Abstract
Background
Ultra-processed food (UPF) consumption increases the risk of type 2 diabetes in various high-income countries, with some variation in the magnitude across studies. Our objective was to investigate the association of UPF consumption and specific subgroups with incident type 2 diabetes in Brazilian adults.
Methods
The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) is a multicenter cohort study of 15,105 adults (35–74 years) enrolled in public institutions in Brazil (2008–2010). We followed participants with two clinic visits (2012–2014; 2017–2019) and annual telephone surveillance. After excluding those with diabetes at baseline, who died or were lost in the follow-up, with missing data, with implausible energy food intake, or reporting bariatric surgery, there were 10,202 participants. We used the NOVA classification to assess UPF consumption based on a food frequency questionnaire. We defined type 2 diabetes by self-report, medication use, or comprehensive laboratory tests. We estimated relative risks (RR) and 95% confidence intervals (95% CI) using robust Poisson regression.
Results
Median UPF consumption was 372 g/day. Over 8.2 (0.7) years of follow-up, we detected 1799 (17.6%) incident cases. After adjustment for socio-demographics, family history of diabetes, and behavioral risk factors, comparing the fourth (≥ 566 g/day) with the first (< 236 g/day) quartile of UPF distribution, RR was 1.24 (1.10–1.39); every 150 g/day increments in UPF consumption resulted in a RR of 1.05 (1.03–1.07). Reclassifying natural beverages with added sweeteners as UPF increased risk (RR 1.40; 1.25–1.58). Among UPF subgroupings, consumption of processed meats and sweetened beverages increased diabetes risk, while yogurt and dairy sweets decreased the risk (p < 0.05).
Conclusions
UPF consumption increased the incidence of type 2 diabetes in Brazilian adults, with heterogeneity across specific food items. These findings add to previous evidence for the role of UPFs in the development of diabetes and other chronic diseases, supporting recommendations to avoid their intake in diabetes prevention and management.
Similar content being viewed by others
Background
Ultra-processed foods (UPFs) have received recent attention in nutritional epidemiology. Being nutritionally unbalanced, rich in additives (flavors, emulsifiers, and dyes, among others), and designed to be highly convenient and attractive to stimulate consumption [1], their intake has increased rapidly over the last few decades [2, 3] with crucial adverse health effects. According to a survey conducted in Brazil, the consumption of UPF accounted for approximately 20% of the total diet, with bread, non-dairy sweets and desserts, processed meats, and sodas being the main contributors [4].
Greater consumption of UPFs relates to higher overall mortality and the incidence of various non-communicable chronic diseases, including cancer and cardiovascular diseases [5, 6]. It also predicted weight [5, 7, 8] and waist [8] gains. In addition, six prospective studies in European and North American countries [9,10,11,12,13] have found that higher consumption of UPFs predicted an increased risk of type 2 diabetes. Based on cohort studies in the United States, one report [13] identified animal-based products, ready-to-eat/heat-mixed dishes, and artificially and sugar-sweetened beverages as the UPF subgroups most related to developing type 2 diabetes.
Eating patterns vary across populations, and the interactions of UPFs with traditional eating patterns in different cultures and their insertion in different nutritional transitions may produce distinct health effects. Thus, it is noteworthy that this association has not been evaluated in low and middle-income countries, where traditional dietary patterns may differ, consumption of UPFs is lower, and where 95% of new cases of diabetes will occur by 2030 [14]. Thus, we aimed to investigate the association between UPF food consumption and the risk of developing type 2 diabetes in Brazil, a large middle-income country currently ranked 6th in the number of persons living with diabetes globally. To this end, we analyzed a cohort of Brazilian adults, the ELSA-Brasil study.
Methods
Study design and population
The Brazilian Longitudinal Study of Adult Health (in Portuguese, ‘Estudo Longitudinal de Saúde do Adulto,’ ELSA-Brasil) is a multicenter prospective occupational cohort aiming primarily to address risk factors for the development and progression of chronic diseases, particularly cardiovascular diseases and diabetes, over a long-term follow-up [15]. Ethics committees of each institution approved the research protocol, and subjects gave written consent to participate in each visit.
Between August 2008 and December 2010, we recruited 15105 active or retired, non-pregnant civil servants, aged 35–74 years, from public institutions of higher education and research located in six Brazilian capital cities (Salvador, Belo Horizonte, Rio de Janeiro, São Paulo, Vitoria, and Porto Alegre), and applied a series of questionnaires as well as laboratory and clinical examinations [15,16,17]. Participants returned twice to the study sites (2012–2014 and 2017–2019) for further investigation. Additionally, they have responded to annual telephone surveillance since 2009.
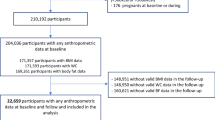
Among the 15105 participants enrolled, we excluded those with prevalent diabetes at baseline (n = 2429), with implausible food intake (< 600 kcal/d or > 6000 kcal/d) (n = 197), who died (n = 321), were lost to follow-up (n = 1361), had missing data on variables of interest (n = 484), or had bariatric surgery between visits (n = 111). The final sample had 10202 participants (Additional file 1: Fig. S1).
Baseline measurements
All measurements followed standardized protocols and regular quality control assessments [18]. In each visit, after an overnight fast, we measured weight, height, and waist circumference following internationally standardized protocols and defined body mass index (BMI) as weight (kg)/height (m)2. We also obtained a fasting blood sample by venipuncture and conducted a standardized 2-h 75-g oral glucose tolerance test (WHO 1999). Plasma glucose was measured using hexokinase and HbA1c by high-pressure liquid chromatography (Bio-Rad, certified by the National Glycohemoglobin Standardization Program).
We interviewed participants using structured questionnaires to ascertain age, sex, self-declared race/color, educational achievement, family income, previous medical history, smoking (current and previous), alcohol consumption, physical activity, and family history of diabetes.
Food consumption was evaluated at baseline through a previously validated food frequency questionnaire, with 114 food items [19]. For each item, we obtained the frequency of consumption in the last year (with eight response options: ‘more than 3 times/day’, ‘2–3 times/day’, ‘once daily’, ‘5–6 times/week’, ‘2–4 times/week’, ‘once/week’, ‘1–3 times/month’ and ‘never/almost never’) and the number of portions consumed, using standardized portion sizes. We then calculated the daily amount consumed for each food item in grams by multiplying its portion number, weight, and frequency. Next, we estimated the nutritional composition and energy using the University of Minnesota Nutrition Data System for Research (NDSR) software. Finally, we imputed the 99th percentile value for a food item when that food's value was above the 99th percentile of its distribution.
Definition of ultra-processed foods
We grouped food items according to the extent and purpose of their industrial processing (the NOVA classification): (i) non- or minimally processed foods and culinary ingredients, (ii) processed foods, and (iii) ultra-processed foods [1]. We aggregated non- or minimally processed foods and culinary ingredients into a single group because our food frequency questionnaire did not distinguish culinary ingredients from the food items that included them (Fig. 1).
In secondary analyses we classified artificially sweetened natural juices, coffee, or tea as UPFs (rather than non- or minimally-processed foods and culinary ingredients), given our previous findings suggesting increased diabetes risk in certain groups [20].
Outcomes
We ascertained diabetes based on laboratory measurements taken during visits and reports from the annual telephone follow-up surveillance. We defined as diabetes cases those who: (i) reported a medical diagnosis of diabetes or current use of medication for diabetes, or (ii) had a laboratory measurement reaching the thresholds for fasting plasma glucose (FPG) (≥ 7.0 mmol/L; 126 mg/dL), 2 h post-load glucose (PG) (≥ 11.1 mmol/L; 200 mg/dL), or HbA1c (≥ 48 mmol/mol; 6.5%) (WHO 2006; ADA 2014). We excluded prevalent diabetes at baseline and ascertained incident diabetes at follow-up visits based on these criteria. We additionally included new cases reporting a diagnosis of diabetes on at least two annual telephone interviews after the last clinic visit.
Statistical analysis
We describe participant characteristics and outcomes using absolute and relative frequencies for categorical variables and mean, standard deviation or median and 25th–75th percentiles for continuous variables. To assess the statistical significance of differences between means or proportions, we employed ANOVA and Chi-square tests, respectively.
We characterized UPF consumption in two ways: first, using mean consumption in grams per day (g/day), expressed as a mean difference of 150 g/day, which represents approximately a 10% difference in consumption in our sample; and second, creating quartiles of consumption (g/day). We used grams instead of kcal to express quantity because some foods and beverages in the UPF group do not provide energy.
We examined the shape of the association along the continuum of UPFs using restricted cubic splines and tested the non-linearity of the associations [21]. We estimated relative risks (RR) and 95% confidence intervals (95% CI) using robust Poisson regression to investigate the associations of UPF intake with incident diabetes. Progressively adjusted models included: in model 2, age (in years), sex (male or female), race/color (white, brown, black, Asian, or Indigenous), school achievement (less than elementary, elementary, secondary or college/university), per capita family income (in Brazilian currency, reais), family history of diabetes (yes or no), smoking (never, former or current), physical activity (in MET minutes/week), and alcohol consumption (in grams/week); in model 3a, model 2 plus energy intake (in kilocalories/day); in model 3b, model 2 plus hypertension (yes or no); and in model 3c, model 2 plus BMI (in kg/m2). We considered model 2 as our final adjustment model; models 3a-c permit a comparison of our results with those of other studies. We drew directed acyclic graphs to understand the variable relationships (Additional file 1: Figure S2).
We tested potential multiplicative interactions by adding multiplicative terms to models. The interactions considered were intermediate hyperglycemia (yes or no), self-reported recent diet change (yes or no), sex (male or female), fruit and vegetable consumption (in grams/day), and (model 3c) with BMI (kg/m2). We assessed multicollinearity between variables, setting a limit of 2 for the variance inflation factor. The overall fit of the model to the data was assessed using the method described by Hosmer and Lemeshow [22].
To assess the robustness of our findings, we performed several sensitivity analyses, based on model 2, by (i) reclassifying natural juices and coffee/tea with added artificial sweeteners from the group of non- or minimally processed foods and culinary ingredients to the UPF group, (ii) alternatively quantifying UPFs as a proportion of grams relative to the total daily grams consumed, (iii) including red meat intake, (iv) fruits and vegetable intake, (v) saturated fat intake, (vi) sugar intake, (vii) fiber intake, and (viii) diet quality as adjustment variables [23], (ix) including 16 cases of incident diabetes who died and were thus excluded in the original analyses, and, finally, (x) performing multiple imputation to permit the addition in analyses of participants with missing values in covariates.
We also evaluated the association between the consumption of specific UPF subgroups and the incidence of diabetes using model 2 for increments of 50 g/d and one standard deviation in each group. We conducted all analyses with the statistical software package SAS Studio® (SAS OnDemand for Academics).
Results
Table 1 provides a detailed description of the sample, overall and according to quartiles of UPF consumption. Briefly, 5846 (57.3%) were women, 5597 (54.9%) were self-declared as being white, 5871 (57.6%) had a complete college/university degree, and 3675 (36%) had a family history of diabetes. Median and IQR values were 372 (235–565) g/day for UPF consumption, 50 (44–57) years for age, and 25.9 (23.4–28.9) kg/m2 for BMI. Compared with those in the first UPF quartile (< 236 g/day), participants in the fourth quartile (≥ 566 g/day) were younger and had higher consumption of overall energy, red meat, saturated fat, and sugar, as well as higher BMI. Those in the top quartile were less frequently women and more frequently white.
After 8.2 (± 0.7) years of follow-up, 1799 (17.6%, 95%CI 16.9–18.4%) participants developed type 2 diabetes. Restricted cubic spline regression models showed a linear relative increase in the incidence of type 2 diabetes with rising values of UPF consumption when adjusting for age, sex, race/color, income, school achievement, family history of diabetes, smoking, physical activity, and alcohol (p non-linearity = 0.20) (Fig. 2).
Restricted cubic splines for the association between ultra-processed food consumption and incidence of type 2 diabetes, adjusted for model 2. The dashed line shows the point estimates of relative risk (RR) for the association along the spectrum of ultra-processed food consumption and the stippled area, the 95% confidence limits. The histogram shows the distribution of ultra-processed foods consumption (% of the study sample; right vertical axis)
Table 2 shows the results of the association between UPF consumption and the incidence of type 2 diabetes. When comparing the fourth (≥ 566 g/day) with the first quartile (< 236 g/day) of UPF consumption and adjusting for sociodemographic, clinical, and behavioral risk factors, we found a RR of 1.24 (95% CI 1.10–1.39, model 2, main analysis). For every 150 g/day increment in UPF consumption, we found a RR of 1.05 (95% CI 1.03–1.07, model 2). Results after adding the potential mediators of energy intake and hypertension (models 3a, b) changed the magnitude of the associations only slightly while adding BMI (model 3c) led to a marked reduction.
We found no multicollinearity among the variables included nor evidence to reject the global fit of the model to the data (p = 0.31). We did not observe effect modification by BMI, intermediate hyperglycemia, self-reported recent change in diet, sex, and fruit and vegetable consumption (p-values of 0.32, 0.95, 0.74, 0.20, and 0.91, respectively).
Table 3 shows the association of UPF subgroups consumption, expressed continuously for 50 g/day and a one SD difference, with the incidence of diabetes. With the division of UPF into smaller groups, only a few associations presented statistical significance. Processed meats and sweetened beverages increased the adjusted risk of diabetes in both analyses. For processed meat, the relative risk for a one SD (21 g) difference was 1.08 (95% CI 1.04–1.13). For sweetened beverages, the relative risk for a one SD (230 ml) difference was 1.14 (95% CI 1.10–1.18). Greater consumption of yogurt and dairy sweets decreased risk in both analyses, with a 61 g/day difference having an adjusted relative risk of 0.94 (95% CI 0.89–0.98).
We also conducted sensitivity analyses (Table 4). When classifying artificially sweetened natural juices, coffee, or tea as UPFs (rather than non- or minimally-processed foods and culinary ingredients), associations increased (third quartile RR = 1.32, 95% CI 1.17–1.49, fourth quartile RR = 1.40, 95% CI 1.25–1.58). All other analyses produced estimates similar to those presented in Table 2.
Discussion
Greater consumption of UPFs increased the incidence of type 2 diabetes over an average of eight years of follow-up in our sample of middle-aged and elderly Brazilian adults. The association was linear over a relevant consumption range and was evident both when comparing extreme quartiles of UPF consumption and increases of 150 g/day in consumption. Within the UPF food subgroups, sweetened beverages and processed meat presented the strongest associations; however, consuming yogurt and dairy sweets was protective.
Our findings align with those of previous observational studies relating UPFs to the incidence of diabetes in France, United Kingdom, Spain, Netherlands, and United States [9,10,11,12,13]. When comparing the extremes of UPF consumption, the relative risks (RRs) varied across studies, from 1.19 (as observed in the Nurses’ Health Study conducted in the US) to 1.56 (in the Lifelines cohort from the Netherlands). Differences may result from specific modeling strategies and population characteristics, and from varying follow-up times, as outlined in Additional file 1: Table S1.
We found weaker associations, not statistically significant after the inclusion of BMI, a potential mediator. We cannot fully explain our smaller associations, but some aspects merit consideration. First, our definition of incident diabetes included not only self-reported information but also laboratory determinations made at a single moment, thus being more sensitive and less specific than the definitions in many other studies. This definition may have diluted the magnitude of our associations. Second, our food frequency questionnaire, not designed to assess UPFs, may have resulted in greater imprecision in our characterization of UPF consumption, biasing the association toward the null. Third, the somewhat stronger association found after reclassification of natural juice/coffee/tea with added sweeteners as UPFs and not as unprocessed or minimally processed foods (sensitivity analyses) suggests that our primary analyses may underestimate the UPF/diabetes association in Brazilian adults because using sweeteners in coffee and natural juices is widespread in Brazil. Finally, other specific aspects of the Brazilian diet may explain the smaller association, although we have adjusted for multiple dietary factors, including an indicator of a healthy diet.
We considered some possibilities to explain the role of UPFs in type 2 diabetes development. UPFs are rich in calories, which can lead to weight gain and obesity, and have a poor nutritional value—high contents of saturated fat, sugar, and sodium, high glycemic index [24], and low content of fiber [25]. However, adjustment for most of these dietary factors, including diet quality, led to an increase in the associations, not a decrease. Although including BMI in the model decreased associations considerably, we cannot distinguish whether this resulted from confounding or from mediation, as adiposity impacts diabetes as a continuous and chronic process.
Thus, additional potential mechanisms of UPFs deserve attention. UPFs contain substances not used in traditional food preparation (such as trans-fat) and numerous additives (such as emulsifiers, nonnutritive sweeteners, and thickeners), some of which have been associated with cardiometabolic effects [26, 27]. Emulsifiers, a common additive in UFPs, have been shown to disrupt the intestinal mucus barrier, leading to the greater passage of pro-inflammatory stimulants, which may further stimulate the development of diabetes [28]. Concerning nonnutritive sweeteners, a recent randomized clinical trial showed them to produce impaired glycemic responses by apparently acting through alteration in the intestinal microbiota [29]. UPFs also increase the intake of substances such as bisphenol A, which migrates into foods from UPF packaging and has been associated with type 2 diabetes incidence [30, 31].
Our findings regarding specific components of UPFs relating differently to the development of type 2 diabetes generally align with those found in cohorts of health professionals in the United States [13]. Processed meats and sweetened beverages were the main drivers of the increased risk of type 2 diabetes in our sample. Meta-analyses of cohort studies support our results [32, 33]. On the contrary, yogurt and dairy sweets acted as protective factors despite being mostly industrialized and with added sugar or artificial sweeteners. Diabetes protection from dairy products is not surprising since meta-analyses in different populations [34, 35] and a previous study from ELSA-Brasil [36] also indicate protection. Unfortunately, these studies do not distinguish dairy products with and without added sugar, and further studies are necessary to unravel these issues.
Though some UPF food subgroups showed positive associations with incident diabetes while others did not, we continue to support the concept that UPFs as a dietary pattern characterized by the NOVA classification are likely harmful. In addition to increasing all-cause mortality, UPFs have been shown to increase the risk of various diseases, ranging from obesity and other cardiometabolic ones to cancer, irritable bowel disease, frailty, and depression [37]. In addition, the UPF concept facilitates public health messaging on nutrition. Further studies of the association of UPF subgroups, particularly yogurt and dairy desserts, with diabetes and the other chronic conditions associated with greater UPF consumption are necessary.
Our study has limitations. First, our food frequency questionnaire was not specifically designed for the NOVA classification. Although these questionnaires are commonly used to assess nutritional intake in epidemiological studies, our lack of specificity in identifying UPFs may lead to an underestimation of the size of the associations reported. Furthermore, it reflects only partially the large amount of UPFs available today. However, the quantity of ultra-processed foods consumed in this cohort is in line with that of a nationwide representative survey assessed with detailed food registries [4]. Second, our approximately eight-year follow-up may be too short to evaluate the total contribution of UPFs to the development of a chronic condition such as diabetes. Third, although we performed multiple adjustments for possible confounders in statistical analyses, we cannot rule out residual or unmeasured confounding, particularly since some potential mediators may also be potential confounders. Fourth, since our cohort started at age 35, we cannot extrapolate our findings to younger groups. Finally, although, following the design of most cohort studies, we did not randomly draw our sample from the Brazilian adult population, it captures Brazil’s racial, social, and regional diversity [17].
Our study also has strengths. To our knowledge, this is the first report of an association between UPF intake and incident type 2 diabetes originating from a country whose food culture is quite different from those of the northern hemisphere high-income countries from which the association has been previously reported. Thus, our findings support the robustness of the association across varying dietary patterns resulting from introducing UPFs within the context of diverse culinary traditions. Our multiple sensitivity analyses also attest to the robustness of the associations found. Finally, our cohort´s follow-up was excellent, and the comparison of results with and without multiple imputation of missing values in covariates attest to the small possibility of bias.
In conclusion, we provide further prospective evidence that consumption of ultra-processed foods and beverages, particularly processed meat and sweetened beverages and foods, is related to the development of type 2 diabetes in adults. Furthermore, our findings from this Brazilian population extend the UPF risk documentation in northern hemisphere high-income countries to different settings and population dietary patterns.
Availability of data and materials
The datasets supporting the conclusions of this article will be made available upon request pending. Considering guidelines placed by the ethics committees responsible for ELSA's study centers, the data used in this study can be made available by a request to ELSA's Publications Committee (pal@ups.br). Additional information can be obtained from the ELSA Coordinator from the Research Center of Rio Grande do Sul (maria.schmidt@ufrgs.br).
Abbreviations
- PG:
-
2H post-load glucose
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ELSA-Brasil:
-
Estudo Longitudinal de Saúde do Adulto
- FPG:
-
Fasting plasma glucose
- g:
-
Grams
- IQR:
-
Interquartile range
- NDSR:
-
Nutrition Data System for Research
- RR:
-
Relative risk
- T2DM:
-
Type 2 diabetes
- UPF:
-
Ultra-processed food
References
Monteiro CA, Cannon G, Levy RB. NOVA. The star shines bright. World Nutr. 2016;7(1–3):28–38.
Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14(S2):21–8.
Moodie R, Stuckler D, Monteiro C, Sheron N, Neal B, Thamarangsi T, et al. Profits and pandemics: Prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet. 2013;381(9867):670–9.
IBGE. Pesquisa de Orçamentos Familiares 2017–2018: análise de consumo alimentar pessoal no Brasil. Instituto Brasileiro de Geografia e Estatística. 2020. 125 p.
Monteiro CA, Cannon G, Lawrence M, Costa Louzada ML, Machado PP. Ultra-processed foods, diet quality, and health using the NOVA classification system. Rome, FAO. 2019;6–9.
Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021;125(3):308–18.
Moradi S, Entezari MH, Mohammadi H, Jayedi A, Lazaridi AV, Kermani MAH, et al. Ultra-processed food consumption and adult obesity risk: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. 2021. https://doi.org/10.1080/10408398.2021.1946005.
Canhada SL, Luft VC, Giatti L, Duncan BB, Chor D, Fonseca MDJMD, et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian longitudinal study of adult health (ELSA-Brasil). Public Health Nutr. 2020;23(6):1076–86.
Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Debras C, Druesne-Pecollo N, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the nutrinet-santé prospective cohort. JAMA Intern Med. 2020;180(2):283–91.
Levy RB, Rauber F, Chang K, da Louzada MLC, Monteiro CA, Millett C, et al. Ultra-processed food consumption and type 2 diabetes incidence: a prospective cohort study. Clin Nutr. 2021;40(5):3608–14.
Llavero-Valero M, Escalada-San Martín J, Martínez-González MA, Basterra-Gortari FJ, de la Fuente-Arrillaga C, Bes-Rastrollo M. Ultra-processed foods and type-2 diabetes risk in the SUN project: a prospective cohort study. Clin Nutr. 2021;40(5):2817–24.
Duan MJ, Vinke PC, Navis G, Corpeleijn E, Dekker LH. Ultra-processed food and incident type 2 diabetes: studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective lifelines cohort. BMC Med. 2022;20(1):1–11.
Chen Z, Khandpur N, Desjardins C, Wang L, Monteiro CA, Rossato SL, et al. Ultra-Processed Food Consumption and Risk of Type 2 Diabetes: Three Large Prospective U.S. Cohort Studies. Diabetes Care. 2023.
International Diabetes Federation. IDF diabetes atlas. IDF. 2021;10 ed.
Aquino EML, Barreto SM, Bensenor IM, Carvalho MS, Chor D, Duncan BB, et al. Brazilian longitudinal study of adult health (ELSA-Brasil): objectives and design. Am J Epidemiol. 2012;175(4):315–24.
Bensenor IM, Griep RH, Pinto KA, de Faria CP, Felisbino-Mendes M, Caetano EI, et al. Routines of organization of clinical tests and interviews in the ELSA-Brasil investigation center. Rev Saude Publica. 2013;47(suppl 2):37–47.
Schmidt MI, Duncan BB, Mill JG, Lotufo PA, Chor D, Barreto SM, et al. Cohort profile: longitudinal study of adult health (ELSA-Brasil). Int J Epidemiol. 2015;44(1):68–75.
Schmidt MI, Griep RH, Passos VM, Luft VC, Goulart AC, de Souza Menezes GM, et al. Strategies and development of quality assurance and control in the ELSA-Brasil. Rev Saude Publica. 2013;47(2):105–12.
Molina MDCB, Benseñor IM, de Cardoso LO, Velasquez-Melendez G, Drehmer M, Pereira TSS, et al. Reproducibility and relative validity of the food frequency questionnaire used in the ELSA-Brasil. Cad Saude Publica. 2023;29(2):379–89.
Yarmolinsky J, Duncan BB, Chambless LE, Bensenor IM, Barreto SM, Goulart AC, et al. Artificially sweetened beverage consumption is positively associated with newly diagnosed diabetes in normal-weight but not in overweight or obese Brazilian adults. J Nutr. 2016;146(2):290–7.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57.
Hosmer D, Lemeshow S. Applied logistic regression. New York: WILEY; 2000. p. 147.
Pires RK, Luft VC, Araújo MC, Bandoni D, Molina MDC, Chor D, et al. Critical analysis of the revised diet quality index for the brazilian population (Dqi-r): Its application in elsa-brasil. Cienc e Saude Coletiva. 2020;25(2):703–13.
Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct. 2016;7(5):2338–46.
da Louzada MLC, Martins APB, Canella DS, Baraldi LG, Levy RB, Claro RM, et al. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev Saude Publica. 2015;49:38.
Bhattacharyya S, O-Sullivan I, Katyal S, Unterman T, Tobacman JK. Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signalling in HepG2 cells and C57BL/6J mice. Diabetologia. 2012;55(1):194–203.
Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189(28):E929–39.
Cani PD, Everard A. Keeping gut lining at bay: Impact of emulsifiers. Trends Endocrinol Metab. 2015;26(6):273–4.
Suez J, Cohen Y, Valdés-Mas R, Mor U, Dori-Bachash M, Federici S, et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. 2022;185(18):3307.
Rancière F, Lyons JG, Loh VHY, Botton J, Galloway T, Wang T, et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Heal A Glob Access Sci Source. 2015. https://doi.org/10.1186/s12940-015-0036-5.
Hwang S, Lim JE, Choi Y, Jee SH. Bisphenol a exposure and type 2 diabetes mellitus risk: a meta-analysis 11 medical and health sciences 1117 public health and health services. BMC Endocr Disord. 2018. https://doi.org/10.1186/s12902-018-0310-y.
Qin P, Li Q, Zhao Y, Chen Q, Sun X, Liu Y, et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose–response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35(7):655–71.
Zhang R, Fu J, Moore JB, Stoner L, Li R. Processed and unprocessed red meat consumption and risk for type 2 diabetes mellitus: an updated meta-analysis of cohort studies. Int J Environ Res Public Health. 2021;18(20):10788.
Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98(4):1066–83.
Alvarez-Bueno C, Cavero-Redondo I, Martinez-Vizcaino V, Sotos-Prieto M, Ruiz JR, Gil A. Effects of milk and dairy product consumption on type 2 diabetes: overview of systematic reviews and meta-analyses. Adv Nutr. 2019;10(12):S154–63.
Drehmer M, Pereira MA, Schmidt MI, Molina MDCB, Alvim S, Lotufo PA, et al. Associations of dairy intake with glycemia and insulinemia, independent of obesity, in Brazilian adults: the Brazilian longitudinal study of adult health (ELSA-Brasil). Am J Clin Nutr. 2015;101(4):775–82.
Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. 2022;7(12):1128.
Acknowledgements
The authors thank the staff and participants of ELSA-Brasil for their essential contributions.
Funding
The study received support from the Brazilian Ministry of Health (Department of Science and Technology) and Ministry of Science, Technology, and Innovation (Financiadora de Estudos e Projetos (FINEP); Grant numbers 01 06 0010.00, 01 06 0212.00, 01 06 0300.00, 01 06 0278.00, 01 06 0115.00, 01 06 0071.00, 405551/2015-0, 405544/2015-4, 405547/2015-3, 405552/2015-7, 405543/2015-8 and 405545/2015-0) and the National Council for Scientific and Technological Development (CNPq). SLC received a fellowship from CAPES (Coordination for the Improvement of Higher Education Personnel). Researchers were independent of funders. The study sponsor/funder was not involved in the study design, collection, analysis, and interpretation of data, writing the report, and did not impose any restrictions regarding the publication.
Author information
Authors and Affiliations
Contributions
SLC performed the statistical analysis, wrote the manuscript, and had primary responsibility for the final content; AV designed the research, helped with statistical analysis, and reviewed the manuscript; BBD and MIS designed the research, wrote and reviewed the manuscript, and had primary responsibility for the final content; RL, VCL, MJMF, LG, and MDCBM designed the research and reviewed the manuscript. All authors have read and approved the final manuscript. SLC and AV are the guarantors and accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committees of each institution approved the research protocol, and subjects gave written consent to participate in each visit.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Flowchart of the analytical sample. Figure S2. Directed acyclic graph for the association between ultra-processed food consumption and the incidence of type 2 diabetes. Table S1. Studies reporting the association between ultra-processed food (UPF) consumption and the incidence of type 2 diabetes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Canhada, S.L., Vigo, Á., Levy, R. et al. Association between ultra-processed food consumption and the incidence of type 2 diabetes: the ELSA-Brasil cohort. Diabetol Metab Syndr 15, 233 (2023). https://doi.org/10.1186/s13098-023-01162-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01162-2