Abstract
Background
Endothelial dysfunction (ED) is a hallmark in type 2 diabetes mellitus (T2DM) that favor both atherogenesis and ischemia and reperfusion injury (IRI). Sodium-glucose-2 co-transporter inhibitors (SGLT2i) may hypothetically improve microvascular and macrovascular functions via a broad spectrum of mechanisms, being superior to traditional antidiabetic therapy such as sulfonylurea, even in subjects under equivalent glycemic control. Hence, the present clinical trial was designed to compare the effect of these two treatments on markers of arterial wall function and inflammation in T2DM patients as well as on the potential mediating parameters.
Method and results
ADDENDA-BHS2 is a prospective, single-center, active‐controlled, open, randomized trial. Ninety-eight participants (40–70 years old) with HbA1c 7–9% were randomized (1:1, stratified by gender, BMI and HbA1c levels) to either dapagliflozin 10 mg/day or glibenclamide 5 mg/day on top of metformin. The primary endpoint was the change of flow-mediated dilation (FMD) after a 12-week period of treatment evaluated at rest and after IRI between dapagliflozin and glibenclamide arms. Secondary outcomes were defined as the difference between treatments regarding: plasma nitric oxide (NO) change after FMD, plasma isoprostane, plasma levels of vascular inflammatory markers and systemic inflammatory markers, plasma levels of adipokines, anthropometric measures, glucose control parameters, office and ambulatory BP control. Safety endpoints were defined as systolic and diastolic function assessed by echocardiography and retinopathy change. Serious adverse events were recorded. The study protocol was approved by the Independent Scientific Advisory Committee.
Conclusion
The ADDENDA-BHS2 trial is an investigator-initiated clinical trial comparing the effect of dapagliflozin versus glibenclamide on several aspects of vascular function in high cardiovascular risk T2DM patients. Besides, a large clinical and biochemical phenotype assessment will be obtained for exploring potential mediations and associations.
Trial registration Clinical trial registration: NCT 02919345 (September, 2016)
Similar content being viewed by others
Background
In type 2 diabetes mellitus (T2DM), endothelial dysfunction results from the direct effects of glucotoxicity, lipotoxicity, oxidative stress, insulin resistance [1] and inflammation [2]. This outcome is potentiated by increased sarcopenic obesity [3] and uncontrolled blood pressure (BP), which are commonly linked to diabetes fueling deterioration of endothelial function [4, 5].
In addition to modulating resting arterial tone, the endothelium plays a central role in controlling arterial flow after temporary occlusion, as in acute coronary syndrome. Decreased post-ischemic blood flow may be particularly vital in T2DM patients in whom ischemia and reperfusion injuries (IRI) are more pronounced and, as a consequence, myocardial infarctions are more extensive [6]. Increased susceptibility to IRI in the context of T2DM is due to several mechanisms, including mitochondrial dysfunction, increased oxidative stress stimuli and impairment of antioxidant capacities at several intracellular and extracellular locations.
Inhibitors of sodium-glucose co-transporter-2 inhibitors (SGLT2) have been shown to be effective in reducing blood glucose, systolic BP and improving body composition [7,8,9,10]. By inference, we may hypothesize that the sum of these effects can improve endothelial function, either by direct or indirect actions. Moreover, improvement of endothelial function may hypothetically mediate the reduction of IRI propensity. The present paper describes the study design, rationale, and baseline characteristics of a prospective, randomized and controlled trial that evaluated whether the addition of dapagliflozin improves endothelial function under resting conditions and after IRI when compared to sulfonylurea in patients with T2DM.
Methods
Study design and population
The Assessment of Dapagliflozin effect on Diabetic Endothelial Dysfunction of brachial Artery-Brazilian Heart Study 2 (ADDENDA-BHS2) trial (NCT 02919345) is a single-center, randomized, open, active-controlled, phase-4 trial. All volunteers signed an informed consent form before entering. Patients were recruited through advertisements in newspapers, radio and television. Clinical and laboratory measurements were performed at the Clinical Research Center (CRC) and Atherosclerosis and Vascular Biology Laboratory (AtheroLab) at UNICAMP. The description of the study follows the recommendations of the CONSORT statement.
Selection criteria
Inclusion criteria were: (i) high cardiovascular risk, defined by stable coronary artery disease (CAD) or subclinical carotid atherosclerotic disease; (ii) T2DM taking up to two oral hypoglycemic agents; (iii) HbA1c between 7 and 9% at the time of randomization; and (iv) age between 40 and 70 years old. CAD was defined as previous myocardial infarction (MI) at least 6 months before inclusion or angiography showing > 70% stenosis in at least one coronary artery. Subclinical carotid atherosclerotic disease was defined by standard guidelines and diagnosed by the presence of [1] carotid plaque; [2] carotid Intima-Media Thickness (cIMT) ≥ 1 mm; or [3] or cIMT values > p75th for age, gender and race according to the distribution in the Brazilian population [11].
Exclusion criteria were: (i) contraindications to metformin use (estimated glomerular filtration rate (eGFR) calculated by CDK-EPI < 45 ml/min, AST or ALT > 3× upper reference limit); (ii) systolic BP (SBP) ≥ 140 or diastolic BP (DBP) ≥ 90 mmHg after 16 weeks of anti-hypertensive medication adjustment during run-in period; (iii) acute coronary syndrome, stroke or coronary artery revascularization within 6 months prior to enrolment; (iv) plasma triglycerides > 500 mg/dl; (v) polyuria, polydipsia, weight loss, or others clinical signs of volume depletion; (vi) insulin use; (vii) pregnancy or women during reproductive age; and (viii) refusal to participate or sign the Informed Consent Form. The informed consent was obtained by the principal investigator.
Clinical care protocol
After assessing eligibility criteria by preliminary telephone interview, the volunteers underwent detailed clinical evaluation, anthropometry, carotid ultrasound, echocardiography, ophthalmologic evaluation and laboratory evaluation. Patients who fulfilled all the selection criteria above were submitted to a run-in phase to adjust the medications seeking at a HbA1c between 7 and 9% by the addition of extended-release metformin ≥ 1.5 g/day (Glifage XR Merck S.A., Brazil), and BP ≤ 130 × 80 mmHg, with losartan 25 to 100 mg/day (Aradois, Biolab-Sanus, Brazil) and the addition of hydrochlorothiazide 12.5 mg/day (Clorana, Sanofi, Brazil) when necessary. If after 16 weeks HbA1c values are < 7% or > 9% or BP is > 130 × 80 mmHg, patients will be discharged from the study. The Fig. 1 depicts the study protocol.
Drug dispensing, adherence and adverse effects
At each visit, the study participants received a 30-day supply of their medications. On delivery of the medicines, patients were instructed to store the used blisters and bring them on the next visit. The number and lot of the pills were recorded on the Clinical Report Form (CRF), and the adherence was calculated based on the percentage of the medication that was taken. Drug adherence was stimulated by telephone calls conducted by a team member every fortnight. In this same telephone contact, patients were questioned about the manifestation of adverse effects and, if they had, advised to come to a medical visit for examination. These phone calls ensured the complete follow-up of enrolled patients. Characteristics and measures taken for adverse events were recorded in the CRF. Serious adverse events, whether or not considered causally related to the investigational therapy, were reported to the Institutional Ethics in Research Committee and AstraZeneca at the same time. As part of the protocol, patients with adverse events should contact the principal investigator and come to the hospital for evaluation shortly after reporting the event.
Data management plan
Data were collected and managed during each visit at CRC using Research Electronic Data Capture (REDCap) tools hosted at Unicamp [12]. Clinical, blood pressure, electrocardiographic, ophthalmologic, anthropometric evaluations were obtained within a week before randomization and after experimental intervention. Echocardiography, laboratory and brachial artery evaluations were obtained on the day of randomization and the day of the 12-week visit. All research data collected is owned by the BHS group. The Principal Investigator of this project, Professor Sposito, takes responsibility for the collection and management of the research data. Study coordinator who is overseen by the Principal Investigator has provided quality assessment in a day-to-day basis. Over the course of the project, data have been collected and stored into the database center of the University following the best practices and standards and in accordance with REDCap rules. Digital video data files generated in the FMD experiments have been processed and stored in AVI format and the same database center. The principal investigators on the project and the BHS group will hold the intellectual property rights for the research data.
Blood samples
After 12-h fasting period blood samples were obtained, centrifuged at 3500 rpm and thereupon measured for urea, creatinine, sodium, potassium, calcium, high-sensitive C-reactive protein (CRP), AST, ALT, glycemia, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) (Cobas c702 Roche, Germany), insulin, thyroid stimulating hormone (TSH) (Cobas e602 Roche, Germany), blood count (Sysmex XN-L-Series, US). Low-density lipoprotein cholesterol (LDL-C) was calculated by a formula [13]. Urinalysis and urinary albumin/creatinine ratio (Dimension RXL MAX, Siemens, Brazil) were performed on the same day. Glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography (HPLC) (D-100, Bio-rad, Brazil). The Homeostasis Model Assessment version 2 (HOMA2) was used to estimate insulin sensitivity (HOMA2S) using fasting plasma insulin and glucose and calculator version 2.2.
At the randomization and the 12-week visit, blood samples were also obtained, centrifuged and frozen at liquid nitrogen in order to measure: plasma leptin, adiponectin (Invitrogen™ eBioscience™ ProcartaPlex Human leptin and adiponectin simplex kit, USA), Interleukin 6 (IL-6), Interleukin 2 (IL-2), tumour necrosis fact alpha (TNF-alpha), Vascular Cell Adhesion Molecule 1 (VCAM-1), Intercellular Adhesion Molecule 1 (ICAM-1) (Invitrogen™ eBioscience™ ProcartaPlex Human VCAM-1, ICAM-1, IL-6, IL-2, TNF-alpha Simplex kit, USA), Endothelin 1 (ET-1) (ET-1 Human ELISA Kit Thermo Scientific, USA), isoprostane (8-Isoprostane ELISA Kit, Cayman Chemical Company, USA).
Anthropometry
Body composition was assessed by measures of weight, height, waist circumference, BMI and dual-energy X-ray absorptiometry (DXA) equipment (Lunar iDXA, GE Healthcare, USA). Briefly, a full-body scanner was performed with information on the body composition and bone mineral density of the femoral neck, Ward triangle, trochanter, and femoral body. The android fat mass was measured between the ribs and the pelvis, and the mass of gynoid fat was measured between the hips and the upper thighs.
Blood pressure and electrocardiogram
Two clinic BP readings were taken at 60-s intervals after 3 min of rest using Omron HEM-705CP (Omron Healthcare, Japan) device, and their mean was defined as the office BP. The participant was then asked to stand up and an orthostatic reading was obtained after 3 min to assess for postural hypotension. Ambulatory blood pressure monitoring (ABPM) was measured as described elsewhere within a week before and after the 12-week experimental treatments (SpaceLabs, model 90207-8Q, USA). Electrocardiogram was recorded at baseline and after follow-up (Micromed Surface Digital Electrocardiogram, Brazil).
Ophthalmologic evaluation
The best corrected visual acuity (BCVA) was tested, following the Early Treatment Diabetic test Retinopathy Study (ETDRS) in the distance evaluation, standardized to 4 m patient, and Jaeger’s table at close range, at a distance of 30 cm [14]. Biomicroscopy was performed on the anterior segment evaluation and, after maximum pupillary dilation (Mydryacil1% 2× e 1× Phelinefrine eye drops), patients underwent evaluation of the lens and retina. Lens opacification was classified based on Locs III [15]. Retina was appraised through posterior pole retinography by VISUCAM® device (NM/FA Carl Zeiss, USA) and indirect ophthalmoscopy, following the International Classification of Diabetic Retinopathy and diabetic macular edema classification, proposed by Wilkison et al. [16].
Finally, patients underwent Optical Coherence Tomography-Spectral Domain (SD-OCT) macular examination, with the SPECTRALIS® SD-OCT (Heidelberg Engineering, Inc., USA), according to International Council of Ophthalmology guideline classification [17]. The retinal layers were analyzed in the macular region, the thickness retinal region in the foveal region and the maximum thickness of the choroid in the subfoveal area was calculated, through the Enhanced Deep Imaging (EDI) method.
Doppler ultrasound assessment of carotid arteries
Patients were screened for clinical or subclinical atherosclerosis by carotid arteries Doppler ultrasound with a 2.4–10 MHz linear array transducer (Vivid-S60, GE Healthcare, Milwaukee, Wisconsin). The patients were evaluated in a supine position with the head slightly extended and turned away from the side of the scanned carotid. After a transversal scan of the arteries, bilateral common, internal and external carotid arteries, as well as vertebral arteries, were imaged longitudinally, and ideally perpendicular to the transducer, by either anterior, lateral or posterior windows, whichever provided the highest image definition of the intima, media and adventitia layers. Images were recorded at frame rates of at least 20 per second and stored in a dedicated workstation for off-line assessments. Intima-media complex thickness (IMT) was assessed at end diastole 20 mm from the carotid bulb and at least 10 mm from the bifurcation. Focus was set to provide the best near and far wall resolution and at a depth of no more than 3–4 cm.
Atherosclerosis occurrence was defined as an IMT above the 75th percentile, IMT ≥ 1 mm, or the presence of plaque in the common carotid artery [11] as preconized by local guidelines. A plaque was defined as a localized protrusion of the arterial wall of more than 1.5 mm into the lumen or thickening of 50% of the arterial compared with an adjacent portion of the vessel wall. Apart from IMT evaluation, manual measurements of the intima, media and adventitia layers, separately, were performed after finding an optimized and zoomed still-frame image in order to individualize and estimate the thickness of each of these layers. These measures were made at the same spot defined for IMTs assessments. Doppler flow velocities of each artery and the respective resistivity index were calculated using the formula [(Peak systolic velocity-end diastolic velocity)/Peak systolic velocity].
Analysis of endothelial function
Brachial arteries were measured using a high-resolution ultrasound (Vivid S6, GE Medical System, Milwaukee, WI, USA) according to previously published guidelines [18]. Briefly, the procedure takes place after over-night fasting and withdrawal of any vasoactive medications for the previous 24 h [19]. After 10 min of quiet resting in a supine position, and in a room with a controlled temperature around 22–25 °C, the brachial artery was located above the antecubital fossa, and a longitudinal image of 6 to 8 cm of the artery was considered as the baseline scan. The probe was then fastened to a probe holder (Quipu, Pizza, Italy) and sustained in an upright position. Continuous recording of real-time 2D image and simultaneous Doppler flow of the brachial artery was started by using a video capture device (Epiphan’s DVI2USB 3.0™, Epiphan Video, Ottawa, Canada) connecting the ultrasound to a dedicated computer. An appropriately sized blood pressure cuff was placed around the forearm and inflated up to 50 mmHg above the systolic blood pressure for 5 min and then deflated. The scan recording was obtained for 11 min (baseline: 1 min, ischemia: 5 min, and hyperemia and dilation: 5 min). Automatic edge-detecting software (LabVIEW 6.02, National Instruments) was used for online and off-line assessment of artery dilation and flow changes. The percentage change in diameter and flow for FMD was calculated in comparison with baseline scans. Other secondary parameters were also assessed offline by means of the same software, namely: the velocity–time integral of the antegrade and retrograde brachial flow, as determined by Doppler flow evaluation; the area under the curve (AUC) of the diameter versus time scatterplot; time to maximum vasodilation; and the derived parameters of blood flow (the product of cross-sectional area and Doppler velocity) and shear rate (4 times velocity divided by arterial diameter). These are validated methods used for covariate adjustment and microvascular endothelial function analysis. Two FMD assessments were performed in two different time-points—at the time of randomization and after 12 weeks of intervention (or control)—totalizing 4 FMDs for each patient. Fifteen minutes after the first FMD, reperfusion injury of the brachial artery was performed by cuff-induced ischemia (50 mmHg above the systolic blood pressure) for 15 min, followed by 15 min of reperfusion. At that moment, the second FMD analysis was repeated and the same parameters described above assessed.
Nitrite and nitrate (NOx) levels in plasma were measured just before FMD and 1 and 5 min after brachial artery cuff deflation. This collection was repeated during the second FMD after ischemia and reperfusion. Samples were collected in a tube containing heparin, centrifuged at 3500 rpm in a refrigerated centrifuge and stored in liquid nitrogen in up to 3 min for further analysis of NOx by a NO chemiluminescence analyzer (Model NOA, Sievers Instruments, Boulder, CO, USA).
Echocardiography assessment
Patients underwent cardiac ultrasound analysis with a 1.5–4.5 MHz phased array transducer (Vivid-S60, GE Healthcare, Milwaukee, Wisconsin) on the day of the randomization and again at the end of the intervention (or control) periods. Still images and cine-loops were acquired, as necessary, for an off-line evaluation by an experienced physician blinded to patients’ characteristics and allocation to intervention or control arms. Cardiac chamber quantification was performed according to the current American Society of Echocardiography (ASE) Guidelines [20]. Left ventricular diastolic function status was determined by evaluation of tissue Doppler myocardial velocities (acquired in early diastole at the anterior, inferior, lateral and septal mitral annulus portions), mitral wave inflow velocities, body surface area-indexed left atrial volumes and tricuspid regurgitation peak velocities, as recommended by ASE guidelines [21]. Left atrial strain complemented left ventricular diastolic function evaluation as proposed recently [22]. Finally, the global longitudinal strain and post-systolic strain (PSS) were assessed by speckle tracking. All the exams were recorded and reviewed by three experienced echocardiographers and in the absence of consensus the evaluations were reprocessed.
Randomization and follow-up
Randomization was made in 1:1 ratio on strata according to gender, baseline levels of HbA1c (7–7.9% or 8–9%) and BMI (below or above 30 kg/m2) for dapagliflozin (10 mg/day) or glibenclamide (5 mg/day) on top of metformin. The REDCap system was used for randomization at the study site by the principal investigator. Glibenclamide was selected as a comparator so that it could be based on the known effect and tested on the RCT with hard events in this population and thus estimate a potential gain with the new therapy [23,24,25]. The doses of dapagliflozin and glibenclamide were chosen in order to obtain similar magnitudes of reduction of glycemia. After randomization, patients were evaluated every 30 days in order to check clinical status, treatment adherence, adverse events, use of concomitant medications.
Objectives and endpoints
The primary objective was the comparison of the FMD change at rest and after IR between a 12-week period of treatment with dapagliflozin or glibenclamide on top of metformin. Secondary outcomes were the difference between treatments regarding: (i) plasma NOx change after FMD; (ii) plasma isoprostane; (iii) plasma levels of the following vascular inflammatory markers ICAM-1, VCAM-1 (iv) plasma ET-1; (v) plasma levels of adipokines: adiponectin and leptin; (vi) systemic inflammatory markers: hs-CRP, TNF-alpha, IL-6 and IL-2; (vii) anthropometric measures: waist circumference, weight, body-mass index, body composition (% of fat mass and % free fat mass) and android/gynoid percent fat ratio, (viii) glucose control parameters: fasting glycemia, insulin, HOMA2S, HbA1c, (ix) office and ambulatory BP control, (x) systolic and diastolic function, and cardiac structure assessed by echocardiography, and (xi) retinopathy change during treatment.
Sample size
Based on previous observations, we assumed a 3% change in FMD between arms (reduction of 1% in glibenclamide and increase of 2% in dapagliflozin), with a mean pre-treatment value of 5.5% and a standard deviation of 3.9% [26]. As we had a double primary outcome, i.e. rest and post-IRI FMD, we performed the Bonferroni’s method of adjustment and, hence, considered an alpha value of 0.025 and beta level of 90%. Under these conditions, it would take 44 patients per arm to achieve the necessary statistical power. However, as the researchers took into account that this study has a large number of secondary endpoints, the total sample was defined in 98 individuals, 49 in each arm. The sample size was calculated with G*Power 3.1 Mac version (Heinrich-Heine-Universität, Germany).
Statistical analyses
Estimates of change from baseline will be compared between treatments for each primary endpoint. Results will be analyzed on an intention to treat principle. This study will be considered a success if one or both of the primary endpoints differ between treatment groups with statistical significance. Similar comparisons of secondary endpoints will be made with adjustment for multiplicity, and statistical significance will be inferred at a two-sided Type I error level of 0.025. Adjustment for potential confounders such as statin use will be employed for the results analyses. The data are shown as the mean ± standard deviation (SD) for normal distribution or median and interquartile range (IQR) for non-normal distribution data. Baseline continuous and categorical data were compared by Wilcoxon–Mann–Whitney test and a two-tailed t test or Fisher exact test. Analyses were performed using the SPSS 22, Mac version (IBM, USA).
Results
Participant baseline characteristics
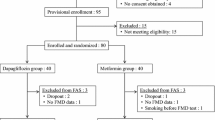
Recruitment of participants for the Addenda-BHS2 trial began in September 2016 and was completed in December 2018. A total of 674 subjects were evaluated and 134 met the selection criteria for the run-in phase when they underwent adjustment of medications and clinical control (Fig. 2). Of these, 98 individuals were enrolled into the trial. The main reasons for screen failure were HbA1c < 7% or > 9% (28.6%), low cardiovascular risk (12.8%), insulin use or age < 40 or > 70 years (6%). At the first visit, enrolled patients were using antidiabetic, antihypertensive and lipid-lowering medications in similar proportions (Table 1). At this time, lipid-lowering therapies were maintained at the same doses and were not introduced for those patients who were not yet in use to avoid interfering upon the primary endpoint of the study. Antidiabetic and antihypertensive medications were replaced with study drugs for a 16-week run-in period.
The baseline characteristics of the participants are shown in Table 2. It is a population with a mean age of 57 years, most males (61%) and about 10 years of T2DM (median 8 and interquartile range of 10 years). Prior coronary artery disease was found in 8 patients (8%) and cIMT above the 75th percentile for Brazilian population was the criteria for 90 patients (92%). Consistent with a tight metabolic control, the majority (98%) had eGFR > 60 ml/min/1.73 m2 (57 to 142 ml/min/1.73 m2). All patients were normoalbuminuric (urinary albumin/creatinine ratio range from 0.01 to 2 mg/g). Systolic and diastolic blood pressure measured in the office were on average 137 and 82 mmHg, respectively, and 79% of the participants (77) were taking antihypertensive medication. The mean systolic and diastolic blood pressure obtained during 24-h ambulatory monitoring was 126 and 77 mmHg, respectively. The mean levels of LDL-C, HDL-C and triglycerides were 95 mg/dl, 41 mg/dl and 181 mg/dl, respectively. A total of 42 (43%) and 1 (1%) participants were taking statins and ezetimibe, respectively. Although no patient enrolled into the trial were active smoker, about 42% smoked in the past and stopped 19 ± 9 years ago.
Discussion
The ADDENDA-BHS2 trial is a randomized, open-label, investigator-initiated clinical trial comparing the effect of dapagliflozin versus glibenclamide on endothelial function in high cardiovascular risk T2DM patients. We hypothesize that treatment with SGLT2i could have a favorable effect on both resting and post-IRI endothelial function as compared to a similar reduction of plasma glycemia by glibenclamide. In addition, the study was designed to explore as secondary endpoints the effect of the treatments on myocardial function, anthropometry, adipose tissue function, blood pressure and retina.
Endothelial function is influenced early and multifactorially by several cardiovascular risk factors, which generates an expectation of high predictive value for this test in risk estimation [27]. Indeed, endothelial dysfunction reveals clinically relevant conditions such as increased 10-year risk scores, microvascular angina and CAD severity [28, 29]. Accordingly, FMD has been used worldwide as a surrogate endpoint in prospective trials assessing the effect of different anti-diabetic and cardiovascular treatments [30,31,32]. However, the variability of FMD methods with induction of systematic errors and subjectivity in the discrimination of luminal edges have challenged its recognition as a reliable tool [33]. Automatic edge detection software allowing continuous recording of brachial artery diameter and guidelines for the method have greatly improved the reproducibility and repeatability of this technique. Still, endothelium evaluation by traditional FMD methods remains very preliminary, either mechanistically or as a surrogate endpoint, because it only gives access to a limited part of vascular physiology [19]. For instance, different populations have diverse time-to-peak dilation despite equal 1-min percent dilation [34].
This variability could be minimized by the ongoing method, in which four conditions were distinctly analyzed in a continuous manner: baseline, ischemia, hyperemia and vasodilation. This approach has several advantages over the traditional FMD protocol: (i) time-to-peak of dilation detection; (ii) peak dilation instead of 1-min single point dilation; (iii) area under the curve (AUC) for the whole extent of dilation; (iv) shear stress (doppler flow), (v) shear rate baseline (dyn/cm2), (vi) maximum shear rate (dyn/cm2), (vii) shear rate delta (0–100%), (viii) shear rate (AUC), (ix) antegrade flow (ml/min), (x) retrograde flow (ml/min), and (xi) oscillatory flow (ml/min). Analyzing several parameters in one experiment could reduce inter and intra-observer variation and, therefore, turn this method more accurate than traditional protocols.
During FMD, NO release is the main mediator of vasodilation concerning macrovascular function. The assessment of the role of NO in FMD has been made by using NO synthase blocker N(G)monomethyl-l-arginine (L-NMMA) [33]. In our approach, we performed a direct measurement of the bioavailability of NO in the brachial vein of the same arm submitted to FMD to estimate its role in altering arterial dilatation after treatment. Endothelial dysfunction is also associated with reduced FMD by increased ET-1 production [35]. The net balance between vasoconstrictor and vasodilatory stimuli will be assessed through the measurement of these molecules. It is also observed that the vascular endothelium phenotype could be addressed by its secretory profile [36]. Many vascular cytokines have a negative correlation to NO availability, such as the ones measured in this study: sICAM-1 and sVCAM-1.
This is the first clinical study assessing the effect of SGLT2i versus active comparator on IRI on equivalent glycemic control. Furthermore, although there is a broad spectrum of interaction between endothelial function and the magnitude of IRI, as mentioned above, this is the first study to simultaneously test endothelial function at rest and after IRI. In situations such as acute coronary syndromes, the behavior of the endothelium after IRI is certainly more relevant than that before acute vascular occlusion. Thus, we believe that our findings will have a greater potential for extrapolation to the potential clinical benefit of the intervention under investigation.
It has been shown that SGTL2i could alter the components of metabolic syndrome, such as BMI and waist circumference [37]. It is observed that both total and central adiposity relates to vascular dysfunction assessed by FMD [38]. In fact, SGLT2i could influence weight loss, and this change is pronounced compared to a sulphonylurea [39]. Moreover, it is possible that SGLT2i could promote visceral fat reduction [40], hence favoring vasodilation [41]. Due to the possible influence of weight and body composition on endothelium [42, 43], BMI, waist circumference and total adiposity evaluated by DXA will be measured. Also, adipose tissue function markers such as leptin, adiponectin and interleukin-6 have been reported to change after SGLT2i treatment [44] and to interplay on mediation of endothelial dysfunction [45]. Therefore, the measurement of these adipokines will be made on an attempt to estimate potential mediations.
In four recent clinical trials, hospitalization for heart failure (HF) was 30% less frequent in SGLT2i users than in the control groups [46,47,48,49]. This effect was equivalent in individuals with or without coronary artery disease or previous HF, suggesting the possibility of primary prevention of HF. In addition, in two small studies, treatment with SGLT2i improved diastolic function at short term [50, 51]. Among the mechanisms proposed are diuretic and hemodynamic action, improvement of myocardial metabolism and effects on cardiac and other ionic channels [52]. In individuals with type 2 or type 1 DM, endothelial function is directly associated with diastolic function [53,54,55]. It is not clear yet the mechanisms by which this association occurs but it is possible that the above-mentioned effects also influence both endothelial and diastolic functions. In fact, measures that improve endothelial function, such as the treatment of hypertension or dyslipidemia, also attenuate diastolic dysfunction [56, 57]. So far the interplay between left ventricle mechanics and the arterial tree buffering of stroke volume has not been addressed in this scenario. Assessing arterial and ventricular elastance, as well as, ventricular-arterial coupling would provide insight into mechanical efficiency of the transfer of blood from the heart to the arterial system. We hypothesize that dapagliflozin may improve ventricular-arterial coupling, LV systolic elastance and arterial elastance after 3 months of use.
The present study aims to test the effect of dapagliflozin versus glibenclamide on the attenuation of endothelial dysfunction in individuals with manifested or subclinical atherosclerosis. If a mitigation effect of endothelial dysfunction is found with the treatments tested in this study, our findings will help explain the reduction of cardiovascular events in patients with T2DM and established cardiovascular disease [46,47,48,49]. Our findings, however, should not be extrapolated to patients with less advanced T2DM duration in whom the development of endothelial dysfunction may not be fully established. Other studies should be done to estimate the effect of these drugs in preventing the decline of endothelial function over time.
Conclusion
In summary, it is expected that the comparison of dapagliflozin with an established and widely used sulfonylurea adds to our current knowledge the description of mechanisms that may justify the reduction of the risk of macrovascular events with SGLT2i therapy. Also, the measurement of the clinical and biochemical profile may help in the understanding of mediators for the potential benefit of these treatments in endothelial function.
Availability of data and materials
The principal investigators on the project and the BHS group will hold the intellectual property rights for the research data. The datasets generated and/or analysed during the current study are not publicly available due to ongoing proprietary work but its availability would be considered by the corresponding author on reasonable request.
Abbreviations
- ED:
-
endothelial dysfunction
- T2DM:
-
type 2 diabetes mellitus
- IRI:
-
ischemia and reperfusion injury
- FMD:
-
flow mediated dilation
- NO:
-
nitric oxide
- SGLT2i:
-
sodium/glucose cotransporter 2 inhibitor
- ADDENDA:
-
Assessment of Dapagliflozin effect on Diabetic Endothelial Dysfunction of brachial Artery
- BHS:
-
Brazilian Heart Study
- CAD:
-
coronary artery disease
- MI:
-
myocardial infarction
- cIMT:
-
carotid Intimia-Media Thickness
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
- CRF:
-
clinical report form
- CRP:
-
C-reactive protein
- TC:
-
total cholesterol
- HDL-c:
-
high-density lipoprotein cholesterol
- TG:
-
triglycerides
- TSH:
-
thyroid stimulating hormone
- LDL-c:
-
low-density
- HbA1c:
-
glycated hemoglobin
- HOMA2:
-
homeostasis model assessment version 2
- HOMA2S:
-
homeostasis model assessment version 2 insulin sensitivity
- IL-6:
-
interleukin 6
- IL-2:
-
interleukin 2
- TNF-alpha:
-
tumour necrosis fact alpha
- VCAM-1:
-
vascular cell adhesion molecule 1
- ICAM-1:
-
intercellular adhesion molecule 1
- ET-1:
-
endothelin 1
- BMI:
-
body mass index
- DXA:
-
dual-energy X-ray absorptiometry (DXA)
- BCVA:
-
best corrected visual acuity
- ETDRS:
-
early treatment diabetic test retinopathy study
- SD-OCT:
-
spectral domain optical coherence tomography
- EDI:
-
enhanced deep imaging
- IMT:
-
Intima-media complex thickness
- AUC:
-
area under the curve
- NOx:
-
nitrate
- ASE:
-
American Society of Echocardiography
- PSS:
-
post-systolic strain
References
Nitenberg A, Cosson E, Pham I. Postprandial endothelial dysfunction: role of glucose, lipids and insulin. Diabetes Metab. 2006;32(Spec No2):2S28–33.
Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19(32):5695–703.
Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, et al. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 2010;56(8):662–6.
Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31(9):2063–9.
Ghiadoni L, Taddei S, Virdis A. Hypertension and endothelial dysfunction: therapeutic approach. Curr Vasc Pharmacol. 2012;10(1):42–60.
Przyklenk K, Maynard M, Greiner DL, Whittaker P. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal. 2011;14(5):781–90.
Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
Leiter LA, Cefalu WT, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62(7):1252–62.
Balakumar P, Sundram K, Dhanaraj SA. Dapagliflozin: glucuretic action and beyond. Pharmacol Res. 2014;82:34–9.
Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2013;16:159–69.
Santos IS, Bittencourt MS, Oliveira IR, Souza AG, Meireles DP, Rundek T, et al. Carotid intima-media thickness value distributions in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Atherosclerosis. 2014;237(1):227–35.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310(19):2061–8.
Ferris FL 3rd, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103(1):181–2.
Chylack LT Jr., Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–6.
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–82.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology Recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608–22.
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65.
Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–12.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1.e14–39.e14.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314.
Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. 2017;11:1405–15.
Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM, Bank AJ. Rosiglitazone improves endothelial function and inflammation but not asymmetric dimethylarginine or oxidative stress in patients with type 2 diabetes mellitus. Vasc Med. 2007;12(4):311–8.
Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168(1):344–51.
Ijzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, de Delemarre-van de Waal HA, Serne EH, et al. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest. 2003;33(7):536–42.
Ceriello A, Genovese S. Atherogenicity of postprandial hyperglycemia and lipotoxicity. Rev Endocr Metab Disord. 2016;17(1):111–6.
Rudolph TK, Fuchs A, Klinke A, Schlichting A, Friedrichs K, Hellmich M, et al. Prasugrel as opposed to clopidogrel improves endothelial nitric oxide bioavailability and reduces platelet-leukocyte interaction in patients with unstable angina pectoris: a randomized controlled trial. Int J Cardiol. 2017;248:7–13.
Shinnakasu A, Yamamoto K, Kurano M, Arimura H, Arimura A, Kikuti A, et al. The combination therapy of fenofibrate and ezetimibe improved lipid profile and vascular function compared with statins in patients with type 2 diabetes. J Atheroscler Thromb. 2017;24(7):735–48.
van Poppel PC, Netea MG, Smits P, Tack CJ. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care. 2011;34(9):2072–7.
Greyling A, van Mil AC, Zock PL, Green DJ, Ghiadoni L, Thijssen DH, et al. Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis. 2016;248:196–202.
Fernandes IA, Sales AR, Rocha NG, Silva BM, Vianna LC, da Nobrega AC. Preserved flow-mediated dilation but delayed time-to-peak diameter in individuals with metabolic syndrome. Clin Physiol Funct Imaging. 2014;34(4):270–6.
Idris-Khodja N, Ouerd S, Mian MOR, Gornitsky J, Barhoumi T, Paradis P, et al. Endothelin-1 overexpression exaggerates diabetes-induced endothelial dysfunction by altering oxidative stress. Am J Hypertens. 2016;29(11):1245–51.
Walczak M, Suraj J, Kus K, Kij A, Zakrzewska A, Chlopicki S. Towards a comprehensive endothelial biomarkers profiling and endothelium-guided pharmacotherapy. Pharmacol Rep. 2015;67(4):771–7.
Davies MJ, Merton KW, Vijapurkar U, Balis DA, Desai M. Canagliflozin improves risk factors of metabolic syndrome in patients with type 2 diabetes mellitus and metabolic syndrome. Diabetes Metab Syndr Obes. 2017;10:47–55.
Arkin JM, Alsdorf R, Bigornia S, Palmisano J, Beal R, Istfan N, et al. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol. 2008;101(1):98–101.
Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38(3):355–64.
Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–31.
Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, et al. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity. 2009;17(11):2054–9.
Dass N, Kilakkathi S, Obi B, Moosreiner A, Krishnaswami S, Widlansky ME, et al. Effect of gender and adiposity on in vivo vascular function in young African Americans. J Am Soc Hypertens. 2017;11(5):246–57.
Hopkins ND, Green DJ, Tinken TM, Sutton L, McWhannell N, Cable NT, et al. Does brachial artery flow-mediated dilation scale to anthropometric characteristics? Eur J Appl Physiol. 2010;110(1):171–6.
Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32–7.
Adya R, Tan BK, Randeva HS. Differential effects of leptin and adiponectin in endothelial angiogenesis. J Diabetes Res. 2015;2015:648239.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295–306.
Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39(12):e212–3.
Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17(1):132.
Tanaka H, Hirata KI. Potential impact of SGLT2 inhibitors on left ventricular diastolic function in patients with diabetes mellitus. Heart Fail Rev. 2018;23(3):439–44.
Shivalkar B, Dhondt D, Goovaerts I, Van Gaal L, Bartunek J, Van Crombrugge P, et al. Flow mediated dilatation and cardiac function in type 1 diabetes mellitus. Am J Cardiol. 2006;97(1):77–82.
Leung M, Phan V, Leung DY. Endothelial function and left ventricular diastolic functional reserve in type 2 diabetes mellitus. Open Heart. 2014;1(1):e000113.
Bedirian R, Neves MF, Oigman W, Gismondi RA, Pozzobon CR, Ladeira MC, et al. Correlation between diastolic function and endothelial function in patients with type 2 diabetes and hypertension. Open Cardiovasc Med J. 2016;10:212–20.
Beck AL, Otto ME, D’Avila LB, Netto FM, Armendaris MK, Sposito AC. Diastolic function parameters are improved by the addition of simvastatin to enalapril-based treatment in hypertensive individuals. Atherosclerosis. 2012;222(2):444–8.
Ginelli P, Bella JN. Treatment of diastolic dysfunction in hypertension. Nutr Metab Cardiovasc Dis. 2012;22(8):613–8.
Acknowledgements
We are indebted to Miss Eliene Dupret for her invaluable contribution to the logistics of this study and of the participants’ visits and appointments.
Funding
The study was designed and proposed by the BHS group and it was funded as an External Sponsored Scientific Research (ESR) by AstraZeneca do Brasil Ltda (ESR-14-10627). As ESR, the BHS group has fully assumed the legal and regulatory responsibility for planning, conducting, managing and analyzing the research, as defined by applicable Brazilian regulations and laws. Prof. Sposito was supported by a Research Career Awards grant from the Brazilian National Research Council (CNPq) (Grant number 301465/2017-7).
Author information
Authors and Affiliations
Consortia
Contributions
RMRC, AASS, IB, JB, DBM, STK-M, PC, RZ, JCB, CM, VWV, IB, JCL-Jr, VLWW, DCO, RH and VH collected outpatient data and contributed on data analyses. RMRC and ACS prepared the manuscript. VH, FRPC and CELA performed the ophthalmologic evaluation and contributed on data analyses. IB, DBM, STK-M, IB, JCL-Jr, DCO and TQ performed the endothelial and echocardiographic evaluations and contributed on data analyses. CM, VWV, IB, JCL-Jr performed the biochemicals evaluations and contributed on data analyses. GG-Jr, ORCF, WN, FRPC, CELA, TQ, ACS reviewed the manuscript. All authors have read and approved the manuscript. ACS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ADDENDA-BHS2 trial was registered at Clinicaltrials.gov in September 29, 2016 by the number NCT 02919345, as a single-center, randomized, open, active-controlled, phase-4 trial. The Institutional Ethics in Research Committee of the Universidade Estadual de Campinas (State University of Campinas-UNICAMP) approved the study (CAAE41618915.1.0000.5404). Accordingly, all patients signed an informed consent term and all methods were performed in accordance with the relevant guidelines and regulations. The first trial randomization occurred at July 27th 2017. The protocol was not modified since the first submission for IERC.
Consent for publication
Not applicable.
Competing interests
The BHS received grant support from AstraZeneca to conduct the Addenda trial. The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cintra, R.M.R., Soares, A.A.S., Breder, I. et al. Assessment of dapagliflozin effect on diabetic endothelial dysfunction of brachial artery (ADDENDA-BHS2 trial): rationale, design, and baseline characteristics of a randomized controlled trial. Diabetol Metab Syndr 11, 62 (2019). https://doi.org/10.1186/s13098-019-0457-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-019-0457-3