Abstract
Background
Point-of-care ultrasound (POCUS) has become a mainstay in the evaluation of critically ill patients in the intensive care unit (ICU). ECMO patients are susceptible to complications during prolonged ICU stay, including cannula malposition, which has deleterious consequences. Although the literature surrounding utility of ultrasound on ECMO patients is expansive, direct comparison between radiographic imaging versus ultrasound for identification of cannula malposition is lacking.
Case presentation
The authors identified four patients with cannula malposition discovered through POCUS that was missed on routine radiographic imaging. Identification and correction of malposition changed their ECMO course.
Conclusion
This case series is the first in literature demonstrating that ultrasound may be superior to radiographic images for ECMO cannula malposition. Further investigation into this subject is warranted.
Similar content being viewed by others
Background
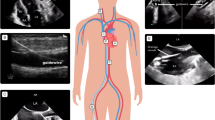
Ultrasound has an important role in the management of critically ill patients; however, there are limited guidelines outlining the use in adults requiring extracorporeal membrane oxygenation (ECMO) [1]. Currently, there are no proposed guidelines for regular point-of-care ultrasound (POCUS) evaluations in the adult ECMO patient population resulting in massive institutional variability in its application. Currently, our institution utilizes POCUS daily to identify and even correct possible ECMO complications in a rapid, cost-effective manner. One such complication, cannula malposition, can have tremendous consequences including direct vessel or cardiac trauma [2], recirculation, and liver and splanchnic organ congestion [3, 4] which can result in cerebral hypoperfusion, hemodynamic instability, and even death [5].
To our knowledge, there are no published reports investigating the superiority of ultrasound in the identification of cannula malposition compared to routine radiography in adult ECMO patients. We present the retrospective review in adult ECMO patients where routine radiographic evaluation was reassuring for proper cannula positioning despite frank malposition identified on POCUS. We demonstrate that POCUS may be superior to routine radiography in the monitoring of ECMO cannula position.
Case presentations
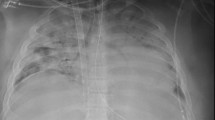
Patient 1 was a 27-year-old female with Hodgkin’s lymphoma and resulting bleomycin-induced lung injury. She underwent cannulation via dual lumen internal jugular (IJ) for veno-venous (VV) ECMO for progressive and refractory hypoxemia, with line verified intraoperatively. Daily chest radiograph (CXR) commented “ECMO cannula in appropriate position”. After developing increasing evidence of recirculation on her circuit on cannulation day 2, she underwent POCUS evaluation which showed outflow jet positioned distally in the hepatic vein. Recirculation improved with retraction of the cannula under ultrasound guidance, however the patient expired after developing acute right ventricular failure of unclear etiology.
Patient 2 was a 31-year-old male with rapidly progressive interstitial lung disease secondary to protein alveolar proteinosis. He underwent intubation and VV ECMO cannulation as a bridge to transplant. Two days after dual lumen IJ cannulation, he showed evidence of recirculation and low flows (SpO2 88%, ScVO2 82%, flow 2.1Lpm). CXR commented “dual lumen IJ ECMO cannula with side hole in the right atrium, distal tip in the IVC.” POCUS showed outflow jet positioned in the hepatic vein. Support immediately improved once the cannula was retracted under ultrasound guidance (SpO2 98%, flow 3.1Lpm). Ultimately, patient survived on VV ECMO until lung transplant was performed and is still alive.
Patient 3 was a 30-year-old-female with acute respiratory distress syndrome (ARDS) secondary to viral pneumonia. Cannulated for VV ECMO via dual lumen IJ. On day 13 of cannulation, had increase in AST/ALT. CXR performed same day reported “ECMO cannulas appear to be in appropriate position.” POCUS showed the return jet had migrated distally and was partially positioned in hepatic vein. He underwent repositioning bedside under ultrasound guidance. The following day, his AST/ALT normalized. She underwent decannulation two days later and survived.
Patient 4 was a 40-year-old female with ARDS secondary to viral pneumonia, complicated by refractory hypoxemia. She underwent bi-femoral VV ECMO cannulation. On day 8 of ECMO, she began cutting out flow and had marginal support despite fluids. CXR on day 6 and 8 both commented “ECMO cannula terminating in the cavo-atrial junction.” POCUS showed tip of the return cannula distal to the hepatic vein with the return jet partially entering the hepatic vein when it had been in the right atrium on days 1–2 ultrasonographically. Her cannula was exchanged for a dual lumen IJ given hematoma formation around groin sites and remained on ECMO for an additional ten days. On ECMO day 24, she developed progressive acidosis and ECMO support failed, resulting in cardiac arrest and patient demise.
Discussion
Multiple position papers, including a 2014 paper by the International ECMO Network “recommend selected members of the ECMO team should be trained in vascular and cardiac ultrasonography for insertion, maintenance, and surveillance of the ECMO device.” [6, 7]. Despite this, a vast majority of guidelines from professional societies are either vague or lacking when discussing the optimal imaging modality for monitoring the position of ECMO cannulas [8]. Practices vary institutionally, although frequent radiographic evaluation appears to be commonplace. Despite numerous studies highlighting the value of POCUS in the evaluation and management of critically ill patients [9, 10], prospective studies regarding POCUS in ECMO patients are lacking. To date, there are several case reports and one prospective study that have highlighted the utility of POCUS in the evaluation of ECMO cannula malposition [3, 4, 11, 12] but none have compared it to radiography. Additionally, these studies did not describe malposition identified by POCUS despite a normal radiograph. Our retrospective review suggests that radiographs may miss clinically important malposition that POCUS rapidly identifies. Additionally, POCUS provides several additional advantages when compared to radiography (e.g., color Doppler to view the outflow jet position, ease of access, minimal patient repositioning, live time cannula repositioning). Radiographic evaluation of ECMO cannulas is limited by underlying ARDS, pneumonia, or pulmonary edema-factors that obscure normal mediastinal and bony landmarks [13]. Situational factors, including poor patient positioning or difficulty maneuvering various lines or tubes in the critically ill patient, can lead to changes in the projected cannula tip position (Figs. 1, 2).
In addition to superiority in the evaluation of cannula malposition, ultrasound provides several other benefits. Ultrasound machines are readily available at the bedside in most ICUs, and the use of portable/hand-held ultrasounds is ever-increasing. POCUS is advantageous in facilities where ultrasound or echocardiography is not always available. Ultrasound also reduces unnecessary radiation exposure to patients and staff. It includes real-time imaging before cannulation, during and after cannula repositioning at the bedside, avoiding unstable patient transfers, less expert consultation use, and subsequent economic savings [9]. There is well-defined utility of ultrasound, specifically echocardiography, throughout all stages of ECMO currently defined in literature [5, 14,15,16,17,18].
Management stage
The use of ultrasound in the management stage of ECMO has the broadest application. In addition to echocardiography, a POCUS exam also includes at least daily assessments of the inferior vena cava (IVC) for volume assessment, cannulated vessels, cannula position(s), surrounding superficial and deep structures, and lungs. Regarding intravascular volume status, providers are tempted to administer additional intravenous fluids (IVF) with sudden decreases in circuit blood flow, mistakenly equating drops in flow to decreased intravascular volume. A plethora of data now indicate a negative fluid balance is associated with improved outcomes in ARDS, a common indication for VV ECMO [19]. Even in the absence of cannula malposition, POCUS can direct a provider to look for other etiologies of decompensation. If POCUS indicates proper cannula position and there is evidence of circuit flow compromise secondary to hypovolemia (negative pressures in the outflow limb, chatter, or collapse of the IVC around the cannula), additional IVF administration could be beneficial [20]. POCUS can also identify clinically relevant cannula site hematomas, peri-cannula thrombus or deep vein thrombosis obstructing circuit flow. Other vascular complications secondary to cannulization including vessel dissection, posterior vessel wall perforation and fistula formation can be identified through POCUS in the hands of an experienced provider [8, 17].
New onset hypotension may be a result of an underlying secondary process for which POCUS is again beneficial. Minor and major hemorrhage is not uncommon in these patients secondary to anticoagulation and platelet dysfunction. Concurrent thrombocytopenia, hepatic dysfunction and renal failure are not uncommon in these critically ill patients, further contributing to coagulopathy [21, 22]. Secondary to prolonged hospital stays, nosocomial infections are common and may contribute to underlying endothelial injury and leakage, further exacerbating hypotension and hypoperfusion. Again, POCUS aids in diagnosis of infections such as pneumonia or parapneumonic effusions [23]. Moreover, pleural ultrasound can assist in the diagnosis of pneumothorax, which is a relatively common cause of decompensation in mechanically ventilated ARDS patients. Other etiologies of shock including new onset cardiac dysfunction, cardiac tamponade, and even pulmonary embolism can be diagnosed with POCUS. Tamponade may occur hours or days after initial cannulation in the setting of anticoagulation initiation, with conventional signs and symptoms not seen secondary to support by the ECMO circuit [14]. Paradoxically, ECMO patients are at high risk of both hemorrhage and thrombosis, with a relatively high rate of PE occurrence estimated at 16.2% [22]. Frequent POCUS evaluation, even in the hemodynamically stable patient, is warranted to ensure that complications such as cannula migration are caught early [5, 20].
Cannula malposition
In our patients, the first sign of cannula malposition was an abrupt drop in circuit blood flow or new hypoxia. A sudden decrease in flow, typically 1–2 L-per-minute (LPM) from baseline, results in a phenomenon known as ‘suck down’ or ‘chugging.’ [19] POCUS distinguishes between the two most common etiologies of reduced circuit flow: intravascular volume depletion and cannula malposition.
Duration of cannulation for patients on ECMO varies depending on the type of support. VA ECMO runs are shorter than VV ECMO runs; on average, patients on VV ECMO are cannulated for 10–14 days [24]. Accidental movement of cannulas can occur at any time [15]. Cannula malposition is estimated to develop in roughly 5% of ECMO patients and is more common in VV ECMO patients [25]. Patients on VV ECMO commonly undergo physical therapy, stand, walk, and are sometimes subjected to intermittent prone ventilation early in the course of illness. Depending on cannulation configuration, subsequent complications related to malposition have varied consequences.
In the dl Vj-V configuration, minor changes in position can have disastrous consequences. Adequate delivery of oxygenated blood to pulmonary circulation relies on the outflow jet facing the tricuspid valve annulus. Proximal or distal migration of the cannula results in recirculation; a phenomenon in which returned, highly oxygenated blood is immediately drained back into the ECMO circuit. This results in decreased oxygen delivery, global hypoxia, and hypoperfusion [1]. Recirculation can also be seen in single lumen cannula configurations such as Vf-Vj, wherein the femoral drainage cannula migrates superiorly toward the IJ return cannula.
In both dual and single lumen jugular venous cannulas, distal migration of the return jet into the hepatic vein can cause several problems, including hepatic congestion, hepatic vein trauma, or decreased circuit blood flow [3, 4, 26]. One patient in our study had an acute elevation of liver enzymes, prompting a POCUS evaluation that identified distal migration of the dl Vj-V cannula. Re-positioning resulted in hepatic enzyme normalization. Migration or displacement into or through the IVC, right atrium (RA), and right ventricle (RV), have been described [2, 11, 26, 27]. This can occur in any configuration, and can result in low circuit flow, chugging, direct vessel trauma, ventricular rupture, and tamponade.
Our review is not the first to explore the utility of POCUS for cannula malposition in decompensating, seriously ill patients on extracorporeal support. A retrospective observational study performed at Massachusetts General Hospital evaluated 189 medical/cardiac ICU patients who underwent rescue POCUS (r-POCUS) during an episode of clinical decompensation. The most common reason for examination was hypotension (49%), followed by evaluation of cannula position (17%). Cannula malposition was identified in 9 patients (5%) with 8 patients undergoing cannula re-positioning because of the r-POCUS findings [12]. Although this study reinforces the utility of POCUS for evaluation of cannula position, it does not compare ultrasound images with standard radiographic images to determine if malposition was suspected.
Although comparisons between POCUS and radiography in adults on ECMO is lacking, a plethora of research in pediatric ECMO supports ultrasound’s superiority to radiographic evaluation of cannula position. A 2020 retrospective study of 39 infants performed by Pawlowski et al. found that POCUS identified cannula malposition in 19 patients who had an optimally positioned cannula determined by plain radiography (kappa of 0.13); the positive predictive value for plain radiography was only 54% [28]. Malposition occurred in half of the infants evaluated, and 52% of patients with malpositioned cannulas had an intervention after POCUS examination. The discordance between POCUS and plain radiography echoed results from multiple prior studies [29, 30].
Study limitations
The retrospective nature of this study limits its applications. Additionally, only 4 patients are included, limiting the power of this study. Time between radiograph and POCUS was unable to be determined through chart review, so positional changes could have occurred after radiographs were taken. POCUS examinations are limited by provider training and accuracy. Finally, all 4 patients included were on VV ECMO, which makes this review less applicable to VA ECMO patients. Despite these limitations, we strongly believe that our case series, in addition to the current literature, warrants evaluation of the current dependence on radiographic studies for ECMO cannula malposition.
Interpretation
This case series indicates that POCUS may be superior to radiography in the identification of ECMO cannula malposition. Additionally, ultrasound remains a fast and readily available tool that provides critical information throughout a patient’s extracorporeal treatment. This includes pre-evaluation and patient selection, cannula insertion, monitoring of progress, detection of complications, determining possibility of recovery, and weaning of support. We recommend a prospective study, directly comparing radiography to ultrasound in ECMO cannula position determination in adults.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Patroniti N, Pesenti A, Sangalli F (2014) ECMO-extracorporeal life support in adults, 1st edn. Springer, Milan
Hirose H, Yamane K, Marhefka G, Cavarocchi N (2012) Right ventricular rupture and tamponade caused by malposition of the avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg 7:36
Winiszewski H, Perrotti A, Chocron S, Capellier G, Piton G (2018) Malposition of the extracorporeal membrane oxygenation venous cannula in an accessory hepatic vein. J Extra Corpor Technol 50(3):167–169
Yastrebov K, Kapalli T (2013) Malposition of double lumen bicaval venovenous extracorporeal membrane oxygenation (VV ECMO) cannula resulting in hepatic venous congestion. Australas J Ultrasound Med 16(4):193–197
Victor K, Barrett NA, Gillon S, Gowland A, Meadows CI, Ioannou N (2015) Critical care echo rounds: extracorporeal membrane oxygenation. Echo Res Pract 2(2):D1–D11
Abrams D, Garan AR, Abdelbary A et al (2018) Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med 44(6):717–729
Combes A, Brodie D, Bartlett R et al (2014) Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 190(5):488–496
Lorusso R, Whitman G, Milojevic M et al (2021) 2020 EACTS/ELSO/STS/AATS expert consensus on post-cardiotomy extracorporeal life support in adult patients. J Thorac Cardiovasc Surg 161(4):1287–1331
Frankel HL, Kirkpatrick AW, Elbarbary M et al (2015) Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically Ill patients-part I: general ultrasonography. Crit Care Med 43(11):2479–2502
Levitov A, Frankel HL, Blaivas M et al (2016) Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically Ill patients-part II: cardiac ultrasonography. Crit Care Med 44(6):1206–1227
Rali AS, Taylor Z, George J, Trevino S, Diaz-Gomez JL (2021) Refractory hypoxemia despite extracorporeal membrane oxygenation: point-of-care ultrasound is needed for patients with COVID-19. Chest 159(4):e289–e291
Lu SY, Dalia AA, Cudemus G, Shelton KT (2020) Rescue echocardiography/ultrasonography in the management of combined cardiac surgical and medical patients in a cardiac intensive care unit. J Cardiothorac Vasc Anesth 34(10):2682–2688
Barnacle AM, Smith LC, Hiorns MP (2006) The role of imaging during extracorporeal membrane oxygenation in pediatric respiratory failure. Am J Roentgenol 186(1):58–66
Zhang Z (2017) Echocardiography for patients undergoing extracorporeal cardiopulmonary resuscitation: a primer for intensive care physicians. J Intensive Care 5:15
Peris A, Lazzeri C, Cianchi G et al (2015) Clinical significance of echocardiography in patients supported by venous-venous extracorporeal membrane oxygenation. J Artif Organs 18(2):99–105
Lee S, Chaturvedi A (2014) Imaging adults on extracorporeal membrane oxygenation (ECMO). Insights Imaging 5(6):731–742
Hussey PT, von Mering G, Nanda NC, Ahmed MI, Addis DR (2022) Echocardiography for extracorporeal membrane oxygenation. Echocardiography 39(2):339–370
Donker DW, Meuwese CL, Braithwaite SA et al (2018) Echocardiography in extracorporeal life support: a key player in procedural guidance, tailoring and monitoring. Perfusion 33(1):31–41
Tonna JE, Abrams D, Brodie D et al (2021) Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO). ASAIO J 67(6):601–610
Krishnan S, Schmidt GA (2019) Hemodynamic monitoring in the extracorporeal membrane oxygenation patient. Curr Opin Crit Care 25(3):285–291
Douraghi-Zadeh D, Logaraj A, Lazoura O et al (2021) Extracorporeal membrane oxygenation (ECMO): radiographic appearances, complications and imaging artefacts for radiologists. J Med Imaging Radiat Oncol 65(7):888–895
Parzy G, Daviet F, Persico N et al (2020) Prevalence and risk factors for thrombotic complications following venovenous extracorporeal membrane oxygenation: a CT scan study. Crit Care Med 48(2):192–199
Via G, Storti E, Gulati G, Neri L, Mojoli F, Braschi A (2012) Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol 78(11):1282–1296
What to Expect. 2022; https://www.elso.org/ecmo-resources/ecmo-what-to-expect.aspx.
Extracorporeal membrane oxygenation (ECMO) in adults. 2021. https://www.uptodate.com/contents/extracorporeal-membrane-oxygenation-ecmo-in-adults#H18.
Tanaka D, Pitcher HT, Cavarocchi N, Hirose H (2015) Migrated avalon veno-venous extracorporeal membrane oxygenation cannula: how to adjust without interruption of flow. J Card Surg 30(11):865–868
Reichelt A, Pichlmaier M, Hagl C, Khaladj N (2013) Malposition of a venous extracorporal life support cannula in a patent foramen ovale. Eur J Cardiothorac Surg 43(2):441
Pawlowski TW, Stoller JZ, Rintoul NE, Hedrick HL, Quartermain MD, Fraga MV (2021) Point-of-care ultrasound for the evaluation of venous cannula position in neonatal extracorporeal membrane oxygenation. J Perinatol 41(7):1645–1650
Irish MS, O’Toole SJ, Kapur P et al (1998) Cervical ECMO cannula placement in infants and children: recommendations for assessment of adequate positioning and function. J Pediatr Surg 33(6):929–931
Thomas TH, Price R, Ramaciotti C, Thompson M, Megison S, Lemler MS (2009) Echocardiography, not chest radiography, for evaluation of cannula placement during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med 10(1):56–59
Acknowledgements
The authors would like to express their gratitude to Kris Greiner for her assistance with editing of this manuscript.
Funding
None reported.
Author information
Authors and Affiliations
Contributions
Study concept and design: Becker, Rappaport, Struble. Acquisition of data: Rappaport and Struble. Analysis and interpretation of data: Becker, Rappaport and Struble. Drafting of manuscript: Becker. Critical revision of the manuscript for important intellectual context: Rappaport and Struble. Administrative, technical, or material support: Becker. Study supervision: Rappaport.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Project was granted a full waiver of HIPPAA Authorization by institutional IRB. IRB#202109561. IRB forms available on request.
Consent for publication
Available on request.
Competing interests
None reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becker, T., Struble, R.D. & Rappaport, C. Veno-venous extracorporeal membrane oxygenation (VV ECMO) cannula malposition identified with point-of-care ultrasound. Ultrasound J 16, 27 (2024). https://doi.org/10.1186/s13089-024-00357-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-024-00357-6