Abstract
In critically ill patients with acute respiratory failure, thoracic images are essential for evaluating the nature, extent and progression of the disease, and for clinical management decisions. For this purpose, computed tomography (CT) is the gold standard. However, transporting patients to the radiology suite and exposure to ionized radiation limit its use. Furthermore, a CT scan is a static diagnostic exam for the thorax, not allowing, for example, appreciation of "lung sliding". Its use is also unsuitable when it is necessary to adapt or decide to modify mechanical ventilation parameters at the bedside in real-time. Therefore, chest X-ray and lung ultrasound are today's contenders for shared second place on the podium to acquire a thoracic image, with their specific strengths and limitations. Finally, electrical impedance tomography (EIT) could soon have a role, however, its assessment is outside the scope of this review. Thus, we aim to carry out the following points: (1) analyze the advancement in knowledge of lung ultrasound use and the related main protocols adopted in intensive care units (ICUs) over the latest 30 years, reporting the principal publications along the way, (2) discuss how and when lung ultrasound should be used in a modern ICU and (3) illustrate the possible future development of LUS.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Lung ultrasound (LUS) is an imaging modality that might impact the physician’s decision-making after a patient’s clinical evaluation and accelerates management changes, such as adjustment of ventilatory setting, fluid therapy, patient’s position (supine vs prone), antibiotic management, chest drainage, thus promoting a "functional approach" potentially leading to an improved patient outcome [1]. In this way, ultrasound has become the fifth pillar of medical examination used by intensive care physicians after inspection, palpation, percussion and auscultation [2].
Although the chest is easily scanned with LUS, by just laying the probe along the intercostal spaces, no tool, including LUS, by itself, can improve patient’s outcome. For this reason, the International Evidence-Based Recommendations for Point-of-Care LUS published in 2012 tried to homogenize the terminology and the techniques used and provided a list of recommendations for a clinical approach to the different illnesses [3].
With the COVID-19 pandemic, LUS has become extremely popular and this was reflected by many publications and by countless theoretical and practical courses, both delivered online and in presence [4]. This narrative review aims to explore the following points: (1) to analyze the advancement in knowledge of LUS signs and the related main protocols used in ICUs over the latest 30 years, reporting the principal publications along the way, (2) to discuss how and when LUS should be used in a modern ICU and (3) to illustrate the possible future development of LUS.
Evolution of LUS in critically Ill patients
From 1995 the American College of Radiologists recommended daily supine chest X-rays in mechanically ventilated patients with acute cardiac and respiratory problems independently from the underlying pathology [5].
At that time, detecting tubes and central line malposition or the discovery of pneumothorax (PNX) with chest X-ray was responsible for a change in patient diagnosis or management in more than 65% of the cases [5]. However, the new millennium brought a breath of fresh air in critical care medicine and heralded the publication in 2000 of the paper "The Acute Distress Syndrome Network" about lung-protective ventilation strategy [6]. This article produced evidence for the protective effect of low tidal volume (6–8 ml/Kg) and its application worldwide, and this saw a reduction in the incidence of volotrauma.
Meanwhile, further important events happened in 2001 and 2002: the use of ultrasound for central venous catheter (CVC) placement was promoted in the USA by the Agency for Healthcare Research and Quality as one of the 11 practices to improve patient care [7], and by the National Institute for Clinical Excellence (NICE) in Europe, and this started to reduce the iatrogenic PNX related to blind CVC placement [8]. Many discoveries by Daniel Lichtenstein, the father of modern LUS use in the ICU, saw the dawn in the new millennium between 1995 and 2009, with the description of "lung sliding", a bedside ultrasound sign ruling out pneumothorax (1995), [9] the comet-tail artifact as a sign of “alveolar-interstitial syndrome” (1997) [10], the “lung point”, an ultrasound sign specific to pneumothorax (2000) [11], the “lung pulse”, an early sign of complete atelectasis (2003) [12], and the dynamic air bronchogram, a lung ultrasound sign of alveolar consolidation ruling out atelectasis (2009) [13]. In his pioneering work first published in 1993, Lichtenstein used ultrasound to examine the abdomen, the pleural space, and the femoral vein at the bedside in ICU [14]. The study results were surprising, with ultrasound showing an immediate impact on management in 33 out of 150 patients (22%), influencing the diagnostic workup and directly impacting the therapeutic decision-making approach; and providing promising results to open the way for ultrasound use in the ICU [14]. Figure 1 (left part).
Lung ultrasound vs Chest X-ray Road map. American College of Radiology (ACR), acute respiratory distress syndrome (ARDS), American College of Chest Physicians (ACCP), intensive care unit (ICU), La Société de Réanimation de Langue Française (SRLF), Bedside Lung Ultrasound in Emergency (BLUE) protocol, point of care lung ultrasound POC-LUS, Extended Focused Assessment with Sonography for Trauma (E-FAST), British Thoracic Society (BTS)
LUS and consolidation, interstitial syndrome, pneumothorax and pleural effusion
Lichtenstein was also the first to compare lung ultrasound sensitivity, specificity and diagnostic accuracy with auscultation and chest X-ray in patients with lung consolidation [15]. His second most impactful article focused on the "Bedside Lung Ultrasound in Emergency” (BLUE) protocol about the relevance of LUS in diagnosing the etiology of acute respiratory failure [16]. With the previously described signs of lung pulse, an early sign of complete atelectasis (2003), and the dynamic air bronchogram (2009), it is easy today to recognize pneumonia as a cause of acute respiratory failure [12]. In this algorithm, for example, comet-tail artifacts—B-lines today—helped differentiate cardiogenic pulmonary oedema from exacerbation of chronic obstructive pulmonary disease (COPD) with a sensitivity of 100% and a specificity of 92% [16]. After this discovery, in 2013 The European Association of Cardiovascular Imaging Recommendations stated that the absence of multiple bilateral B-lines excludes cardiogenic pulmonary oedema with a negative predictive value close to 100% [17].
On the contrary, B-lines were significantly correlated with a new onset acute congestive heart failure if their number was ≥ 15 per scan. This cut-off could be considered for a quick and reliable assessment of decompensation in outpatients with heart failure (HF) [18]. That was followed in 2016 by the European Society of Cardiology Guidelines stating that for the diagnosis and treatment of acute and chronic heart failure, chest X-ray is only of limited use in the diagnostic work-up of patients with suspected HF and probably most helpful in identifying an alternative pulmonary explanation for patient's symptoms and signs [19].
The diagnosis of PNX with LUS deserves a further separate examination. Considering that supine antero-posterior (A-P) chest X-ray may misdiagnose up to 30% of cases of PNX detected with a computed tomographic (CT) scan, LUS can be extremely useful in everyday clinical practice [20]. In the context of trauma, the case of pneumothorax not detected by plain radiography but later confirmed by CT, was first described in 2001 by Kirkpatrick [21]. In a comparative study, the same author first described the superiority of LUS vs chest X-ray for PNX detection in trauma patients when extending the abdominal ultrasound examination to the lung, applying the Extended Focused Assessment with Sonography for Trauma (E-FAST) protocol in 2004 [22]. In this study, LUS showed double sensitivity compared with chest-X ray (48.8% vs 20.9%), while specificity was high for both diagnostic images 99.6% vs 98.7% respectively. Soldati et al. in 2008, reported that out of 218 trauma patients, 25 showed PNX on CT scans. Taking the diagnostic accuracy of CT scan as the gold standard, the authors compared it with chest X-ray and LUS, and found that only 52% of PNX were revealed by chest X-ray with a sensitivity of 52% and specificity of 100%. In comparison, LUS detected 23 of 25 PNX with a sensitivity of 92% and specificity of 99.4% [23]. These findings have been confirmed today by evidence arising from 3 meta-analyses [24,25,26].
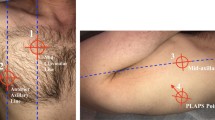
Pleural effusion (PE) is another example of the superiority of LUS compared to supine chest X-ray in ICU, where patients' physical examination with percussion and auscultation have shown low sensitivity and specificity compared to CT scan as the gold standard [27,28,29,30] (Figure 2). In practice, comparing LUS with a supine chest X-ray for pleural effusion produced important findings. In fact, this last becomes abnormal when PE is ≥ 200 mL, obliterating the hemi-diaphragmatic sinus [15, 31]. Furthermore, the possibility of coexisting parenchymal lung opacities further decreases chest X-ray sensitivity, while 'normal' appearances do not exclude the presence of an effusion [15, 29,30,31]. In 2008, Rocco et al. published a trial comparing bedside chest X-ray and LUS to diagnose PE in trauma patients, finding that LUS was more accurate than chest X-rays [32]. Another study by Xirouchaki et al. comparing the diagnostic accuracy of LUS and bedside chest X-ray in ICU patients showed excellent sensitivity, specificity, and an accuracy of 100%. In contrast, chest X-rays showed suboptimal corresponding values of 65%, 81%, and 69%, respectively [33]. Therefore, evidence suggests that LUS is more accurate for detecting pleural effusion than a supine chest X-ray. However, with ultrasound is not possible to distinguish PE type, i.e., exudate vs transudate, and after 50 years, the report by Light et al. is still the landmark in determining the different origins of effusion [34].
Estimating pleural effusion and improving accuracy in chest drainage positioning
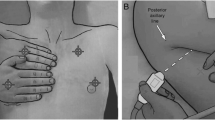
An essential task of LUS is the possibility of quantifying PE, and for this purpose many formulas exist [35,36,37,38,39]. All the authors in Table 1 found a good correlation between PE volume estimated by their formulas and effective volume measured after drainage. No superiority of one formula over another has been demonstrated to date. The Balik formula has gained popularity for its simplicity by measuring PE volume by multiplying the maximal interpleural distance (D) at lung base with a constant factor (V(ml) = 20 × D (mm)) [37]. The average error of this formula can be calculated at around 158–160 mL. Moreover, the formula overestimates the volume in some conditions, like in tall males with large thoracic circumferences, small effusions under 200 mL, and more significant effusions above 1000 mL [38, 39]. In the context of PE, another important event occurred in 2010 with the publication of the British Thoracic Society (BTS) Pleural Disease Guideline [28], now revisited, in response to the rapid system report of 12 deaths related to chest drain insertion, and 15 cases of serious harmful events between January 2005 and March 2008 [40]. The BTS strongly recommended that all chest drains for pleural effusion should be inserted under ultrasound guidance with small-bore chest drains for all fluid types in the thorax [40, 41]. In this context, the main purpose of the ultrasound is to identify a safe site for fluid aspiration followed by an accurate positioning of the chest drain insertion. A detailed illustration has been previously described [29]. In a recent ICU study, pleural drainage in terms of diagnostic and therapeutic impact has been showed to improve the pre-drainage diagnosis in 91 out of 119 (76.5%) patients, with 62 (52.1%) of these resulting in a complete change in the diagnosis, and 80 out of 137 procedures (58.4%) resulting in a change in treatment. However, extubation success and weaning from non-invasive ventilation (NIV) were not affected by drainage—17.5% vs 30.9% (p = 0.13) and 40% vs 66.7% (p = 0.38), respectively, as well as no difference was found regarding in-hospital mortality between those treated and not, 27.3% vs 27.3% (p > 0.99), respectively [42]. It should also be noted that thoracic ultrasound is only of limited utility in guiding the insertion of a chest drain in the presence of PNX because of the difficulty in obtaining valuable images due to the poor transmission of sound waves through the air. Table 2 [3]
LUS and fluid tolerance
Detecting fluid responsiveness is an important task in the management of critically ill patients. Moreover, detecting fluid tolerance, defined as the extent to which a patient can tolerate fluid administration without falling into organ dysfunction, is also of paramount importance [43]. Therefore, fluid resuscitation in ICU is a double-edged sword; under-resuscitation with hypoperfusion and overhydration with venous congestion are both dangerous, and they require a careful assessment of what intravenous volume status and fluid tolerance are [44, 45]. We can study fluid tolerance and intolerance with LUS through evaluating the presence of B-lines [46, 47]. In this review, we will emphasize that LUS can better detect interstitial syndrome compared with chest X-ray with an accuracy of 93% [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. As described by Lichtenstein, it is important to know that assessing interstitial syndrome requires the study of the anterolateral zones of the chest as the posterior zone suffers from fluid gravity [48, 49].
Interstitial syndrome suggests cardiogenic pulmonary oedema with a sensitivity of 97% and specificity of 95% when B-profile arises from the base to the apex and the pleural is thin (Fig. 3). Fluids in this case are not recommended [43,44,45,46,47,48,49].
Interstitial syndrome is also present with B-lines with anterolateral B-profile in case of isolated or diffuse ARDS. Copetti et al. helped in recognizing interstitial syndrome due to ARDS from pulmonary oedema by describing those pleural lines as thickened with reduced "gliding" in the context of "spared A-lines areas" in cases of ARDS [49]. The optimal fluid management in ARDS patients remains challenging and controversial because it should provide adequate oxygen delivery to the body while avoiding an inadvertent increase in lung oedema. Monitoring B-lines in this setting also requires echocardiographic evaluation and, if appropriate, an advanced invasive hemodynamic monitoring tool [50],because these patients can also develop a low cardiac output (CO) state with a reduced left ventricular ejection fraction (EF%) or isolated diastolic dysfunction (DD), respectively, in 39% and 20% [51, 52]. Volpicelli et al. first noted that B-lines correlate with extravascular lung water (EVLW) measured with the invasive monitoring PiCCO tool [53]. In that way, restrictive strategies, including fluid restriction guided by the monitoring of extravascular lung water, have been shown to improve oxygenation and reduce mechanical ventilation duration significantly, but with no significant effect on mortality [54, 55]. On the contrary, the absence of B-profile—“lung tolerance”—in patients with shock is a clear indication for intravenous fluids [43, 56, 57]. A new concept about organ congestion known as the venous excess with ultrasound score (VExUS) evaluating lung, liver and kidney is also developing. VexUS is outside the scope of this review and requires further literature evidence [47].
Lung ultrasound score in ICU
Lung ultrasound can scan the lung surface through the anterior, lateral and posterior areas giving a score based on the different aeration patterns from 0 to 3 from the best to the worst as follows: A-lines plus sliding = 0, well-separated B-lines = 1, coalescent B-lines = 2, and for C-pattern, consolidation = 3 [58]. An increase in score indicates a decrease in aeration and vice-versa. LUS score is feasible and easily obtained at the bedside to understand the effect of modification of the ventilation parameters, of patient’s positioning (supine vs prone), and of weaning outcome [59, 60]. This score was first proposed by Soummer et al. in a work that highlighted its use during the weaning phase from mechanical ventilation. LUS changes during a spontaneous breathing trial accurately predict post-extubation distress and the first time a switch in LUS use from diagnostic to monitoring was proposed in 2012 [61]. In 2010 Via et al. showed the LUS score to be reliable in evaluating lung aeration changes in patients who underwent whole lung lavage [62]. Bouhemad et al., in 2010, studied antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. The authors compared CT scan, LUS score and chest X-ray regarding reaeration of lungs following 7 days of antimicrobial therapy [63]. The authors use a 12 regions exam between days 0 and 7. They found that an ultrasound score > 5 was associated with a computed tomography reaeration > 400 mL and successful antimicrobial therapy. While an ultrasound score < 10 was associated with a loss of computed tomography aeration > 400 mL and a failure of antibiotics. Computed tomography and ultrasound lung reaeration showed a highly significant correlation (Rho = 0.85, p < 0.0001), while chest X-ray was inaccurate in predicting lung reaeration changes [63]. With the SARS-CoV2 pandemic leading to interstitial pneumonia characterized by superficial and subpleural lung lesions, interest in the LUS score has rapidly spread. The most famous study in this area was conducted in Israel in a medical ward and intensive care setting. In 280 patients, Lichter et al. found that LUS score predicted clinical deterioration and death [64]. In another study from a Brazilian group, de Alencar et al. found in 180 patients a correlation between the LUS score at admission and the duration of mechanical ventilation, intubation, and the probability of death. This study considered a broader population spectrum admitted to the emergency department with only 74 ICU patients [65]. We also found that COVID-19 patients with a lower LUS score after ICU admission had a better survival rate than those with a high score [66]. Furthermore, we identify four sub-phenotypes: (a) those with clinical improvement independently from the LUS score; (b) those who presented a moderate improvement in LUS score; (c) those who responded very clearly, after mechanical ventilation with a significant reduction in LUS score; (d) those who, while improving their clinical condition, did not show an evident improvement from an ultrasound point of view and presented an apparent worsening in LUS. This could mean that different components can influence the LUS score. For example, underlying cardiac and pulmonary diseases such as HF, COPD, chronic asthma, and the development of new pulmonary fibrosis could be crucial. The fact that some patients showed an immediate improvement in the LUS score after mechanical ventilation probably underlines a misdiagnosed cardiac involvement; on the contrary, patients who showed no improvement in the LUS score could have pulmonary fibrosis [67]. Interestingly, a recent multicenter prospective observational study proposed a new score in non-COVID-19 patients called the LUS-ARDS score [68]. It is based on LUS aeration scores of the left and right lung plus the anterolateral pleural line abnormalities—that score has been compared with the performance of chest X-rays in ARDS patients. The LUS-ARDS score showed an area under the receiver operating characteristics (ROC) curve of 0.90 (CI 0.85–0.95), a comparable performance to the current practice with experienced chest X-ray readers, but with more objectifiable diagnostic accuracy at each cut-off [68].
Lung ultrasound: basic and advanced skills
The use of ultrasound in ICU was first classified in 2009 by the American College of Chest Physicians (ACCP) and “La Société de Réanimation de Langue Française” (SRLF) [69]. Clinical ultrasound competencies were validated using the Delphi methodology and divided into two main branches: the general critical care ultrasound (GCCU) focusing on the thorax, abdominal and vascular level assessment, and the critical care echocardiography (CCE) with two levels of expertise, basic and advanced. Pleural and LUS were components of the GCCU. For pleural ultrasound, critical steps guided thoracentesis and pleural device insertion, estimating the remaining pleural fluid and identifying intrapleural device placement. LUS was explicitly oriented to discover PNX after the procedure [69]. In 2012, the international consensus conference also introduced the important concept of monitoring lung aeration and de-aeration with LUS scores to quantify the effect of ventilatory strategies [3]. However at the moment, it is difficult to separate LUS knowledge into basic and advanced on a continuum, and citing the recent work of Kraaijenbrink et al., “the high negative predictive value of ruling-out a PNX with lung sliding suggests this is straightforward, but ruling-in a PNX is much more complicated needing a different number of exams to become proficient showing that artifacts have a different learning curve.” [70]. Therefore, the low incidence of pneumothorax makes the recognition of the lung point difficult, with the need for a long training. The same is true for consolidation, an essential skill to differentiate between pneumonia, atelectasis, contusion and pulmonary embolism. It requires advanced skills supporting the idea that competence cannot easily be divided into basic and advanced skills [71, 72].
Chest X-ray in ICU after 2012–2014
In 2006, Graat et al. first demonstrated in 2,457 daily routine chest X-rays performed in 754 consecutive ICU patients that this imaging modality did not reveal any new predefined significant findings [73]. This work was followed by a trial in which one patient group underwent a supine chest X-ray daily and a second group on demand. The results showed that on-demand chest X-rays did not delay the diagnosis, length of stay, ventilator-free days or increased mortality between the two groups. Subsequently, Oba and Zaza performed a meta-analysis involving 7,078 patients and found that the elimination of daily routine chest X-rays did not affect ICU LOS (WMD = 0.19 days; 95% CI –0.13, 0.51; P = 0.25), hospital LOS (WMD = –0.29 days; 95% CI –0.71, 0.13; P = 0.18), ventilator days (WMD = 0.33 days; 95% CI –0.12, 0.78; P = 0.15), ICU or hospital mortality (OR, 1.02;[95% CI 0.89, 1.17; P = 0.78 and OR, 0.92; 95% CI 0.76, 1.11; P = 0.4, respectively) [74].
Hendrikse et al. analyzed the data on 1,780 daily chest X-rays in 559 hospital admissions, underlining the diagnostic efficacy of daily routine chest X-rays at 4.4% [75]. Following this evidence, the American College of Radiology (ACR) amended its recommendations in December 2011 by assigning a “usually not appropriate” rating with some exceptions to routine daily chest X-rays [76].
In 2014, the category of patients receiving mechanical ventilation was removed from ACR recommendations, and routine chest X-rays in all stable patients in the ICU were categorized as “usually not appropriate.” [77]. And then, “Do not order diagnostic tests at regular intervals (such as every day)”, including daily chest X-rays, was among the top 5 Choosing Wisely list [78]. However, scepticism persists [4, 61]. Figure 1. (Right part).
Chest X-ray vs LUS cost
Hejblum et al. assessed chest radiographs in mechanically ventilated patients in 21 ICUs; 11 were using daily chest radiographs and 10 a clinical-driven strategy. Four hundred and twenty-four patients received 4,607 routine chest radiographs, and 425 received 3,148 clinical-driven chest X-rays. There was a 32% reduction in the second group with a 35% reduction in chest X-rays and $9,900 per bed per year without any change in the quality of care or safety [79]. Scott et al. showed that by restricting daily chest X-rays in ICU, the average monthly cost decreased from $11,633 before the intervention to $7,348 after the intervention, with a 37% cost saving [80]. Peris et al., after the introduction of LUS to their ICU, showed a significantly decreased use of diagnostic chest X-rays and CT scans by 26% and 47%, respectively, with a 39% cost saving in radiological examination (around €27,000) [81]. The authors also found a trend in the reduction of CT scans. We also analyzed the cost of chest X-rays after implementing LUS use showing a reduced related cost of 57% (€22,104) without affecting patient outcomes. Significantly, the number of CT scans remains the same [82].
Limitations of chest X-ray vs LUS
The limitations of bedside portable chest X-ray should also be highlighted in terms of image quality and, more importantly, the inability to accurately diagnose critical causes of dyspnoea, such as pleural effusion, pneumothorax, pulmonary edema and embolism. With a chest X-ray, 10% to 25% of pleural effusion can be misdiagnosed entirely, and 30% of pneumothoraxes are not visible because air moves up and medially between the lung and the heart, and only after filling these spaces free air gather the usual apical-lateral position. Chest X-ray is moderately specific (specificity 76%, 83%) but not sensitive enough (67–68%) for diagnosing heart failure, where the crucial exam is echocardiography. Chest X-ray also showed low sensitivity for pulmonary embolism [83]. Although LUS, as shown above, has several advantages, it has relatively lower sensitivity compared to chest CT. Only 70% of the lung surface can be explored, which explains the relatively low sensitivity to detect intra-parenchymal pneumonia not adherent to the pleural surface. LUS may be more challenging in obese patients due to the thickness of their ribcage and soft tissues. However, the primary enemy of LUS is that subcutaneous emphysema prevents the propagation of the ultrasound beams to the subpleural lung parenchyma [84].
Chest X-ray and irradiation
When dealing with radiological imaging techniques, it is imperative not to separate appropriateness from radioprotection issues. Specifically, critical care physicians and radiologists must always be mindful of the risk of exposure-induced biological effects resulting from ionizing radiations. Such effects are considered stochastic in diagnostic imaging, i.e., they can randomly derive from damage in a single cell, possibly resulting in cancer in the exposed individuals and hereditary diseases in their descendants [85]. For such effects, the International Commission on Radiological Protection (ICRP) has adopted a linear-no-threshold dose–response relationship, meaning that increasing the dose corresponds to an increased event frequency but not severity [86]. In other words, the excess risk of stochastic adverse effects is directly proportional to the radiation dose received, without any threshold below which there is zero excess risk [86]. While data on the rate of radiation-induced cancer are more solid in cases of high-dose exposures, such as those observed in cohorts of atomic bomb survivors, [86] there is a lack of clear evidence regarding the stochastic cancer risk for low-dose exposures, i.e., those below an effective dose of 100 milliSievert (mSv). As a point of reference, a single posteroanterior X-ray is estimated to deliver a dose of 0.02 mSv, whereas a chest CT is equivalent to 300–400 CXRs (about 6–8 mSv) [87]. While the burden of stochastic risk associated with radiological diagnostic imaging may be overestimated, it is prudent to err on the side of caution when addressing radiation exposure issues [88]. Therefore, radiation exposure should always be kept to a minimum. Indeed, even when the risk inherent to a given exposure is shallow, using close reiterated radiological examinations in patients with chronic conditions may lead to non-negligible cumulative radiation exposure. In addition, the patient’s biological risk for a given dose is highly dependent on age and gender, with children and women at greater risk, thus making the ICRP-promoted “as low as reasonably achievable—ALARA” recommendation even more important [89]. This cautious theoretical approach translates into the assumption that one can define chest X-ray and CT scan use in ICU diagnostic practice as appropriate only when coupled with increasing knowledge of patient radiation-inherent risks and benefits. Indeed, the responsible use of radiological examinations requires an awareness that, while they can be life-saving, alternative imaging techniques that do not involve ionizing radiation, such as LUS, should be preferred when the desired information can be obtained with comparable accuracy [90].
Further direction with LUS in ICU
As stated before, LUS use in ICU is now relevant to providing image modality that, integrated with patient clinical information, may impact physician decision-making and accelerate management changes in terms of adjustments of ventilatory setting, fluid therapy, patient’s position (supine vs prone), antibiotic management, and chest drainage [91] It is not possible to neglect LUS use today in ICU when also considering the economic and environmental impact over chest X-ray [92]. Many studies will be soon available showing the usefulness of this instrument in patients with pneumonia, ARDS or to evaluate patient weaning from mechanical ventilation. LUS is an operator-dependent exam, and the quality of images may vary depending on the technique and skill, which requires a steep learning curve, making challenging ultrasound studies replications and generalizable conclusions on its utility. Developing dedicated algorithms with artificial intelligence (AI) that automatically evaluate LUS video acquisition through real-time feedback could help in this direction [93]. At the same time, another exciting area of research could be using contrast-enhanced ultrasound (CEUS) imaging to characterize consolidations, helping choose optimal patient treatment and reduce the need for radiation exposure regarding CT scan exams [94]. Finally, in the ICU, experimental studies investigating LUS sliding velocity as a sign of lung dissection could improve ventilation setting and PEEP titration during mechanical ventilation [95].
Conclusion
In this review, we have analyzed the progress of LUS and the decline of chest X-ray use in ICU over the latest 30 years, highlighting the turning points brought about by new discoveries. LUS has shown to be an essential tool in enhancing patients’ safety and helping clinicians reach a bedside diagnosis and monitor patients over time. We have also discussed the problem related to the economic point of view and patients’ radiation exposure so that a supine chest X-ray could be reduced to the minimum while LUS should be incorporated into the ICU physician’s armamentarium. We should consider that at the present time, the major limitation is the need for a reasonable number of expert supervisors in the ICU team, together with the homogenisation of the image acquisition modality and the standardization of the exam’s report. In the near future, with the widespread use of technologies, it could be possible to review and discuss the LUS images at the patient’s bedside and use these images to monitor progress throughout the patient’s journey in the ICU.
Availability of data and materials
Not applicable.
References
Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D (2019) Lung ultrasound for critically Ill Patients. Am J Respir Crit Care Med 199(6):701–714. https://doi.org/10.1164/rccm.201802-0236CI.Erratum.In:AmJRespirCritCareMed.2020Apr15;201(8):1015.Erratumin:AmJRespirCritCareMed.2020Jun1;201(11):1454. (PMID: 30372119)
Narula J, Chandrashekhar Y, Braunwald E (2018) Time to add a fifth pillar to bedside physical examination: inspection, palpation, percussion, auscultation, and insonation. JAMA Cardiol 3(4):346–350. https://doi.org/10.1001/jamacardio.2018.0001. (PMID: 29490335)
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. 2012 International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 38(4): 577–91. doi: https://doi.org/10.1007/s00134-012-2513-4.
Vetrugno L, Mojoli F, Boero E, Berchialla P, Bignami EG, Orso D et al (2022) Level of diffusion and training of lung ultrasound during the COVID-19 pandemic - a national online italian survey (ITALUS) from the lung ultrasound working group of the italian society of anesthesia, analgesia, resuscitation, and intensive care (SIAARTI). Ultraschall Med 43(5):464–472. https://doi.org/10.1055/a-1634-4710
http://www.acr.org ACR panel American College of Radiology Thoracic Expert Panel Report. 1996. Last accessed on 5 November 2023.
Acute Respiratory Distress Syndrome Network; Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8. doi: https://doi.org/10.1056/NEJM200005043421801. PMID: 10793162
Clinical Practice Guidelines Archive. Content last reviewed July 2018. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/prevention/guidelines/archive.html Last accessed on 5 November 2023.
Guidance on the use of ultrasound locating devices for placing central venous catheters. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/ta49 Last accessed on 5 November 2023.
Lichtenstein DA, Menu Y (1995) A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding Chest 108(5):1345–1348. https://doi.org/10.1378/chest.108.5.1345. (PMID: 7587439)
Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O (1997) The comet-tail artefact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 156(5):1640–1646. https://doi.org/10.1164/ajrccm.156.5.96-07096
Lichtenstein D, Mezière G, Biderman P, Gepner A (2000) The, “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med 26(10):1434–1440. https://doi.org/10.1007/s001340000627. (PMID: 11126253)
Lichtenstein DA, Lascols N, Prin S, Mezière G (2003) The, “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive Care Med 29(12):2187–2192. https://doi.org/10.1007/s00134-003-1930-9. (Epub 2003 Oct 14 PMID: 14557855)
Lichtenstein D, Mezière G, Seitz J (2009) The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 135(6):1421–1425. https://doi.org/10.1378/chest.08-2281. (Epub 2009 Feb 18 PMID: 19225063)
Lichtenstein D, Axler O (1993) Intensive use of general ultrasound in the intensive care unit. Prospective study of 150 consecutive patients. Intensive Care Med 19(6):353–355. https://doi.org/10.1007/BF01694712. (PMID: 8227728)
Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ (2004) Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 100(1):9–15. https://doi.org/10.1097/00000542-200401000-00006. (PMID: 14695718)
Lichtenstein DA, Mezière GA (2013) Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 134(1):117–125. https://doi.org/10.1378/chest.07-2800
Neskovic AN, Hagendorff A, Lancellotti P, Guarracino F, Varga A, Cosyns B, et al. 2013 European Association of Cardiovascular Imaging. Emergency echocardiography the European Association of Cardiovascular Imaging recommendations. Eur Heart J Cardiovasc Imaging. 14(1): 1–11.
Miglioranza MH, Gargani L, Sant’Anna RT, Rover MM, Martins VM, Mantovani A et al (2013) Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 6(11):1141–1151. https://doi.org/10.1016/j.jcmg.2013.08.004. (Epub 2013 Oct 2 PMID: 24094830)
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016 Jul 14;37(27):2129-2200. doi: https://doi.org/10.1093/eurheartj/ehw128. Epub 2016 May 20.
Ball CG, Kirkpatrick AW, Laupland KB, Fox DI, Nicolaou S, Anderson IB, et al. Incidence, risk factors, and outcomes for occult pneumothoraces in victims of major trauma. J Trauma. 2005 Oct;59(4):917–24; discussion 924–5. doi: https://doi.org/10.1097/01.ta.0000174663.46453.86. PMID: 16374282.
Kirkpatrick AW, Ng AK, Dulchavsky SA, Lyburn I, Harris A, Torregianni W et al (2001) Sonographic diagnosis of a pneumothorax inapparent on plain radiography: confirmation by computed tomography. J Trauma 50(4):750–752. https://doi.org/10.1097/00005373-200104000-00029. (PMID: 11303179)
Kirkpatrick AW, Sirois M, Laupland KB, Liu D, Rowan K, Ball CG et al (2004) Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the Extended Focused Assessment with Sonography for Trauma (EFAST). J Trauma 57(2):288–295. https://doi.org/10.1097/01.ta.0000133565.88871.e4. (PMID: 15345974)
Soldati G, Testa A, Sher S, Pignataro G, La Sala M, Silveri NG (2008) Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest 133(1):204–211. https://doi.org/10.1378/chest.07-1595. (Epub 2007 Oct 9 PMID: 17925411)
Ding W, Shen Y, Yang J, He X, Zhang M (2011) Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest 140(4):859–866. https://doi.org/10.1378/chest.10-2946. (Epub 2011 May 5 PMID: 21546439)
Alrajhi K, Woo MY, Vaillancourt C (2012) Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 141(3):703–708. https://doi.org/10.1378/chest.11-0131. (Epub 2011 Aug 25 PMID: 21868468)
Alrajab S, Youssef AM, Akkus NI, Caldito G (2013) Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care 17(5):R208. https://doi.org/10.1186/cc13016.PMID:24060427;PMCID:PMC4057340
Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH (1992) Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 159(1):29–33. https://doi.org/10.2214/ajr.159.1.1609716. (PMID: 1609716)
Roberts ME, Rahman NM, Maskell NA, Bibby AC, Blyth KG, Corcoran JP et al (2023) British thoracic society guideline for pleural disease. Thorax 78(11):1143–1156. https://doi.org/10.1136/thorax-2023-220304. (Epub 2023 Aug 8 PMID: 37553157)
Vetrugno L, Guadagnin GM, Orso D, Boero E, Bignami E, Bove T (2018) An easier and safe affair, pleural drainage with ultrasound in critical patient: a technical note. Crit Ultrasound J 10(1):18. https://doi.org/10.1186/s13089-018-0098-z.PMID:30066098;PMCID:PMC6068051
Brogi E, Gargani L, Bignami E, Barbariol F, Marra A, Forfori F et al (2017) Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care 21(1):325. https://doi.org/10.1186/s13054-017-1897-5.PMID:29282107;PMCID:PMC5745967
Blackmore CC, Black WC, Dallas RV, Crow HC (1996) Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol 3(2):103–109. https://doi.org/10.1016/s1076-6332(05)80373-3. (PMID: 8796649)
Rocco M, Carbone I, Morelli A, Bertoletti L, Rossi S, Vitale M et al (2008) Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand 52(6):776–784. https://doi.org/10.1111/j.1399-6576.2008.01647.x. (Epub 2008 May 12 PMID: 18477080)
Xirouchaki N, Magkanas E, Vaporidi K, Kondili E, Plataki M, Patrianakos A et al (2011) Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med 37(9):1488–1493. https://doi.org/10.1007/s00134-011-2317-y. (Epub 2011 Aug 2 PMID: 21809107)
Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr (1972) Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 77(4):507–513. https://doi.org/10.7326/0003-4819-77-4-507. (PMID: 4642731)
Vignon P, Chastagner C, Berkane V, Chardac E, François B, Normand S et al (2005) Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med 33(8):1757–1763. https://doi.org/10.1097/01.ccm.0000171532.02639.08. (PMID: 16096453)
Roch A, Bojan M, Michelet P, Romain F, Bregeon F, Papazian L et al (2005) Usefulness of ultrasonography in predicting pleural effusions > 500 mL in patients receiving mechanical ventilation. Chest 127(1):224–232. https://doi.org/10.1378/chest.127.1.224. (PMID: 15653988)
Balik M, Plasil P, Waldauf P, Pazout J, Fric M, Otahal M et al (2006) Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med 32(2):318. https://doi.org/10.1007/s00134-005-0024-2
Usta E, Mustafi M, Ziemer G (2010) Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg 10(2):204–207. https://doi.org/10.1510/icvts.2009.222273. (Epub 2009 Nov 10 PMID: 19903687)
Remérand F, Dellamonica J, Mao Z, Ferrari F, Bouhemad B, Jianxin Y et al (2010) Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med 36(4):656–664. https://doi.org/10.1007/s00134-010-1769-9. (Epub 2010 Feb 6 PMID: 20140421)
Lamont T, Surkitt-Parr M, Scarpello J, Durand M, Hooper C, Maskell N (2009) Insertion of chest drains: summary of a safety report from the National Patient Safety Agency. BMJ 2(339):b4923. https://doi.org/10.1136/bmj.b4923. (PMID: 19955139)
Vetrugno L, Guadagnin GM, Barbariol F, D’Incà S, Delrio S, Orso D et al (2019) Assessment of pleural effusion and small pleural drain insertion by resident doctors in an intensive care unit: an observational study. Clin Med Insights Circ Respir Pulm Med 3(13):1179548419871527. https://doi.org/10.1177/1179548419871527.PMID:31516312;PMCID:PMC6724497
Fysh ETH, Smallbone P, Mattock N, McCloskey C, Litton E, Wibrow B et al (2020) Clinically Significant pleural effusion in intensive care: a prospective multicenter cohort study. Crit Care Explor 2(1):e0070. https://doi.org/10.1097/CCE.0000000000000070.PMID:32166290;PMCID:PMC7063904
Kattan E, Castro R, Miralles-Aguiar F, Hernández G, Rola P (2022) The emerging concept of fluid tolerance: a position paper. J Crit Care 71:154070. https://doi.org/10.1016/j.jcrc.2022.154070. (Epub 2022 Jun 2 PMID: 35660844)
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C et al (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40(12):1795–1815. https://doi.org/10.1007/s00134-014-3525-z
Vincent JL, Weil MH (2006) Fluid challenge revisited. Crit Care Med 34(5):1333–1337. https://doi.org/10.1097/01.CCM.0000214677.76535.A5. (PMID: 16557164)
Lichtenstein DA (2015) BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 147(6):1659–1670. https://doi.org/10.1378/chest.14-1313. (PMID: 26033127)
Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R et al (2020) Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 12(1):16. https://doi.org/10.1186/s13089-020-00163-w.PMID:32270297;PMCID:PMC7142196
Lichtenstein D, Mezière GA (2009) Diagnosis of cardiogenic pulmonary edema by sonography limited to the anterior lung. Chest 135(3):883–884. https://doi.org/10.1378/chest.08-2733. (PMID: 19265105)
Copetti R, Soldati G, Copetti P (2008) Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 29(6):16. https://doi.org/10.1186/1476-7120-6-16.PMID:18442425;PMCID:PMC2386861
Franchi F, Vetrugno L, Scolletta S (2017) Echocardiography to guide fluid therapy in critically ill patients: check the heart and take a quick look at the lungs. J Thorac Dis 9(3):477–481. https://doi.org/10.21037/jtd.2017.02.94.PMID:28449449;PMCID:PMC5394071
Geri G, Vignon P, Aubry A, Fedou AL, Charron C, Silva S et al (2019) Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 45(5):657–667. https://doi.org/10.1007/s00134-019-05596-z. (Epub 2019 Mar 19 PMID: 30888443)
Vincent JL, De Backer D (2013) Circulatory shock. N Engl J Med 369(18):1726–1734. https://doi.org/10.1056/NEJMra1208943. (PMID: 24171518)
Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V et al (2014) Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 121(2):320–327. https://doi.org/10.1097/ALN.0000000000000300. (PMID: 24821071)
Ingelse SA, Juschten J, Maas MAW, Matute-Bello G, Juffermans NP, van Woensel JBM et al (2019) Fluid restriction reduces pulmonary edema in a model of acute lung injury in mechanically ventilated rats. PLoS ONE 14(1):e0210172. https://doi.org/10.1371/journal.pone.0210172.PMID:30653512;PMCID:PMC6336323
Vignon P, Evrard B, Asfar P, Busana M, Calfee CS, Coppola S et al (2020) Fluid administration and monitoring in ARDS: which management? Intensive Care Med 46(12):2252–2264. https://doi.org/10.1007/s00134-020-06310-0
Lichtenstein D (2013) FALLS-protocol: lung ultrasound in hemodynamic assessment of shock. Heart Lung Vessel 5(3):142–147
Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A (2009) A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 136(4):1014–1020. https://doi.org/10.1378/chest.09-0001. (PMID: 19809049)
Bouhemad B, Mongodi S, Via G, Rouquette I (2015) Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology 122(2):437–447. https://doi.org/10.1097/ALN.0000000000000558. (PMID: 25501898)
Cammarota G, Bruni A, Morettini G, Vitali L, Brunelli F, Tinarelli F et al (2023) Lung ultrasound to evaluate aeration changes in response to recruitment maneuver and prone positioning in intubated patients with COVID-19 pneumonia: preliminary study. Ultrasound J 15(1):3. https://doi.org/10.1186/s13089-023-00306-9.PMID:36693978;PMCID:PMC9873545
Vetrugno L, Baciarello M, Bignami E, Bonetti A, Saturno F, Orso D et al (2020) The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J 12(1):39. https://doi.org/10.1186/s13089-020-00185-4.PMID:32785855;PMCID:PMC7422672
Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q et al (2012) Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med 40(7):2064–2072
Via G, Lichtenstein D, Mojoli F, Rodi G, Neri L, Storti E et al (2010) Whole lung lavage: a unique model for ultrasound assessment of lung aeration changes. Intensive Care Med 36(6):999–1007. https://doi.org/10.1007/s00134-010-1834-4. (Epub 2010 Mar 11 PMID: 20221746)
Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M et al (2010) Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med 38(1):84–92. https://doi.org/10.1097/CCM.0b013e3181b08cdb. (PMID: 19633538)
Lichter Y, Topilsky Y, Taieb P, Banai A, Hochstadt A, Merdler I et al (2020) Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 46(10):1873–1883. https://doi.org/10.1007/s00134-020-06212-1
de Alencar JCG, Marchini JFM, Marino LO, da Costa Ribeiro SC, Bueno CG, da Cunha VP et al (2021) COVID U. S. P. Registry Team Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann Intensive Care 11(1):6. https://doi.org/10.1186/s13613-020-00799-w
Vetrugno L, Meroi F, Orso D, D’Andrea N, Marin M, Cammarota G et al (2022) Can lung ultrasound be the ideal monitoring tool to predict the clinical outcome of mechanically ventilated COVID-19 patients? an observational study. Healthcare (Basel) 10(3):568. https://doi.org/10.3390/healthcare10030568.PMID:35327046;PMCID:PMC8955357
Leote J, Judas T, Broa AL, Lopes M, Abecasis F, Pintassilgo I et al (2022) Time course of lung ultrasound findings in patients with COVID-19 pneumonia and cardiac dysfunction. Ultrasound J 14(1):28. https://doi.org/10.1186/s13089-022-00278-2.PMID:35796809;PMCID:PMC9261145
Smit MR, Hagens LA, Heijnen NFL, Pisani L, Cherpanath TGV, Dongelmans DA et al (2023) DARTS consortium lung ultrasound prediction model for acute respiratory distress syndrome a multicenter prospective observational study. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202210-1882OC
Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A et al (2009) American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 135(4):1050–1060. https://doi.org/10.1378/chest.08-2305. (Epub 2009 Feb 2 PMID: 19188546)
Kraaijenbrink BVC, Mousa A, Bos LD, Paulus F, Tuinman PR (2022) Defining basic (lung) ultrasound skills: not so basic after all? Intensive Care Med 48(5):628–629. https://doi.org/10.1007/s00134-022-06666-5. (Epub 2022 Mar 30 PMID: 35355097)
Mongodi S, Bonomi F, Vaschetto R, Robba C, Salve G, Volta CA et al (2022) Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ 22(1):647. https://doi.org/10.1186/s12909-022-03708-w.PMID:36031630;PMCID:PMC9420188
Slemko JM, Daniels VJ, Bagshaw SM, Ma IWY, Brindley PG, Buchanan BM (2021) Critical care ultrasound training: a survey exploring the “education gap” between potential and reality in Canada. Ultrasound J 13(1):48. https://doi.org/10.1186/s13089-021-00249-z.PMID:34897552;PMCID:PMC8665911
Graat ME, Kröner A, Spronk PE, Korevaar JC, Stoker J, Vroom MB et al (2007) Elimination of daily routine chest radiographs in a mixed medical-surgical intensive care unit. Intensive Care Med 33(4):639–644. https://doi.org/10.1007/s00134-007-0542-1
Oba Y, Zaza T (2010) Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology 255(2):386–395. https://doi.org/10.1148/radiol.10090946. (PMID: 20413752)
Hendrikse KA, Gratama JW, Wt H, Rommes JH, Schultz MJ, Spronk PE (2007) Low value of routine chest radiographs in a mixed medical-surgical ICU. Chest 132(3):823–828. https://doi.org/10.1378/chest.07-1162
Amorosa JK, Bramwit MP, Mohammed TL, Reddy GP, Brown K, Dyer DS et al (2013) ACR appropriateness criteria routine chest radiographs in intensive care unit patients. J Am Coll Radiol 10(3):170–174. https://doi.org/10.1016/j.jacr.2012.11.013. (PMID: 23571057)
Suh RD, Genshaft SJ, Kirsch J, Kanne JP, Chung JH, Donnelly EF et al (2015) ACR Appropriateness criteria® intensive care unit patients. J Thorac Imaging 30(6):W63–W65. https://doi.org/10.1097/RTI.0000000000000174. (PMID: 26439890)
Halpern SD, Becker D, Curtis JR, et al; Critical Care Societies Collaborative. Five things physicians and patients should question. http://www.choosingwisely.org/doctor-patient-lists/critical-care-societies-collaborative-critical-care/. [31 March 2023]
Hejblum G, Chalumeau-Lemoine L, Ioos V, Boëlle PY, Salomon L, Simon T et al (2009) Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet 374(9702):1687–1693. https://doi.org/10.1016/S0140-6736(09)61459-8. (Epub 2009 Nov 4 PMID: 19896184)
Brogi E, Bignami E, Sidoti A, Shawar M, Gargani L, Vetrugno L et al (2017) Could the use of bedside lung ultrasound reduce the number of chest x-rays in the intensive care unit? Cardiovasc Ultrasound 15(1):23. https://doi.org/10.1186/s12947-017-0113-8.PMID:28903756;PMCID:PMC5597991
Peris A, Tutino L, Zagli G, Batacchi S, Cianchi G, Spina R et al (2010) The use of point-of-care bedside lung ultrasound significantly reduces the number of radiographs and computed tomography scans in critically ill patients. Anesth Analg 111(3):687–692. https://doi.org/10.1213/ANE.0b013e3181e7cc42. (PMID: 20733164)
Scott J, Waite S, Napolitano A (2021) Restricting daily chest radiography in the intensive care unit: implementing evidence-based medicine to decrease utilizationt. J Am Coll Radiol. https://doi.org/10.1016/j.jacr.2020.05.035
Cardinale L, Volpicelli G, Lamorte A, Martino J, Veltri A (2012) Revisiting signs, strengths and weaknesses of standard chest radiography in patients of acute dyspnea in the emergency department. J Thorac Dis 4(4):398–407. https://doi.org/10.3978/j.issn.2072-1439.2012.05.05
Blazic I, Cogliati C, Flor N, Frija G, Kawooya M, Umbrello M et al (2023) The use of lung ultrasound in COVID-19. ERJ Open Res 9(1):00196–02022. https://doi.org/10.1183/23120541.00196-2022.PMID:36628270;PMCID:PMC9548241
Council Directive 2013/59/Euratom of 5 December 2013 laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation. https://eur-lex.europa.eu/eli/dir/2013/59/2014-01-17. Accessed 15 Mar 2023.
ICRP Publication 105. Radiation protection in medicine. Ann ICRP. 2007;37(6):1-63. doi: https://doi.org/10.1016/j.icrp.2008.08.001. PMID: 18762065.
Lowe D, Roy L, Tabocchini MA, Rühm W, Wakeford R, Woloschak GE et al (2022) Radiation dose rate effects: what is new and what is needed? Radiat Environ Biophys 61(4):507–543. https://doi.org/10.1007/s00411-022-00996-0
Ozasa K, Grant EJ, Kodama K (2018) Japanese legacy cohorts: the life span study atomic bomb survivor cohort and survivors’ offspring. J Epidemiol 28(4):162–169. https://doi.org/10.2188/jea.JE20170321
European Commission, Directorate-General for Environment, Dixon, A., Referral guidelines forimaging : radiation protection No 118, Dixon, A. (editor), Publications Office, 2001.
European Society of Radiology (ESR) (2011) White paper on radiation protection by the European society of radiology. Insights Imaging 2(4):357–362. https://doi.org/10.1007/s13244-011-0108-1
Picano E, Vañó E, Rehani MM, Cuocolo A, Mont L, Bodi V et al (2014) The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 35(10):665–672. https://doi.org/10.1093/eurheartj/eht394. (Epub 2014 Jan 8 PMID: 24401558)
Gargani L, Picano E (2015) The risk of cumulative radiation exposure in chest imaging and the advantage of bedside ultrasound. Crit Ultrasound J 28(7):4. https://doi.org/10.1186/s13089-015-0020-x.PMID:25883779;PMCID:PMC4392040
Cammarota G, Simonte R, Longhini F, Spadaro S, Vetrugno L, De Robertis E (2023) Advanced point-of-care bedside monitoring for acute respiratory failure. Anesthesiology 138(3):317–334. https://doi.org/10.1097/ALN.0000000000004480. (PMID: 36749422)
Cammarota G, Vetrugno L, Longhini F (2023) Lung ultrasound monitoring: impact on economics and outcomes. Curr Opin Anaesthesiol 36(2):234–239. https://doi.org/10.1097/ACO.0000000000001231. (Epub 2022 Dec 28 PMID: 36728722)
Russell FM, Ehrman RR, Barton A, Sarmiento E, Ottenhoff JE, Nti BK (2021) B-line quantification: comparing learners novice to lung ultrasound assisted by machine artificial intelligence technology to expert review. Ultrasound J 13(1):33. https://doi.org/10.1186/s13089-021-00234-6.PMID:34191132;PMCID:PMC8245599
Safai Zadeh E, Dietrich CF, Kmoth L, Trenker C, Alhyari A, Ludwig M, Görg C (2022) Peripheral pulmonary lesions in confirmed pulmonary arterial embolism: follow-up study of B-mode ultrasound and of perfusion patterns using contrast-enhanced ultrasound (CEUS). J Ultrasound Med 41(7):1713–1721. https://doi.org/10.1002/jum.15852. (Epub 2021 Oct 25 PMID: 34694040)
García-de-Acilu M, Santafé M, Roca O (2023) Use of thoracic ultrasound in acute respiratory distress syndrome. Ann Transl Med 11(9):320. https://doi.org/10.21037/atm-22-4576.PMID:37404985;PMCID:PMC10316096
Acknowledgements
Not applicable
Funding
None to declare.
Author information
Authors and Affiliations
Contributions
LV conceived the review, searched for literature, wrote the manuscript, gave important intellectual content and retrieved images. DGB conceived the review, searched for literature, wrote the manuscript, gave important intellectual content and retrieved images. CD wrote the manuscript, gave important intellectual content and retrieved images. SS wrote the manuscript and gave important intellectual content. FAL wrote the manuscript and gave important intellectual content. FL wrote the manuscript and gave important intellectual content. LP wrote the manuscript and gave important intellectual content. LC wrote the manuscript and gave important intellectual content. GC wrote the manuscript and gave important intellectual content. SMM wript and gave important intellectual content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable due to the type of article.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vetrugno, L., Biasucci, D.G., Deana, C. et al. Lung ultrasound and supine chest X-ray use in modern adult intensive care: mapping 30 years of advancement (1993–2023). Ultrasound J 16, 7 (2024). https://doi.org/10.1186/s13089-023-00351-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-023-00351-4