according to a gut-brain-liver neuronal mechanism and lipid-sensing system. Lipids in the lumen of rats fed a lipid-rich diet stimulate the release of cholecystokinin (CCK) from intestinal cells lining the mucosa of the duodenum [41]; the CCK then binds to the CCK-A receptor in the gastrointestinal system and indirectly regulates hepatic glucose synthesis. Lipid-induced CCK can activate the release of glutamate (the principal excitatory neurotransmitter) from vagal afferent terminals [42]. This neuronal axis represents one of the first lines of metabolic defense against nutrient excess, providing metabolic balance by lowering glucose production upon nutrient exposure. This suggested involvement of CCK in the attenuation of the lipid-sensing mechanism and the reported elevation of MDA in HLD-fed ampicillin-treated rats can be supported by the current work of Tamás et al. [43], in which MDA was significantly elevated in the pancreatic tissue of a CCK-treated group compared to untreated animals. Interestingly, the suggested involvement of a gut-brain neuronal mechanism in ampicillin-treated rats fed an HLD can easily be related to glutamate excitotoxicity, which is a mechanism that has recently been highlighted in the etiology of autism. Wyeth et al. [44] reported that CCK induced an up-regulation of excitatory glutamatergic neurons and a down-regulation of inhibitory gamma amino butyric acid (GABA) ergic neurons in mice with spontaneous seizures. This finding raises the possibility that, in the present study, the suggested amplification of CCK in response to a lipid-rich diet could explain the epileptic seizures observed in most autistic patients and indicate the possibility of avoiding them through dietary changes. The observed effects of HCD and HPD reported in the present study are inconsistent with previous reports from Kitabchi et al. [45] that a low-carbohydrate (rather than a high-protein) diet is more efficacious in lowering MDA in obese, premenopausal non-diabetic women.
Antioxidant enzymes are an appropriate indirect measure to evaluate the pro-oxidant/antioxidant status associated with PPA- and ampicillin-induced toxicity. The activity of GST decreased in PPA-treated rats, while it was increased in ampicillin-treated rats and in ampicillin-treated rats fed HC, HP and HL diets. The remarkable increase in GST in the brains of antibiotic-treated rats and antibiotic-treated rats fed a modified diet could be related to the induction of GST in the medium culture as a type of defense against ampicillin toxicity. This explanation could be supported by the work of Shaffer et al. 46], which reported the efficiency of GST in decreasing the activity of many antibiotics, including ampicillin, resulting in increased values of the minimum inhibitory concentrations (MIC). A single exposure caused the leakage of proteins from brain mitochondria, a high rate of lipid peroxidation, and a significant increase in cytosolic antioxidant enzymes, thus demonstrating that antioxidant defenses act as an early manifestation of antibiotic neurotoxicity that is remarkably affected by diet. This explanation is consistent with the reported ampicillin-induced neurotoxicity noted in very low birth weight neonates, which has been attributed to immature transport mechanisms and renal immaturity, as well as increased permeability of the blood brain barrier [46]. The decrease of GST activity in PPA-treated rats is a reproducible neurotoxic effect of this short-chain fatty acid [38].
Catalase is an important enzyme in the elimination of H2O2from tissues, and it has been suggested that catalase and not glutathione peroxidase (GPX) is the major antioxidant enzyme responsible for H2O2 degradation [47]. Because H2O2 induces catalase expression, it is likely that PPA-treated rats, ampicillin-treated rats and ampicillin-treated rats fed either an HPD or HLD diet have elevated levels of this ROS. This result could be related to the pathological effects of PPA and ampicillin because similarly increased levels of catalase were found in H2O2-stressed Chinese hamster fibroblasts, A549 human lung adenocarcinoma cells and U87MG glioblastoma cells [48,49]. The increased catalase activity observed in PPA-treated rats does not contradict the observed decrease in catalase activity that has previously been reported as an autistic feature in PPA-treated rat pups [38].This finding is expected because it is well known that for in vivo systems, if the oxidative stress is not very strong or very prolonged, the catalase activity increases, but if it is persistent or its level is very high, the protein damage becomes profound, and a decrease in the catalase activity may occur (either via direct oxidative damage of the catalase molecules, via oxidative stress-altered gene expression, or both). This assumption could be supported by the depletion of GSH and elevation of MDA (two important markers of oxidative stress) reported in the present study and in our previous study. Rats fed a HC diet plus ampicillin show significantly reduced catalase activity. This finding is supported by the work of Francini et al. [50], which reported significant reductions of 24% and 18% in the total GSH content and CAT activity, respectively, in the livers of rats fed a high-fructose diet.
The results of the present study show the LDH activity increased to 40-50% in the 5 groups relative to control (p < 0.001), whereas CK activity was significantly decreased only in the ampicillin-treated rats fed a high-lipid diet (p < 0.002). As LDH is an important cytoplasmic enzyme that regulates energy metabolism in the cell, the remarkable increase in the activity of this enzyme shows that PPA and ampicillin both induce changes in the plasma membrane permeability with consequent cellular damage. The neurotoxicity of both agents can be explained by an increase in LDH activity causing a low pH due to high lactate production. Lactic acidosis could in turn induce the denaturation of integral and structural proteins, injuring neurons [51] With the exception of HCD; there was no significant dietary effect on LDH activity. The remarkable increase in LDH activity in rats fed an HCD compared to the tested groups could be explained by the idea that LDH, as a glycolytic enzyme, is activated in response to the increase in glucose in an attempt to enhance its catabolism. This assumption is consistent with a previous study by Walton [52], in which the activation of certain glycolytic enzymes, including LDH, was recorded in response to a high-carbohydrate/low-protein diet in the rainbow trout Salmogairdneri.
Biochemically, HLD-induced disturbances in metabolism cause an increase in the flux of free fatty acids (FFA) into a variety of tissues. This response mediates brain disturbances through a reduction in the ability of brain to respond to various stressors and a loss of the capacity to repair damaged brain cells. In the present study, the decrease in CK activity in animals fed an HLD after ampicillin treatment is not in good agreement with the previous work of Amin et al. [40], in which an HLD induced an increase in CK secondary to the induction of glycolytic enzymes. The significant decrease in CK in rats fed an HLD could be attributed to the synergistic effect of the antibiotic. Although ampicillin alone did not induce a significant impairment in CK, rats fed an HLD post ampicillin treatment exhibited a highly significant decrease in CK compared to those of control untreated and unfed rats. The suggested synergistic effect of HLD can be supported by the previous work of Ribeiro et al. [53], which demonstrated that HLD aggravates the toxicity of hydrochlorothiazide (HCTZ) in treated rat brains.
Voltage-gated K+ channels (Kvs), together with a presynaptic Ca2+ influx, control the release of neurotransmitters [54], and these channels play a critical role in glucose homeostasis. Blocking Kvs reduces IL-2 and tumor necrosis factor production, resulting in improved autoimmune encephalitis and the inhibition of microglial-mediated neuronal death in experimental models [55,56]. The reported low level of K+ in PPA-neuro-intoxicated rats could indicate the importance of maintaining K+ within a controlled concentration range for normal brain synaptic function. The significant increase in K+ reported in ampicillin-treated rats could be attributed to the potent effect of ampicillin under various nutritional conditions on activation of the Na+/K+ ATPase and the total ATPase activity [40].
The positive correlations of lipid peroxides (MD, as a marker of oxidative stress) with catalase, GST and K+ and its negative correlation with CK(as a marker of energy metabolism) (Table 4) show that oxidative stress is an etiological mechanism involved in the neurotoxicity of PPA and ampicillin.
In addition to the AUC, the specificity and sensitivity values listed in Table 5 demonstrate the possibility of using GSH, MDA, GST and K+ as markers of PPA and ampicillin neurotoxicity. In addition, K+ was the most potent marker of ampicillin neurotoxicity,showing effectiveness when testing both toxic agents together in conjunction with the three dietary regimens.
Conclusion
The present work proposes that ampicillin-induced neurotoxicity might be among the etiologies of late-onset autism due to alterations in gut microbiota and the induction of propionibacteria. Examining how disturbances in the gut flora can induce oxidative stress as a mechanism that remarkably affects the brain in rodent models of autism can reveal promising targets for the development of diagnostic markers of this disorder. As ampicillin is used to treat a wide variety of infections caused by bacteria, such as ear infections (which are commonly observed in autistic patients), bladder infections, and pneumonia, a good balanced diet rich in a variety of nutrients is crucial to ameliorate or avoid the neurotoxic effects of antibiotics, which are the most frequently used pharmaceuticals during brain development in cases of frequent infection.
Methods
Animals
The experimental assays for this study were performed on 60 young (approximately 21 days old) male western albino rats (45 to 60 g). Rats were obtained from the animal house of the Pharmacy College of King Saud University and were randomly assigned to six groups of ten rats each. The first group of rats received only phosphate buffered saline and were used as a control group (n = ten). The second group was given oral neurotoxic doses of PPA (250 mg/kg body weight/day for three days; n = ten) [57] and were referred to as the oral buffered PA-treated group. The third group received an orogastric dose of ampicillin (50 mg/kg for three weeks) with standard diet and referred to as the ampicillin group. Groups 4, 5 and 6 were given orogastric doses of ampicillin (50 mg/kg for three weeks). This dosage was appropriate and selected according to clinical guidelines and the diagnosis and treatment manual (30–100 mg/kg in humans) [58,59]. The ampicillin-treated animals were fed a high-carbohydrate, high-protein or high-lipid diet for 10 weeks. All groups of rats were individually housed under a controlled temperature (21 ± 1°C) with ad libitum access to food and water. All of the procedures described were reviewed and approved by the King Saud University animal ethical committee.
Diet
The standard rats/mice chow consisted of 47% complex carbohydrates, 21% protein, 4.0% fat, 5.0% fiber, and 8.0% ash. The special diets characterized as high-carbohydrate and high-protein were made by mixing pulverized regular chow with sucrose and casein, respectively, and then pelleting. The pellets were baked at 55°C for 7 hours before use. The lipid-rich diet was made by adding corn oil to the regular chow.
Tissue preparation
At the end of the feeding trials, the brain was dissected out and submitted to biochemical analysis. The brains isolated from sacrificed animals were washed, dissected into small pieces and homogenized in distilled water using a Teflon homogenizer. The homogenate was centrifuged at 3,000 g for 20 minutes to remove debris and kept at −80°C until further use.
Biochemical analyses
The following biochemical estimations were made from the brain tissue samples. Glutathione was assayed by the method of Beutler et al. [60] using 5,5′-dithiobis 2-nitrobenzoic acid (DTNB) with sulfhydryl compounds to produce a relatively stable yellow color. Lipid oxidation was estimated by the formation of thiobarbituric acid reactive substances (TBARS) by the method of Ruiz-Larrea et al. [61]. Glutathione S-transferase activity (GST) activity was assessed using an assay kit (Biovision, USA) that was based upon the GST-catalyzed reaction between GSH, GST substrate, and CDNB (1-chloro-2,4-dinitrobenzene). The activity of catalase was determined by the method of Chance [62], in which the levels of catalase activity were expressed as μmoles of H2O2 dissociated/minute/dl. Lactate dehydrogenase was assayed using the lactate-to-pyruvate kinetic method described by Henry et al. [63]. An assay of creatine kinase was performed using the CK kit from the National Scientific Company (NSC) [64]. Potassium levels were measured by producing a turbid suspension in a protein-free alkaline medium by reaction with sodium tetraphenyl boron [65].
Microbiological examination
The cecal contents of the all experimental groups were collected in sterile tubes and immediately stored at −20°C. The frozen tubes were then analyzed. The process of bacterial cultivation involves the use of optimal artificial media and incubation conditions to isolate and identify the bacterial etiologies of an infection as rapidly and accurately as possible. Fecal samples were cultured under aerobic and anaerobic conditions and continuously monitored for one week. Positive cultures were plated with appropriate media, and the species were identified by Scepter micro dilution and standard bacteriological techniques. All of the plates were examined after 24 and 48 h of incubation at 37°C. This work was performed in a microbiology lab at Almishari Hospital, Riyadh, KSA.
Statistical analysis
The SPSS software program was used. The results were expressed as the mean ± SD, and all statistical comparisons were made using independent t-tests with P ≤ 0.05considered as significant. Receiver Operating Characteristic (ROC) analysis was performed as a comprehensive way to measure the effectiveness of the studied parameters in terms of either the neurotoxicity of PPA and ampicillin or the effects of the various dietary regimens. The area under the curve (AUC) provides a useful metric to compare various biomarkers. Whereas an AUC value close to 1 indicates an excellent predictive marker, a curve that lies close to the diagonal (AUC = 0.5) has no diagnostic utility. An AUC of 0.8-0.9 represents a good test, 0.7-0.8 indicates fair, 0.6-0.7 indicates poor, and 0.5-0.6 indicates a worthless test. AUC analysis is always accompanied by the relative values of specificity and sensitivity of the biomarker [66]. Multiple regression analysis using the Stepwise method was used to evaluate the relationship between MDA as a dependent variable and the remaining measured parameters as independent variables. The value of R2 usually indicates the percentage of the variance of the dependent variable associated with the regression of the recorded independent variables.
References
Sommer F, Bäckhed F. The gut microbiota — masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38.
Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G and Pacheco-LópezG: Themicrobiota–gut–brain axis: neurobehavioral correlates, health and sociality Front. Integr. Neurosci. 2013. | doi:10.3389/fnint.2013.00070.
Gur TL, Worly BL, Bailey MT. Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front Psychiatry. 2015;2(6):5.
De Theije CG, Wopereis H, Ramadan M, Van Eijndthoven T, Lambert J, Knol J, et al. Altered gutmicrobiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206.
Martin Jr HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, et al. Vaginal lactobacilli, microbial ora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8.
Marshall JC. Gastrointestinal flora and its alterations in critical illness. Curr Opin Clin Nutr Metab Care. 1999;2:405–11.
Vollaard EJ, Clasener HAL, VanSaene HKF, Muller NF. Effect on colonization resistance: an important criterion in selecting antibiotics. DICP. 1990;24:60–6.
Das UN. Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids. Nutrition. 2013;29:1275–85.
Frye RE, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, et al. Effectiveness of methylcobalamin and folinic acid treatment on adaptive behavior in children with autistic disorder is related to glutathione redox status. Autism Res Treat. 2013. Article ID 609705.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63.
Louis P. Does the human gut microbiota contribute to the etiology of autism spectrum disorders? Dig Dis Sci. 2012;57:1987–9.
Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15(7):429–35.
Claus SP, Ellero SL, Berger B, Krause L, Bruttin A, Molina J, et al. Colonization-induced host-gut microbial metabolic interaction. mBio. 2011;2:e00271–310.
Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinalmicrobiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–87.
15) Russell SL, Gold MJ, Reynolds LA, Willing BP, Dimitriu P, Thorson L, Redpath SA, Perona-Wright G, Blanchet MR, Mohn WW, Brett Finlay B, McNagny KM. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol. 2015. S0091-6749(14)00893-8. doi:10.1016/j.jaci.2014.06.027. [Epub ahead of print].
Finegold SM. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses. 2011;77(2):270–4.
Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. 2012;2:260–2.
Foley KA, MacFabe DF, Kavaliers M, Ossenkopp KP. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: Relevance to autism spectrum disorders. Behav Brain Res. 2014;78C:244–56.
MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boond F, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149.
Shultz SR, MacFabe DF, Ossenkopp KP, Scratch S, Whelan J, Taylor R, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54:901.
Davis TN, O’Reilly M, Kang S, Lang R, Rispoli M, Sigafoos J, et al. Chelation treatment for autism spectrum disorders: A systematic review. Res Autism Spectrum Disorders. 2013;7:49–55.
Yassa HA. Autism: A form of lead and mercury toxicity. Environ Toxicol Pharmacol. 2014;38:1061–24.
Seko Y, Miura T, Takahashi M, Koyama T. Methyl mercury decomposition in mice treated with antibiotics. Acta Pharmacol Toxicol (Copenh). 1981;49(4):259–65.
Deth R, Muratore C, Power Charnitsky VA BJ, Waly M. How environmental and genetic factors combine to cause autism:aredox/methylation hypothesis. Neurotoxicology. 2008;29:190–201.
Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol. 2014;5:150.
Rodríguez-Rodríguez A, Egea-Guerrero JJ, Murillo-Cabezas F, Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr Med Chem. 2014;21(10):1201–11.
Mortensen LS, Schmidt H, Farsi Z, Barrantes Freer A, Rubio ME, Ufartes R, et al. KV 10.1 opposes activity-dependent increase in Ca(2+) influx into the presynaptic terminal of the parallel fibre-Purkinje cell synapse. J Physiol. 2015;593(1):181–96.
Eleftherios Terry P, Meyer C l. Fermentation equation for propionic acid bacteria and production of assorted oxychemicals from various sugars. Biotechnol Bioeng. 1985;XXVII:67–80.
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain developmentand behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–52.
Lian Hua Luo J-WS, Sun YH, Baek Rock O, Dae-Hyuk K, Chul Ho K. Identification and characterization of Klebsiellapneumoniae aldehyde dehydrogenases increasing production of 3-hydroxypropionic acid from glycerol. Bioprocess Biosyst Eng. 2013;36(9):1319–26.
Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in klebsiellapneumoniae Biofilm resistance to ampicillin and CiprofloxacinAntimicrob. Agents Chemother. 2000;44(7):1818–24.
Guentzel MN. Escherichia, Klebsiella, Enterobacter, Serratia Citrobacter, and Proteus. In: Barron’s Medical Microbiology Univ of Texas Medical Branch(Barron ’s et al., eds.) (4th ed). 1996.
Sherman JM, Shaw RH. The propionic acid fermentation of lactose. J Biol Chem. 1923;56:695–700.
Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–6.
Ono H, Sakamoto A, Sakura N. Plasma total glutathione concentrations in healthy pediatric and adult subjects. Clin Chim Acta. 2001;312:227–9.
Micke P, Beeh KM, Schlaak JF, Buhl R. Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. Eur J Clin Invest. 2001;31:171–8.
Alzoubi KH, Khabour OF, Salah HA, Hasan Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: the role of oxidative stress. Physiol Behav. 2013;119:72–8.
El-Ansary AK, Ben Bacha A, Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation. 2012;9:74.
CalderónGuzmán D, BarragánMejía G, Hernández García E, JuárezOlguín H. Effect of nutritional status and ozone exposure on some biomarkers of oxidative stress in rat brain regions. Nutr Cancer. 2006;55(2):195–200.
Amin KA, Kamel HH, AbdEltawab MA. Protective effect of Garcinia against renal oxidative stress and biomarkers induced by high fat and sucrose diet. Lipids Health Dis. 2011;14(10):6.
Cheung GWC, Kokorovic A, Lam CKL. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109.
Allchin RE, Batten TF, McWilliam PN, Vaughan PF. Electrical stimulation ofthevagus increases extracellular glutamate recovered from the nucleus tractussolitariiof the cat by in vivo microdialysis. Exp Physiol. 1994;79:265–8.
TamásLetoha CS, Tamás T, Szabolcs A, Katalin J, Rakonczay Jr Z, Péter H, et al. A nuclear import inhibitory peptide ameliorates the severity of cholecystokinin-induced acute pancreatitis. World J Gastroenterol. 2005;11(7):990–9.
Wyeth MS, Zhang N, Houser CR. Increased cholecystokinin labeling in the hippocampus of amouse model of epilepsy maps to spines and glutamatergic Terminals. Neuroscience. 2012;202:371–83.
Kitabchi AE, Mcdaniel KA, Wan JY, Tylavsky FA, Jacovino CA, Sands CW, et al. Effects of high-protein VersusHigh-carbohydrate diets on markers ofb-cell function, oxidative stress, LipidPeroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes. Diabetes Care. 2013;12:1–7.
Shaffer CL, Davey AM, Ransom JL, Brown YL, Gal P. Ampicillin-induced neurotoxicity in very-low-birth-weight neonates. Ann Pharmaco ther. 1998;32:482–4.
Spitz DR, Elwell JH, Sun Y, Oberley LW, Oberley TD, Sullivan SJ, et al. Oxygen toxicity in control andH2O2-resistant Chinese hamster fibroblast cell lines. Arch BiochemBiophys. 1990;279:249–60.
Hunt CR, Sim JE, Sullivan SJ, Featherstone T, Golden W, Von Kapp-Herr C, et al. Genomic instability and catalasegene amplification induced by chronic exposure to oxidativestress. Cancer Res. 1998;58:3986–92.
Bojes HK, Suresh PK, Mills EM, Spitz DR, Sim JE, Kehrer JP, et al. resistant A549 and U87MGcells. Toxicol Sci. 1998;42:109–16.
Francini F, Castro MC, Schinella G, García ME, Maiztegui B, Raschia MA, et al. Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Life Sci. 2010;86(25–26):965–71.
Moody WA. Effects of intracellular H+ on the electrical properties of excitable cells. Rev Neurosci. 1984;7:257.
Walton MJ. Metabolic effects of feeding a high protein/low carbohydrate diet as compared to a low protein/high carbohydrate diet to rainbow troutSalmogairdneriFish. Physiol Biochem. 1986;1(1):7–15.
Ribeiro MC, Barbosa NB, de Almeida TM, Parcianello LM, Perottoni J, de Avila DS, et al. High-fat diet and hydrochlorothiazide increase oxidative stress in brain of rats. Cell Biochem Funct. 2009;7:473–8.
Yang YM, Wang W, Fedchyshyn MJ, Zhou Z, Ding J, Wang LY. Enhancing the fidelity of neurotransmission by activity-dependent facilitation of presynaptic potassium. Curr Nat Commun. 2014;5:4564.
NutileMcMenemy N, Elfenbein A, Deleo JA. Minocycline decreases in vitro microglial motility, beta1-integrin, and Kv1.3 channel expression. J Neurochem. 2007;103(5):2035–46.
Xu J, Wang P, Li Y, Li G, Kaczmarek LK, Wu Y, et al. The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. Proc Natl Acad Sci U S A. 2004;101(9):3112–7.
Wyatt I, Farnworth M, Gyte AJ, Lock EA. L-2-chloropropionic acid metabolism and disposition in male rats: relevance to cerebellar injury. Arch Toxicol. 1997;71:668.
Clinical guidelines, diagnosis and treatment manual, for curative programmes in Hospitals and dispensaries. Guidance for prescribing 2013 Edition,
Hentges DJ, Stein AJ, Casey SW, Que JU. Protective role of intestinal flora against infection with Pseudomonas aeruginosa in mice: influence of antibiotics on colonization resistance. Infect Immun. 1985;47(1):118–22.
Beutler E, Duran O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882.
Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsome. Steroids. 1994;59:383.
Chance B. Catalases and peroxidases, part II. Special methods. Methods Biochem Anal. 1954;1:408.
Henry JB. Clinical Diagnosis and Management by Laboratory Method. Sixteenthedtion. Philadelphia, PA: WB Saunders and Company; 1974.
Szasz G. Laboratory measurement of creatine kinase activity. In: Tietz NW, Weinstock A, Rodgerson DO, editors. Proceedings of the Second International Symposium on Clinical Enzymology. Washington DC: American Association for Clinical Chemistry; 1975. p. 43–179.
Terri AE, Sesin PG. Determination of potassium in blood serum. Am J Clin Pathol. 1958;29:86–9. 1.
Fawcett T. An introduction to ROC analysis. Pattern Recogn Lett. 2006;27:861–74.
Acknowledgments
This research project was supported by a grant from the research center of the Center for Female Scientific and Medical Colleges at King Saud University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AE: designed the study and drafted the manuscript. RSB: performed the practical work and suggested the dietary regimens. SA: co-drafted the manuscript. AA: performed the statistical analysis and helped perform the microbiology work. All authors have read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
El-Ansary, A., Bhat, R.S., Al-Daihan, S. et al. The neurotoxic effects of ampicillin-associated gut bacterial imbalances compared to those of orally administered propionic acid in the etiology of persistent autistic features in rat pups: effects of various dietary regimens. Gut Pathog 7, 7 (2015). https://doi.org/10.1186/s13099-015-0054-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-015-0054-4