Abstract
Aims
The Stress Hyperglycemia Ratio (SHR) potently predicts adverse outcomes in patients with cardiovascular and cerebrovascular diseases. However, the relationship between SHR and short-term mortality risk in patients with a first diagnosis of acute myocardial infarction (AMI) remains contentious. This study sought to understand better the relationship between SHR and short-term mortality risk in patients with a first diagnosis of AMI.
Methods
We conducted a cohort study using data from 1961 patients with a first diagnosis of AMI from the MIMIC-IV (version 2.2) database. Patients were divided into three groups based on SHR tertiles. The Cox proportional hazards model and a two-segmented Cox proportional hazards model were used to elucidate the nonlinear relationship between SHR in patients with a first diagnosis of AMI and mortality.
Results
Of the surveyed population, 175 patients (8.92%) died within 90 days, and 210 patients (10.71%) died within 180 days. After multivariate adjustments, elevated SHR levels were significantly and non-linearly associated with a higher risk of 90-day and 180-day mortality in patients with a first diagnosis of AMI, showing a J-shaped correlation with an inflection point at 0.9. Compared to participants with SHR levels below the inflection point, those with higher SHR levels had a fivefold increased risk of 90-day mortality (hazard ratio [HR] 5.74; 95% confidence interval [CI] 3.19, 10.33) and a fourfold increased risk of 180-day mortality (HR 4.56; 95% CI 2.62, 7.95). In the subgroup analysis, patients with pre-diabetes mellitus (pre-DM) and higher SHR levels had increased 90-day (HR 6.90; 95% CI 1.98, 24.02) and 180-day mortality risks (HR 5.30; 95% CI 1.96, 14.27).
Conclusion
In patients with a first diagnosis of AMI, there is a J-shaped correlation between SHR and 90-day and 180-day mortality, with an adverse prognostic inflection point of SHR at 0.9.
Similar content being viewed by others
Introduction
As the global population ages and expands, cardiovascular diseases (CVD) have become the leading causes of mortality and morbidity worldwide [1]. However, individuals with cardiovascular diseases often exhibit metabolic disturbances due to unhealthy dietary and lifestyle habits, potentially exacerbating adverse cardiovascular outcomes [2, 3]. Previous research has shown that elevated blood glucose levels upon hospital admission in patients with CVD are independently associated with poor prognosis [4, 5]. However, hyperglycemia often arises from a combination of acute stress and chronic glucose levels and thus might not necessarily reflect an acute rise in glucose. Consequently, some researchers have employed a novel marker of hyperglycemic state, the Stress Hyperglycemia Ratio (SHR), to represent genuine acute hyperglycemia [6].
Stress-induced hyperglycemia is a relative increase in glucose caused by inflammation and neurohormonal disruptions during severe illness. The interaction between hyperglycemia and illness severity partly mirrors the more intense inflammatory and neurohormonal responses seen with more severe disease stimuli [7]. Many recent studies have demonstrated that acute hyperglycemia upon admission in patients with acute coronary syndrome (ACS) is independently associated with early and late adverse prognoses [8]. A study from the COACT registry revealed that SHR is an effective predictor of post-percutaneous coronary intervention (PCI) major adverse cardiac and cerebrovascular events (MACCE), especially in patients with non-diabetic ST-segment elevation myocardial infarction (STEMI) [9]. Yet, another report indicated no significant correlation between SHR bifurcation and short-term survival in diabetic patients [10]. Thus, the relationship between SHR and short-term mortality risk in patients with a first diagnosis of AMI remains contentious and merits further investigation. Moreover, past research has had limitations, including small sample sizes and overlooking potential confounding factors like the influence of other diseases and medication use, which could affect the results. Hence, in this study, using the MIMIC-IV (version 2.2) database, we aim to understand better the relationship between SHR and short-term mortality risk in patients with a first diagnosis of AMI.
Materials and methods
Data source and study population
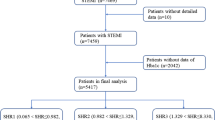
This retrospective study utilized health-related data from the MIMIC-IV (version 2.2) database, a comprehensive large-scale database developed and managed by the MIT Computational Physiology Laboratory. The database comprises over 50,000 high-quality medical records of patients admitted to the Intensive Care Unit (ICU) at the Beth Israel Deaconess Medical Center [11]. Notably, all personal identifying information has been anonymized to safeguard patient privacy. In light of the nature of this study, the Institutional Review Board of the Beth Israel Deaconess Medical Center waived the requirement for informed consent [11]. To access the database, the author (XHC) obtained the necessary certification and then extracted the required variables (Certification No.: 58951192). For this study, patients with a first diagnosis of AMI based on the International Classification of Diseases (ICD), ICD-9, and ICD-10 were included. Participants under the age of 18 years (n = 0), those with chronic kidney disease stage 5 (n = 119), with malignant tumors (n = 9), and those with missing glucose and HbA1c data (n = 1580) were excluded. In the final analysis, 1961 individuals were included and categorized into three groups based on the tertiles of their SHR levels (Fig. 1).
Data collection
Data extraction was performed using the PostgreSQL tool (v.14, PostgreSQL Global Development Group, Berkley, California, USA). Diagnoses of patients were retrieved as per ICD-9 and ICD-10 using Structured Query Language (SQL). Baseline characteristics, laboratory indicators, and medication usage records were queried through the item IDs stored in MIMIC-IV and matched to selected patients using a unique identifier (subject_id). Extracted variables included: (1) Baseline characteristics: age, sex, height, weight, systemic inflammatory response (SIRS), sequential organ failure assessment (SOFA); (2) Comorbidities: hypertension, cardiogenic shock, atrial fibrillation, cardiac arrest, heart failure, diabetes, chronic kidney disease stage 5, malignant tumor; (3) Laboratory parameters: red blood cell (RBC), white blood cell (WBC), platelet, creatinine, glucose, hemoglobin A1C (HbA1C); (4) Medication use: vasoactive drugs, antihypertensive drugs, antilipidemic drugs, antiplatelets, insulin; (5) Hospital and ICU admission data: admission and discharge dates, ICU admission and discharge dates, death date and time. All measurements utilized in this study were from the initial 24 h post-admission. Specific details and query codes for each metric are available in Table S1.
Data definitions
The Body Mass Index (BMI) was calculated as weight (kg) divided by the square of height (m). The SHR was determined using the following formula: SHR = admission blood glucose (ABG) (mmol/L)/[1.59 × HbA1c (%) − 2.59] [6]. Diabetes was defined as a history of diabetes or HbA1c > 6.5%. Pre-DM was defined as patients without a history of diabetes but with HbA1c levels between 5.7 and 6.4%. Normoglycemia (NGR) was identified in patients without a history of diabetes or with HbA1c ≤ 5.7% [12].
Outcomes
The primary outcomes were all-cause mortality at 90 and 180 days post-admission, calculated based on the first AMI diagnosis time and the follow-up death dates from MIMIC-IV 2.2.
Statistical analysis
Based on the baseline SHR tertiles, data are expressed as mean (SD) or median (interquartile range) for continuous variables and frequency (percentage) for categorical variables. Differences between SHR tertiles groups were assessed using the chi-squared test (for categorical variables), one-way ANOVA (for normally distributed data), and the Kruskal–Wallis H test (for skewed data). The lowest tertile of SHR served as the reference group. To analyze the association between the SHR index and the risk of 90-day and 180-day death, multivariable Cox proportional hazards regression models were utilized to calculate Hazard ratios (HRs) and 95% confidence intervals (CIs). Model 1 did not adjust for any covariates. Any covariate altering the resulting estimate by more than 10% was included in Model 2 as a potential confounder. If the covariate changed the resulting estimate by more than 10% or had a regression coefficient P-value < 0.1, it was considered a potential confounder in Model 3 [13]. To prevent multicollinearity, variables with a variance inflation factor (VIF) greater than five were excluded from the models. The Kaplan–Meier survival analysis was also employed to assess endpoint occurrence rates based on different SHR levels.
Likewise, analyses were divided into groups according to the following factors: age, sex, BMI, hypertension, heart failure, atrial fibrillation, and diabetes. Except for the stratification variable, the adjustment technique was the same as in Model 3. Log-likelihood ratio tests were employed to evaluate interactions between SHR and outcomes between subgroup factors.
Additionally, restricted cubic splines were used with three knots (10th, 50th, and 90th percentiles) to examine dose–response associations. We then calculated HR for SHR and outcomes by running a log-likelihood ratio test for the non-linearity of the smooth curve fit, contrasting the segmented regression model to the single-linear (non-segmented) model while accounting for relevant confounders.
The highest covariate missing was the BMI (29.63%). We employed a multiple imputation and chained equation technique based on five replications in the R MI process to account for the missing data (BMI, WBC, RBC, PLT, and creatinine) in to prevent a deterioration in the effectiveness and bias of the statistical analyses owing to the direct exclusion of missing values [13]. Cox regression analysis was performed on the five newly created data sets, and the outcomes were merged using Rubin's rules [14, 15]. We performed sensitivity analyses to assess how reliable our results were. Firstly, distributions of variables with missing data comparing observed complete case data. Secondly, the association between SHR and the risk of 90-day or 180-day mortality was investigated using data before multiple imputations (n = 1358). Thirdly, further adjustments were made to the SIRS and SOFA scores based on clinical data (n = 986). Finally, recognizing the varying diabetes status among patients, we performed subgroup analyses. All analyses have been conducted using the data after multiple imputations except Table S3, which used raw data for analyses. R 4.3.0 (http://www.R-project.org) was used for all analyses. A two-sided P-value of less than 0.05 was deemed statistically significant.
Results
Baseline characteristics of patients with AMI
Data were derived from 1961 AMI patients (mean age 66.65 years; 69.10% male). Those with elevated SHR tended to be older males with increased white blood cell counts and glucose levels. Notable differences in age, sex, white blood cell count, red blood cell count, platelets, creatinine, glucose, HbA1c, heart failure, cardiogenic shock, cardiac arrest, atrial fibrillation, hypertension, diabetes, antilipidemic drugs, antiplatelets, vasoactive drugs, 90-day and 180-day mortality were statistically significant across the three patient groups (all P < 0.05) (Table 1).
Associations between SHR and outcomes
Over the 90-day and 180-day follow-up periods post-admission, there were 175 and 210 recorded deaths, respectively. After multivariable adjustment for age, sex, BMI, cardiogenic shock, cardiac arrest, and hypertension, compared with the reference tertiles, the third tertiles showed a significant association with SHR concerning 90-day and 180-day mortality in Model 2. With further adjustments for potential confounder, the results were consistent in Model 3, multivariable-adjusted HRs (95% CIs) tertiless of across SHR were 1.00 (reference), 0.81 (0.50, 1.30), and 1.94 (1.33, 2.82) (P trend < 0.001); and 1.00 (reference), 0.88 (0.58, 1.34), and 1.92 (1.37, 2.70) (P trend < 0.001), respectively (Table 2).
The dose–response relationship between SHR and the adjusted hazard ratio for 90-day and 180-day mortality in AMI patients was depicted using restricted cubic splines. A J-shaped association between SHR and the 90-day and 180-day mortality rates was observed (Fig. 2). Additionally, a combination of Cox proportional hazard models with a two-segmented Cox proportional hazards model was employed to study the non-linear relationship between SHR levels in AMI patients and the mortality mentioned above rates (P for log-likelihood ratio < 0.05) (Table 3). An inflection point was detected at an SHR of 0.9. When the SHR exceeds 0.9, for each unit increase in the SHR level, the adjusted HRs for 90-day and 180-day mortality increase by fivefold (HR 5.74; 95% CI, 3.19–10.33) and fourfold (HR 4.56; 95% CI, 2.62–7.95), respectively. However, when the SHR is below 0.9, there is no significant association with 90-day and 180-day mortality. Survival analysis further suggested a positive relationship between baseline SHR levels, when categorized into tertiles, and the 90-day and 180-day mortality rates (Kaplan–Meier, log-rank P < 0.001) (Fig. 3). Similar results were also observed when categorized into two groups according to the inflection point (Figure S1).
Restricted cubic spline analyses of the association of SHR with all-cause mortality (A all-cause death in 90 days, B all-cause death in 180 days). Heavy central lines represent the estimated adjusted hazard ratios. The 95% confidence interval is represented by the red band. The adjustment strategy is the same as the Model 3
Stratified analysis
After stratification of studies by age, sex, BMI, hypertension, heart failure, atrial fibrillation, and diabetes to explore the associations with 90-day and 180-day mortality (Figs. 4 and 5). Consistent results were observed in people under age 60 who were female, BMI (> 25 kg/m2), with hypertension, without heart failure. The model's interaction tests for covariates with the SHR were non-significant except for hypertension in 180-day mortality (P for interaction = 0.03) (Fig. 5). Hypertensive patients exhibited a heightened adverse effect from an elevated SHR compared to non-hypertensive individuals.
Sensitivity analysis
The characteristics of raw data and data after multiple imputations (Table S2). Similar results were observed when we used data before multiple imputations to investigate the association between SHR and the risk of 90-day and 180-day mortality using multivariate Cox regression models (Table S3). Similar results were also observed when further adjusting for SIRS and SOFA (Table S4). When we conducted a subgroup analysis for individuals with different diabetes statuses, the results were consistent among those with pre-diabetes and diabetes (Tables S5 and S6).
Discussion
Using data from the MIMIC-IV database (version 2.2), our study revealed an independent association between SHR and 90-day and 180-day mortality in patients with a first diagnosis of AMI. This association was especially evident in those with pre-DM. Additionally, we observed a J-shaped curve relationship between SHR levels and 90-day and 180-day mortality for AMI-diagnosed patients. When the SHR was below 0.9, the association was not significant; however, when it exceeded 0.9, a positive correlation emerged. Understanding and optimizing the distribution of SHR levels in AMI patients may facilitate improvements in subsequent health outcomes for these individuals.
Stress hyperglycemia has been identified as a potent predictor of adverse outcomes in ACS patients [16, 17], significantly correlating with adverse cardiovascular and cerebrovascular events [18]. Unlike admission glucose, SHR—when adjusted for background glucose—may be a superior biomarker for severe illness [6]. For instance, a study by Marenzi et al., which included 1553 AMI patients, with the primary endpoint being a composite of cardiogenic shock, acute pulmonary edema, and in-hospital mortality, identified SHR as a better biomarker for in-hospital mortality and morbidity than absolute glucose levels upon admission [19]. Their findings underscored the predictive value of stress hyperglycemia and its role as an independent predictor of post-AMI in-hospital mortality, which can potentially help identify AMI patients at elevated risk for subsequent adverse outcomes. In a longer-term prognosis, Kojima et al. recruited 6287 STEMI patients discharged safely. With endpoints being all-cause mortality and readmission due to heart failure (median follow-up of 1522 days), they found that in non-diabetic patients, those in the highest quartile of SHR faced significantly worse long-term outcomes than those in the lower quartile [20]. In our study, RCS demonstrated a J-shaped relationship between SHR and short-term mortality in AMI patients. Another study involving 5562 ACS patients who underwent PCI reported a U-shaped or J-shaped association between SHR and early and late adverse outcomes [21]. Furthermore, a large cohort study from China demonstrated a J-shaped correlation between SHR and adverse outcomes in diabetes [22].
Our findings align with these previous studies to some extent. Given the above positive relationship between SHR and acute cardiac events, our study's observation of a J-shaped association between SHR and short-term mortality is not unexpected, with an SHR of 0.90 as the inflection point. Moreover, our findings emphasize a strong association between SHR and short-term mortality, especially among pre-DM patients. Compared to diabetic patients, those without diabetes exhibited a significantly heightened in-hospital mortality risk. Such differences have been reported in prior studies. For example, Kerby et al. proposed that stress hyperglycemia correlates closely with mortality in patients without diabetes rather than those with diabetes [23]. Similarly, Wei et al. reported a significant association between SHR and in-hospital mortality risk in STEMI patients without diabetes [24]. The underlying mechanisms remain elusive and may be attributed to several factors. Firstly, our study focused on mortality at 90-day and 180-day post-admission, representing short-term mortality rates. Secondly, patients with diabetes, due to their adaptation to chronic inflammation and oxidative stress over time, may exhibit insensitivity to SHR [25]. Lastly, patient heterogeneity across different regions and the potential beneficial outcomes in patients with diabetes receiving insulin or other anti-inflammatory drugs should be considered [26].
During stress, the hypothalamic–pituitary–adrenal axis and the sympathetic-adrenal system are activated, increasing pro-inflammatory cytokine release, which induces stress hyperglycemia together [27]. Elevated blood glucose during an AMI is considered a primary determinant for the instability and rupture of atherosclerotic plaques, subsequently influencing an increase in coronary thrombus burden [28, 29]. Moderate stress hyperglycemia is a protective response to stress [30]. In animal shock models, the external application of hypertonic glucose can enhance cardiac output and improve survival rates [31]. However, mounting evidence indicates that stress hyperglycemia is associated with larger myocardial infarction in patients with non-obstructive coronary artery myocardial infarction and poor short- and long-term outcomes [32]. This relationship might be linked to decreased endothelium-dependent vasodilation, impaired platelet anti-aggregation, and over-activation of the sympathetic nervous system with pro-inflammatory pathways. Hyperglycemia may manifest as overactivity of the sympathetic nervous system, accompanied by glucose-mediated pro-inflammatory pathways, affecting outcomes [33, 34]. Based on the stratified analysis, we observed a increased risk of mortality in patients with a first diagnosis of AMI with an elevated SHR, especially in patients aged below 60, females, those with a BMI > 25 kg/m2, those with hypertension or pre-DM, without heart failure,. Thus, we should consider SHR levels clinically for patients diagnosed with AMI, especially in populations under 60, female, overweight, with hypertension or pre-DM, and without heart failure.
There are several limitations in our study. Firstly, the study design was retrospective, prohibiting definitive conclusions about causality. Secondly, being a single-center study with limited sample size, even with multivariable adjustment and subgroup analyses, potential biases due to residual confounders may persist. Third, given the inherent limitations of the MIMIC-IV database, considerations such as disease severity, baseline characteristics at admission, and sociodemographic factors like socioeconomic status and education level were not taken into account, possibly introducing potential biases in study outcomes. Additionally, selection bias may have been introduced due to the exclusion of individuals with deficiencies in glucose or HbA1c data. Finally, distinctions between specific types of acute myocardial infarctions the patients presented with and their relationships with evaluated endpoints were not made.
Conclusions
In summary, this study revealed a J-shaped relationship between SHR and short-term outcomes in patients with a first diagnosis of AMI, underscoring the importance of optimal blood glucose control in this context. Our findings emphasize that elevated SHR levels are associated with an increased mortality rate. The inflection point for adverse prognosis with SHR is 0.9. Moreover, the causal relationship between SHR levels and short-term mortality in adult AMI patients requires further investigation.
Availability of data and materials
The datasets were accessible from the MIMIC-IV (version 2.2) database. Corresponding author will provide the datasets upon reasonable request.
Abbreviations
- SHR:
-
Stress hyperglycemia ratio
- AMI:
-
Acute myocardial infarction
- HR:
-
Hazard ratio
- pre-DM:
-
Pre-diabetes mellitus
- CVD:
-
Cardiovascular diseases
- ACS:
-
Acute coronary syndrome
- PCI:
-
Percutaneous coronary intervention
- ABG:
-
Admission blood glucose
- MACCE:
-
Major adverse cardiac and cerebrovascular events
- STEMI:
-
ST-segment elevation myocardial infarction
- ICD:
-
International classification of diseases
- SQL:
-
Structured query language
- SIRS:
-
Systemic inflammatory response
- SOFA:
-
Sequential organ failure assessment
- RBC:
-
Red blood cell
- WBC:
-
White blood cell
- HbA1C:
-
Hemoglobin A1C
- BMI:
-
Body mass index
- NGR:
-
Normoglycemia
- SD:
-
Standard deviation
- CIs:
-
Confidence intervals
- VIF:
-
Variance inflation factor
References
McClellan M, Brown N, Califf RM, Warner JJ. Call to action: urgent challenges in cardiovascular disease: a presidential advisory from the American heart association. Circulation. 2019;139(9):e44–54.
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12.
Rodriguez-Monforte M, Sanchez E, Barrio F, Costa B, Flores-Mateo G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2017;56(3):925–47.
Luo J, Li Z, Qin X, Zhang X, Liu X, Zhang W, Xu W, Liu B, Wei Y. Association of stress hyperglycemia ratio with in-hospital new-onset atrial fibrillation and long-term outcomes in patients with acute myocardial infarction. Diabetes-Metab Res. 2023;40:e3726.
Fu R, Cui K, Yang J, Xu H, Yin D, Song W, Wang H, Zhu C, Feng L, Wang Z, et al. Fasting stress hyperglycemia ratio and in-hospital mortality after acute myocardial infarction in patients with different glucose metabolism status: Results from China acute myocardial infarction registry. Diabetes Res Clin Pr. 2023;196:110241.
Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, Burt MG, Doogue MP. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocr Metab. 2015;100(12):4490–7.
Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–807.
Jensen CJ, Eberle HC, Nassenstein K, Schlosser T, Farazandeh M, Naber CK, Sabin GV, Bruder O. Impact of hyperglycemia at admission in patients with acute ST-segment elevation myocardial infarction as assessed by contrast-enhanced MRI. Clin Res Cardiol. 2011;100(8):649–59.
Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, Seung KB, Lee SH, Chang K. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. 2017;241:57–63.
Zhang C, Shen HC, Liang WR, Ning M, Wang ZX, Chen Y, Su W, Guo TT, Hu K, Liu YW. Relationship between stress hyperglycemia ratio and allcause mortality in critically ill patients: results from the MIMIC-IV database. Front Endocrinol. 2023;14:1111026.
Johnson A, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, Pollard TJ, Hao S, Moody B, Gow B, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10(1):1.
Kilpatrick ES, Bloomgarden ZT, Zimmet PZ. International expert committee report on the role of the A1C assay in the diagnosis of diabetes: response to the international expert committee. Diabetes Care. 2009;32(12):e159.
Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. Bmj-Brit Med J. 2014;348:g14.
EA YS. Multiple imputation with diagnostics (mi) in R: opening windows into the blackbox. J Stat Softw. 2011;45(2):1–31.
Barnard J, Meng XL. Applications of multiple imputation in medical studies: from AIDS to NHANES. Stat Methods Med Res. 1999;8(1):17–36.
Huang YW, An YH, Yin XS, Li ZP. Association of the stress hyperglycemia ratio and clinical outcomes in patients with cardiovascular diseases: a systematic review and meta-analysis. Eur Rev Med Pharmaco. 2022;26(24):9258–69.
Cui K, Fu R, Yang J, Xu H, Yin D, Song W, Wang H, Zhu C, Feng L, Wang Z, et al. Stress hyperglycemia ratio and long-term mortality after acute myocardial infarction in patients with and without diabetes: a prospective, nationwide, and multicentre registry. Diabetes-Metab Res. 2022;38(7):e3562.
Mi D, Li Z, Gu H, Jiang Y, Zhao X, Wang Y, Wang Y. Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. Cns Neurosci Ther. 2022;28(3):372–81.
Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, Rubino M, Campodonico J, Moltrasio M, Marana I, et al. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. 2018;41(4):847–53.
Kojima T, Hikoso S, Nakatani D, Suna S, Dohi T, Mizuno H, Okada K, Kitamura T, Kida H, Oeun B, et al. Impact of hyperglycemia on long-term outcome in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2020;125(6):851–9.
Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, Wang W, Shao C, Tang YD. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. 2022;45(4):947–56.
Liu B, Chen Y, Yu L, Zhou M. Stress hyperglycemia ratio is associated with systemic inflammation and clinical outcomes in diabetic inpatients with pneumonia on admission. J Diabetes. 2023;15(7):545–56.
Kerby JD, Griffin RL, MacLennan P, Rue LR. Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann Surg. 2012;256(3):446–52.
Wei QC, Chen YW, Gao QY, Ren KD, Liu YB, He F, Shi JT, Jiang J. Association of stress hyperglycemia with clinical outcomes in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention: a cohort study. Cardiovasc Diabetol. 2023;22(1):85.
Bahadoran Z, Mirmiran P, Ghasemi A. Role of nitric oxide in insulin secretion and glucose metabolism. Trends Endocrin Met. 2020;31(2):118–30.
Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Insulin as an anti-inflammatory and antiatherogenic modulator. J Am Coll Cardiol. 2009;53(5 Suppl):S14–20.
Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305.
D’Onofrio N, Sardu C, Paolisso P, Minicucci F, Gragnano F, Ferraraccio F, Panarese I, Scisciola L, Mauro C, Rizzo MR, et al. MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J Cell Physiol. 2020;235(2):1438–52.
Sardu C, D’Onofrio N, Mauro C, Balestrieri ML, Marfella R. Thrombus aspiration in hyperglycemic patients with high inflammation levels in coronary thrombus. J Am Coll Cardiol. 2019;73(4):530–1.
Paolisso P, Bergamaschi L, Rambaldi P, Gatta G, Foa A, Angeli F, Fabrizio M, Casella G, Barbieri M, Galie N, et al. Impact of admission hyperglycemia on heart failure events and mortality in patients with takotsubo syndrome at long-term follow-up: data from HIGH-GLUCOTAKO investigators. Diabetes Care. 2021;44(9):2158–61.
McNamara JJ, Mills D, Aaby GV. Effect of hypertonic glucose on hemorrhagic shock in rabbits. Ann Thorac Surg. 1970;9(2):116–21.
Gao S, Huang S, Lin X, Xu L, Yu M. Prognostic implications of stress hyperglycemia ratio in patients with myocardial infarction with nonobstructive coronary arteries. Ann Med. 2023;55(1):990–9.
Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, Chirkov YY, Zeitz CJ, Horowitz JD. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(3):304–10.
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54.
Acknowledgements
We thank the Ascetic Practitioners in Critical Care (APCC) team, and the easy Data Science for Medicine (easyDSM) team for sharing their knowledge and codes in big data of critical care, along with the cross-platform Big Data Master of Critical Care (BDMCC) software (https://github.com/ningyile/BDMCC_APP). We especially appreciate the MIMIC-IV official team's efforts to open-source the database and codes.
Funding
This work was supported by no funding.
Author information
Authors and Affiliations
Contributions
Data analysis, and manuscript writing were all assisted by BH and JF. Xinghua Chen extracted data. The analytical procedures were confirmed by LLH and JF, who also read the text. JF edited the text for key information. YHW and XW gathered some data. The final manuscript was reviewed and approved by all writers.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
The use of the MIMIC-IV database was approved by the review committee of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Based on our use of public datasets, this study was exempted from approval by the Ethics Committee of the Second People's Hospital of Hefei.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted without commercial or financial relationships construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, B., Chen, X., Wang, Y. et al. J-shaped relationship between stress hyperglycemia ratio and 90-day and 180-day mortality in patients with a first diagnosis of acute myocardial infarction: analysis of the MIMIC-IV database. Diabetol Metab Syndr 16, 132 (2024). https://doi.org/10.1186/s13098-024-01380-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01380-2