Abstract
Background
Disease-modifying antirheumatic drugs (DMARDs) are widely used for treating rheumatoid arthritis (RA). However, there are no established biomarkers to predict a patient’s response to these therapies. Prostanoids, encompassing prostaglandins, prostacyclins, and thromboxanes, are potent lipid mediators implicated in RA progression. Nevertheless, the influence of DMARDs on prostanoid biosynthesis in RA patients remains poorly understood. This study aims to assess the impact of various DMARDs on urinary prostanoids levels and to explore whether urinary prostanoid profiles correlate with disease activity or response to therapy.
Methods
This study included 152 Swedish female patients with early RA, all rheumatoid factor (RF) positive, enrolled in the NORD-STAR trial (registration number: NCT01491815). Participants were randomized into four therapeutic regimes: methotrexate (MTX) combined with (i) prednisolone (arm ACT), (ii) TNF-α blocker certolizumab pegol (arm CZP), (iii) CTLA-4Ig abatacept (arm ABA), or (iv) IL-6R blocker tocilizumab (arm TCZ). Urine samples, collected before start of treatment and at 24 weeks post-treatment, were analyzed for tetranor-prostaglandin E metabolite (tPGEM), tetranor-prostaglandin D metabolite (tPGDM), 2,3-dinor thromboxane B2 (TXBM), 2,3-dinor-6-keto prostaglandin F1a (PGIM), leukotriene E4 (LTE4) and 12-hydroxyeicosatetraenoic acid (12-HETE) using liquid chromatography–mass spectrometry (LC–MS). Generalized estimating equation (GEE) models were used to analyze the change in urinary eicosanoids and their correlations to clinical outcomes.
Results
Patients receiving MTX combined with CZP or TCZ exhibited significant elevations in urinary tPGEM and TXBM levels after 24 weeks of treatment. Other eicosanoids did not show significant alterations in response to any treatment. Baseline urinary eicosanoid levels did not correlate with baseline clinical disease activity index (CDAI) levels, nor with changes in CDAI from baseline to week 24. Their levels were also similar between patients who achieved CDAI remission and those with active disease at week 24.
Conclusions
Treatment with anti-TNF or anti-IL6R agents in early RA patients leads to an increased systemic production of proinflammatory and prothrombotic prostanoids. However, urinary eicosanoid levels do not appear to be predictive of the response to DMARDs therapy.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is an autoimmune disease affecting around 1% of the global population [1]. Disease-modifying antirheumatic drugs (DMARDs), including synthetic DMARDs, and biological DMARDs, are first-line therapies for RA. Several clinical trials have revealed remission rates of 50-80% in RA patients treated with DMARDs [2,3,4,5]; however, at least 20% do not respond to certain DMARD therapies, underscoring the importance of identifying predictive biomarkers, which could enable clinicians to provide more effective, early-stage therapies for RA.
The pathogenesis of RA is complex and involves both environmental and genetic factors [6]. Arachidonic acid (AA) derived eicosanoids, such as prostanoids and leukotrienes, have been shown to mediate RA progression [7, 8]. Their biosynthesis involves the release of AA from membrane glycerophospholipids by cytosolic phospholipase A2 (cPLA2), cyclooxygenation and hydroperoxidation into prostaglandin H2 (PGH2) by cyclooxygenases (COX), and hydroperoxidation and dehydration into leukotriene A4 (LTA4) by 5-lipoxygenase (5-LO) and 5-LO-activating protein (FLAP). As the levels of these lipids are low in blood and their measurements could be challenging and irreproducible due to many factors, analyzing their urinary metabolites presents a viable alternative reflecting the systemic biosynthesis over a longer period of time [9]. Levels of urinary leukotriene E4 (LTE4), 12-hydroxyeicosatetraenoic acid (12-HETE), and metabolites of prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), prostacyclin (prostaglandin I2) and thromboxane B2 (TXB2) are increased in several inflammatory diseases [10]. Although urinary tetranor-prostaglandin D metabolite (tPGDM), TXB2 metabolite (11-dehydro TXB2), and LTE4 have been shown to be elevated in RA patients [11, 12], it is still unclear how these metabolites might be affected by treatments of DMARDs.
Previous study described higher levels of plasma 6-trans leukotriene B4 (6-trans LTB4), 6-trans-12-epi LTB4, LTE4, PGE2, PGD2, and TXB2 in RA patients unresponsive to DMARDs [13]. However, these findings are questionable due to methodological flaws and technical challenges in measuring these lipids in blood [9, 14]. In the present research, we aim to investigate the effects of DMARDs on urinary levels of tPGEM, tPGDM, 2,3-dinor-6-keto prostaglandin F1α (PGIM), 2,3-dinor thromboxane B2 (TXBM), LTE4 and 12-HETE in RA patients. In addition, we aim to determine if baseline urinary eicosanoid levels differ between responders and non-responders to DMARDs therapy.
Materials and methods
The study population
This study included 152 Swedish patients enrolled in the NORD-STAR trial (registration number: NCT01491815) (Table 1) [15]. All patients included are females and are positive for rheumatoid factor (RF). All patients recruited in the NORD-STAR trial fulfilled the 2010 ACR/EULAR classification criteria and had symptom duration of less than 24 months. These participants, stratified by anti-citrullinated protein antibodies (ACPA) positivity, were evenly randomized into four groups, each receiving methotrexate (MTX) from day one alongside one of four DMARDs: (i) prednisolone (arm ACT), (ii) TNF-α blocker certolizumab pegol (arm CZP), (iii) CTLA-4Ig abatacept (arm ABA), or (iv) IL-6R blocker tocilizumab (arm TCZ). While intra-articular corticosteroid injections were permitted, they were not allowed within four weeks prior to sample collection. Clinical outcomes include clinical disease activity index (CDAI), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Clinical remission is defined as CDAI ≤ 2.8 [16]. Urine samples were collected at baseline and 24 weeks post-treatment, and stored at -80℃ for subsequent analysis. All patients recruited in the study were free from urinary tract infections within 1 month prior to urine sample collection.
Lipid extraction and liquid chromatography–mass spectrometry (LC-MS)
Urine samples were thawed on ice or in a fridge (4oC) and centrifuged at 1,000 g for 5 min at 4℃ to remove any precipitant. Each 250 µl urine sample was spiked with 50 µl of an internal standard (IS) mixture containing 0.4 µg/ml of tPGEM-d6, tPGDM-d6, 2,3-dinor-6-keto PGF1α-d9, 2,3-dinor TXB2-d9, LTE4-d5 and 12-HETE-d8 (Cayman Chemicals, Michigan, United States). Samples were diluted with 700 µl of 10mM ammonium acetate (AmAc) at pH 9.5 (Merck, Darmstadt, Germany), and 5 µl of 5% NH4OH (Sigma Aldrich, Missouri, United States) was added to ensure a final pH at 10 ± 0.5. The prepared samples were then loaded to a pre-activated and equilibrated Oasis MAX 96-well plate (Waters, Massachusetts, United States), washed with 1 ml AmAc (10mM pH 9.5) and 1 ml 100% acetone (Merck, Darmstadt, Germany). Oxylipins were eventually eluted in 1 ml acetone containing 3% acetic acid, then evaporated with SpeedVac and reconstituted in 50 µl 10% methanol in Milli-Q water. All urine samples were extracted in duplicates.
Reconstituted samples were analyzed by liquid chromatography on a Waters ACQUITY UPLC I-Class PLUS System (Waters Corporation, Massachusetts, United States) coupled to a triple quadrupole mass spectrometer, Waters Xevo TQXS Mass Spectrometer (Waters Corporation, Massachusetts, United States). Separation was achieved on a Waters ACQUITY UPLC BEH C18 column (2.1*50 mm, 1.7 μm) with a guard cartridge (Waters Corporation, Massachusetts, United States). The temperature of the analytical column was set to 60 °C and the flow rate was set to 0.5 ml/min. Mobile phase A was 0.05% formic acid in Milli-Q water, and mobile phase B was acetonitrile: isopropanol 9:1 with 0.05% formic acid. For LC-MS, two different methods were used in this study. Samples from arm CZP and arm TCZ were analyzed in the following gradient: initial 5% B, held at 5% B from 0.0 to 2.0 min, 5 to 10% B from 2.0 to 3.0 min, 10 to 25%B from 3.0 to 3.5 min, 25 to 35% B from 6.5 to 6.5 min, 35 to 95% B from 6.5 to 8.0 min, held at 95% B from 8.0 to 9.0 min, 95 to 5% B from 9.0 to 9.5 min and held 10%B from 9.5 to 11.0 min. After all samples were injected and analyzed for tPGEM and tPGDM, 10 µl methoxyamine hydrochloride (0.5 g/ml, Sigma-Aldrich, Missouri, United States) was added into the remaining samples and were incubated for 15 min at room temperature for full derivatization. Derivatized PGIM and TXBM were analyzed in the same gradient introduced above. Samples form arm ACT and ABA were analyzed in an optimized method, where tPGEM, tPGDM, LTE4 and 12-HETE were first analyzed in the following gradient: initial 5% B, held at 5% B from 0.0 to 1.5 min, 5 to 10% B from 1.5 to 2.5 min, 10 to 34%B from 2.5 to 4.0 min, 34 to 36% B from 4.0 to 8.5 min, 36 to 95% B from 8.5 to 14.0 min, held at 95% B from 14.0 to 15.0 min, 95 to 5% B from 15.0 to 15.5 min and held 10%B from 15.5 to 17.0 min. After derivatization, PGIM and TXBM were analyzed in a different gradient: initial 5% B, held at 5% B from 0.0 to 1.5 min, 10 to 35% B from 1.5 to 5.5 min, 35 to 95% B from 5.5 to 7.0 min, held at 95% B from 7.0 to 8.0 min, 95 to 5% B from 8.0 to 8.5 min and held 10% B from 8.5 to 11.0 min. Oxylipins were detected in multiple reaction monitoring (MRM) mode. The quantification transition, retention time (RT), lowest limit of detection (LLOD) and lowest limit of quantification can be found in Supplementary Table 1. Mass spectrometry data were analyzed with MassLynx (Version 4.20) software and quantifications were done using external standard curves with IS. In the present study, tPGEM and tPGDM were quantifiable in all samples. However, TXBM, PGIM, 12-HETE, and LTE4 were only quantifiable in some samples, with their positivity rather than absolute levels being analyzed. The levels of tPGEM and tPGDM were normalized to creatinine concentrations, which were measured by ELISA (#500,701, Cayman Chemicals, Michigan, United States) according to the manufacturer’s instructions. Total prostanoids were calculated by summing up all quantifiable prostanoids, including tPGEM, tPGDM, PGIM and TXBM, and normalized to creatinine concentrations.
Statistical analysis
This study employed generalized estimating equation models to examine the impact of DMARDs on oxylipin production and to distinguish between responders (patients who achieved remission at week 24) and non-responders (patients with active disease at week 24). These models were adjusted for covariates including the use of nonsteroidal anti-inflammatory drugs (NSAIDs), creatinine concentrations, and baseline CDAI levels. Additionally, various statistical methods were used as appropriate and stated in the legends of each table/figure. These statical models include the student’s t-test, paired t-test, Pearson Chi-square test, Mann-Whitney U test, Spearman’s correlation test, repeated measures ANOVA, one-way ANOVA, and Kruskal–Wallis test. Statistical significance was determined at P values below 0.05. All analyses were conducted using SPSS Statistics software (IBM, New York, United States).
Results
Characteristics of the selected study population
We have selected 152 Swedish female patients who were RF positive across four treatment arms (Table 1). These patients exhibited comparable age, body mass index (BMI), smoking status, ACPA positivity, and baseline levels of CDAI, CRP, and ESR across the different arms, and seem to be representative for the NORD-STAR trial. When comparing the therapeutic effects between the treatments, we didn’t find any difference in CDAI at weeks 12 and 24, the percentage of patients achieving remission at week 24, or the percentage of patients considered to have a good response according to EULAR recommendations. However, a trend was noted indicating that patients treated with biological DMARDs were more likely, albeit not statistically significant, to achieve CDAI remission compared to those in the ACT arm. Notably, tocilizumab demonstrated a superior effect in reducing acute phase reactants compared to other drug combinations (Table 1). These findings align with those reported in the whole NORD-STAR trial [17], suggesting that our selection of patients represent the NORD-STAR cohort well.
Urinary tPGEM, tPGDM, and TXBM were elevated specifically in patients who received anti-TNF and anti-IL6R treatments
To understand the effects of DMARDs on prostanoids production, we first studied the potential influence of NSAIDs uptake on prostanoid levels. Samples were considered NSAID-affected if NSAIDs had been used 2 weeks prior to sample collection. The number of NSAID-affected samples can be found in Supplementary Table 2. We found that NSAID-affected samples exhibited markedly lower levels of tPGEM and tPGDM, and were less likely to be tested positive for PGIM and TXBM (Supplementary Table 3). This highlights the necessarily to include “use of NSAIDs” as a covariate in the statistical model. As PGIM, TXBM, LTE4 and 12-HETE were not quantified in all samples and were analyzed based on their positivity, it is important to know if their detection is affected by urine concentration variances. We found that samples testing positive for these eicosanoids were significantly more concentrated than those testing negative, as evidenced by creatinine concentration comparisons (Supplementary Fig. 1A). Additionally, creatinine levels varied across samples from different therapeutic regimens and collection time points (Supplementary Fig. 1B), necessitating the inclusion of creatinine concentration as another covariate in the GEE models where PGIM, TXBM, LTE4 and 12-HETE were analyzed.
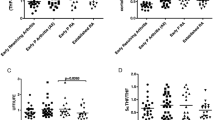
To determine the effects of DMARDs on urine eicosanoid production, we first compared the levels of eicosanoids at baseline and 24 weeks post-treatment in 152 patients from all therapeutic arms. We didn’t find any changes in tPGEM, tPGDM and total prostanoid, or positivity for PGIM, TXBM, LTE4 and 12-HETE (Fig. 1; Table 2). As CZP and TCZ are known to target upstream signal pathways of eicosanoid biosynthesis, we further analyzed the effects of different treatment combinations separately. Surprisingly, we observed significant increase, instead of decrease, in tPGEM, tPGDM and total prostanoid among patients in arm CZP and TCZ (Fig. 1). Correspondingly, the proportion of TXBM-positive samples notably increased in these two arms (Table 2). Despite PGIM positivity did not significantly rise in these two arms, there’s a trend of increase. To unequivocally rule out any potential interference from NSAIDs, we conducted a parallel analysis on patients who had not consumed any NSAIDs during the initial 24 weeks. This analysis confirmed that increased production of urinary tPGEM and TXBM was exclusive to patients treated with CZP and TCZ (see Supplementary Table 4). Collectively, these results indicate that COX derived AA metabolites are increased in patients treated with anti-TNF and anti-IL6 drugs.
Effects of 4 therapeutic regimens on levels of tPGEM, tPGDM and total urinary prostanoids. Levels of tPGEM (A), tPGDM (B) and total urinary prostanoids (C) concentrations were measured in urine samples collected at baseline (Day 0) and week 24. Prostanoid concentrations were normalized to creatinine levels. Total prostanoids also include PGIM and TXBM when their levels are quantifiable (> LLOQ). Comparisons were performed across all patients and within individual arms. GEE models were used and use of NSAIDs were adjusted in the model. *P < 0.05, **P < 0.01. ACT, Active Conventional Therapy arm; CZP, Certolizumab Pegol arm; ABA, Abatacept arm; TCZ, Tocilizumab arm; GEE, Generalized Estimating Equations; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs
Baseline levels of urinary eicosanoids are not associated with baseline CDAI, and are not predictive of CDAI reduction or CDAI remission
Age of diagnosis, ACPA positivity and smoking status have been shown to be independent prognostic markers in RA [18,19,20]. Therefore, we first determined the association between levels of urinary eicosanoids and age/ACPA/smoking. Our analysis revealed no correlation between urinary eicosanoids and either age, ACPA positivity, or smoking (Supplementary Table 5). We next explored the correlation between levels of urinary eicosanoids and disease severity, and found no association between any eicosanoid and CDAI (Table 3). In addition, baseline levels of urinary eicosanoids did not correlate with a reduction in CDAI, nor did they vary between responders and non-responders (Table 3). Given the influence of muscle mass on creatinine production, which was used to adjust urine concentrations, we conducted further analysis incorporating BMI as an additional covariate. These analyses reaffirmed the absence of a correlation between baseline urinary eicosanoid levels and patient responses to treatment (Table 3). Considering that different DMARDs affect urinary prostanoids differently, we conducted the same correlation analysis within the separate treatment arms. This analysis also revealed no correlation with baseline CDAI, reduction in CDAI, or remission status at week 24 (Supplementary Table 6). In summary, our data do not support the use of urinary eicosanoids as biomarkers for treatment response of the analyzed drugs in early RA.
Discussion
RA synovial tissues are known to express high levels of COX-2 and 5-LO [21,22,23]. Consequently, higher levels of AA metabolites derived from these oxygenase pathways have been detected in RA synovial fluid [8, 24]. Studies in animal models of inflammatory arthritis revealed significant pro-inflammatory and arthritogenic effects of PGE2/EP4 and LTB4/BLT1 signaling pathways [25,26,27]. Additionally, thromboxane and prostacyclin are also potent mediators of inflammation, and they might contribute to the cardiovascular comorbidity and pain behaviors in RA [28, 29]. In contrast, PGD2 and its metabolite 15-deoxy-∆12,14-prostaglandin J2 (15dPGJ2) exhibit anti-inflammatory effects in animal models of inflammatory arthritis [30, 31]. Therefore, these pro-inflammatory and anti-inflammatory lipid mediators derived from AA are considered as potential therapeutic targets and prognostic biomarkers in RA. NSAIDs, which inhibit inflammation by suppressing COXs, have been prescribed to RA patients since 1930s and remain in widespread use [32]. In our selection of patients, nearly 60% had used NSAIDs during the first 24 weeks of the trial (Supplementary Table 2). However, despite their efficient analgesic actions, NSAIDs can’t prevent or delay bone destructions themselves and may raise a series of gastrointestinal and cardiovascular side effects [33]. The adverse effects of NSAIDs are caused by disrupted TXA2/PGI2 balance, and might be avoided by using more selective inhibitors of PGE2 synthase, which might not only reduce production of pro-inflammatory PGE2, but also promote shunting to anti-inflammatory prostaglandins [34]. However, this therapeutic approach is not yet clinically available.
The potential of prostanoids and leukotrienes as prognostic biomarkers is understudied in RA patients. To our knowledge, only a single study has investigated the potential of plasma eicosanoids in predicting the response to DMARDs [13]. This study identified significantly higher levels of various eicosanoids derived from AA, including PGE2, PGF2α, PGD2, TXB2, LTB4, 6-trans LTB4, 6-trans-12-epi LTB4, LTE4, 5,12-diHETE and 5,15-diHETE, in the plasma collected from patients who didn’t respond to DMARDs [13]. However, analyzing eicosanoids in blood samples could be unreliable due to many reasons. Firstly, levels of eicosanoids in blood are notably low (usually less than 10 pg/ml) and unstable due to β- and ω-oxidations. More importantly, both prostanoids and leukotrienes can be formed ex vivo during blood collection, potentially leading to measured concentrations that are over tenfold higher than in samples collected with COX and 5-LO inhibitors [35]. Eicosanoid metabolites in the urine, on the contrary, are much more stable and offer more reproducible results [9, 35]. Urinary eicosanoids could be both excreted from the kidney and formed by the kidney. It is usually assumed that the more intermediate metabolites (ex. 6-keto PGF1α) are synthesized locally and the end products (ex. 2,3-dinor-6-keto PGF1α) reflect systemic production of the body [10]. Despite that the secretion of these eicosanoids might be affected under extreme kidney failure conditions, measuring urinary eicosanoids still represents the most reliable approach to study their systemic production. To this end, we measured urinary β-oxidation metabolites of PGE2, PGD2, thromboxane, and prostacyclin in RA patients and found no differences between those who responded to DMARDs and those who didn’t (Table 3 and Supplementary Table 6). Our data clearly negate the predictive values of these urinary oxylipins metabolites in RA. However, it should be noted that eicosanoids exert their biological effects in a localized manner, meaning that the systemic production of eicosanoids measured in the urine or blood might not be relevant to joint inflammation in RA. This is further evidenced by the lack of associations between urinary eicosanoids and clinical disease activity index (Table 3 and Supplementary Table 6), or CRP and ESR (data not shown). Therefore, the potential of eicosanoids in synovial fluid as predictors of DMARD response in RA patients warrants further investigation.
Expression of COX-2 is up-regulated in RA synovial tissue as a result of surrounding pro-inflammatory environment [8]. Pro-inflammatory cytokines, such as TNF-α and IL-6, induce expression of COX-2 in synovial fibroblasts and macrophages [36,37,38]. Therefore, anti-TNF and anti-IL6 treatments are expected to reduce production of COX-2 derived prostanoids. Contrary to this expectation, we observed an increase in urinary prostanoids in RA patients treated with CZP and TCZ (Fig. 1; Table 2). Notably, this elevation was not present in patients in arm ACT and ABA, suggesting that the increase is a specific effect of anti-TNF and anti-IL6 treatments rather than MTX. This finding aligns partially with our previous observations where TNF blockers, such as etanercept and infliximab, did not reduce COX-2 expression in RA synovial tissue, despite their significant inhibitory effects in vitro [23]. However, the mechanisms underlying the increase in prostanoid production by anti-TNF and anti-IL6 therapies remain unclear.
The presented research has several limitations: (i) Our study exclusively included seropositive female patients, necessitating validation of our results in a broader RA population; (ii) The sensitivity of our analytical protocol was insufficient to quantify TXBM, PGIM, LTE4 and 12-HETE in all samples, which hinders our ability to accurately assess changes in these eicosanoids. In addition, we analyzed our samples in two separate batches, with arm CZP and TCZ analyzed in the first batch, and arm ACT and ABA in second. Some samples were analyzed in both batches to exclude any batch effects. However, we employed different LC-MS methods for TXBM and PGIM across these two batches. After the first batch, we optimized the method to enhance sensitivity, leading to differences in baseline positivity rates for various regimens (Table 2). To ensure the validity of our conclusion that TXBM and PGIM positivity did not increase in the ACT and ABA arms, we redefined ‘positive’ with the LLOQ of the first batch method (80 fmol for TXBM and PGIM) in the data from the second batch. This adjustment yielded consistent results, indicating no significant differences in TXBM and PGIM positivity between baseline and 24 weeks post-treatment (data not shown). Nonetheless, the use of different analytical methods across batches may limit the direct comparability of data between different treatment arms.
Conclusions
To the best of our knowledge, we are the first to study the effects of various DMARDs on urinary eicosanoids production in RA patients. We discovered that patients treated with certolizumab pegol and tocilizumab had significantly increased production of urinary prostanoids after 24 weeks of therapies. However, baseline levels of eicosanoids are not associated with clinical disease activity or response to treatments.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author (Per-Johan Jakobsson).
Abbreviations
- 15dPGJ2: 15:
-
deoxy-∆12,14-prostaglandin J2
- AA:
-
Arachidonic Acid
- ABA:
-
Abatacept
- ACPA:
-
Anti-Citrullinated Protein Antibodies
- ACR:
-
American College of Rheumatology
- ACT:
-
Active Conventional Treatment
- AmAc:
-
Ammonium Acetate
- BMI:
-
Body Mass Index
- CDAI:
-
Clinical Disease Activity Index
- cPLA2 :
-
cytosolic phospholipase A2
- COX:
-
Cyclooxygenases
- CRP:
-
C-Reactive Protein
- CZP:
-
Certolizumab Pegol
- DMARDs:
-
Disease-Modifying Antirheumatic Drugs
- ESR:
-
Erythrocyte Sedimentation Rate
- EULAR:
-
European League Against Rheumatism
- GEE:
-
Generalized Estimating Equation
- HETE:
-
Hydroxyeicosatetraenoic acid
- IL6:
-
Interleukin 6
- IS:
-
Internal Standard
- LC-MS:
-
Liquid Chromatography–Mass Spectrometry
- LLOD:
-
owest Limit of Detection
- LLOQ:
-
Lowest Limit of Quantification
- LO:
-
Lipoxygenase
- LTA4 :
-
Leukotriene A4
- LTB4 :
-
Leukotriene B4
- LTE4 :
-
Leukotriene E4
- MO:
-
Methoxyamine
- MRM:
-
Multiple Reaction Monitoring
- MTX:
-
Methotrexate
- NORD-STAR:
-
Nordic Rheumatic Diseases Strategy Trials and Registries
- NSAIDs:
-
Nonsteroidal Anti-Inflammatory Drugs
- PGD2 :
-
Prostaglandin D2
- PGE2 :
-
Prostaglandin E2Prostaglandin E2
- PGH2 :
-
Prostaglandin H2
- PGIM:
-
2,3-dinor-6-keto prostaglandin F1α
- PGI2 :
-
Prostaglandin I2, prostacyclin
- RF:
-
Rheumatoid Factor
- RT:
-
Retention Time
- TCZ:
-
Tocilizumab
- TNF-α:
-
Tumor Necrosis Factor-α
- tPGDM:
-
tetranor-Prostaglandin D metabolite
- tPGEM:
-
tetranor-Prostaglandin E metabolite
- TXB2 :
-
Thromboxane B2
- TXBM:
-
2,3-dinor thromboxane B2
References
Finckh A, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591–602.
Kerschbaumer A et al. Efficacy of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis, 2023. 82(1).
Østergaard M, et al. Certolizumab pegol, abatacept, tocilizumab or active conventional treatment in early rheumatoid arthritis: 48-week clinical and radiographic results of the investigator-initiated randomised controlled NORD-STAR trial. Annals of the Rheumatic Diseases; 2023.
Fleischmann RM, et al. Anti-GM-CSF otilimab versus tofacitinib or placebo in patients with active rheumatoid arthritis and an inadequate response to conventional or biologic DMARDs: two phase 3 randomised trials (contRAst 1 and contRAst 2). Ann Rheum Dis. 2023;82(12):1516–26.
Emery P, et al. Pragmatic randomised controlled trial of very early etanercept and MTX versus MTX with delayed etanercept in RA: the VEDERA trial. Ann Rheum Dis. 2020;79(4):464–71.
Alivernini S, Firestein GS, McInnes IB. The pathogenesis of rheumatoid arthritis. Immunity. 2022;55(12):2255–70.
Hikiji H, et al. The roles of prostanoids, leukotrienes, and platelet-activating factor in bone metabolism and disease. Prog Lipid Res. 2008;47(2):107–26.
Korotkova M, Jakobsson P-J. Persisting eicosanoid pathways in rheumatic diseases. Nat Rev Rheumatol. 2014;10(4):229–41.
Gómez C, et al. Quantitative metabolic profiling of urinary eicosanoids for clinical phenotyping. J Lipid Res. 2019;60(6):1164–73.
Idborg H, Pawelzik S-C. Prostanoid metabolites as biomarkers in Human Disease. Metabolites, 2022. 12(8).
Cho C, et al. Prostaglandin D2 metabolites as a biomarker of in vivo mast cell activation in systemic mastocytosis and rheumatoid arthritis. Immun Inflamm Dis. 2016;4(1):64–9.
Nakamura H, et al. Difference in urinary 11-dehydro TXB2 and LTE4 excretion in patients with rheumatoid arthritis. Prostaglandins Leukot Essent Fat Acids. 2001;65(5–6):301–6.
Gomez EA, et al. Blood pro-resolving mediators are linked with synovial pathology and are predictive of DMARD responsiveness in rheumatoid arthritis. Nat Commun. 2020;11(1):5420.
O’Donnell VB, et al. Failure to apply standard limit-of-detection or limit-of-quantitation criteria to specialized pro-resolving mediator analysis incorrectly characterizes their presence in biological samples. Nat Commun. 2023;14(1):7172.
Glinatsi D, et al. Head-to-head comparison of aggressive conventional therapy and three biological treatments and comparison of two de-escalation strategies in patients who respond to treatment: study protocol for a multicenter, randomized, open-label, blinded-assessor, phase 4 study. Trials. 2017;18(1):161.
Felson DT, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70(3):404–13.
Hetland ML, et al. Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial. BMJ (Clinical Res ed). 2020;371:m4328.
Innala L, et al. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: a prospective study. Arthritis Res Therapy. 2014;16(2):R94.
Gottenberg JE et al. Brief Report: Association of Rheumatoid Factor and Anti-Citrullinated Protein Antibody Positivity With Better Effectiveness of Abatacept: Results From the Pan-European Registry Analysis. Arthritis & Rheumatology (Hoboken, N.J.), 2016. 68(6): p. 1346–52.
Saevarsdottir S, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum. 2011;63(1):26–36.
Siegle I, et al. Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum. 1998;41(1):122–9.
Gheorghe KR, et al. Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res Therapy. 2009;11(3):R83.
Korotkova M, et al. Effects of antirheumatic treatments on the prostaglandin E2 biosynthetic pathway. Arthritis Rheum. 2005;52(11):3439–47.
Mustonen A-M, Nieminen P. Fatty acids and oxylipins in Osteoarthritis and Rheumatoid Arthritis-a Complex Field with significant potential for future treatments. Curr Rheumatol Rep. 2021;23(6):41.
Trebino CE, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA. 2003;100(15):9044–9.
McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Investig. 2002;110(5):651–8.
Miyabe Y, Miyabe C, Luster AD. LTB4 and BLT1 in inflammatory arthritis. Semin Immunol. 2017;33:52–7.
Stiller C-O, Hjemdahl P. Lessons from 20 years with COX-2 inhibitors: importance of dose-response considerations and fair play in comparative trials. J Intern Med. 2022;292(4):557–74.
Pulichino A-M, et al. Prostacyclin antagonism reduces pain and inflammation in rodent models of hyperalgesia and chronic arthritis. J Pharmacol Exp Ther. 2006;319(3):1043–50.
Maicas N, et al. Prostaglandin D2 regulates joint inflammation and destruction in murine collagen-induced arthritis. Arthritis Rheum. 2012;64(1):130–40.
Silva Quinteiro M, et al. 15-deoxy-∆12,14-prostaglandin J2 reduces albumin-induced arthritis in temporomandibular joint of rats. Eur J Pharmacol. 2014;740:58–65.
Burmester GR, et al. Managing rheumatic and musculoskeletal diseases - past, present and future. Nat Rev Rheumatol. 2017;13(7):443–8.
Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Therapy. 2013;15(Suppl 3):S2.
Liu J, et al. Microsomal prostaglandin E synthase-1 inhibition promotes shunting in arachidonic acid metabolism during inflammatory responses in vitro. Prostaglandins & Other Lipid Mediators; 2023. p. 106738.
Tsikas D. A.A. Zoerner 2014 Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: a historical retrospect and a discussion. J Chromatogr B Analyt Technol Biomed Life Sci 964 79–88.
Fournier T, Fadok V, Henson PM. Tumor necrosis factor-alpha inversely regulates prostaglandin D2 and prostaglandin E2 production in murine macrophages. Synergistic action of cyclic AMP on cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Biol Chem. 1997;272(49):31065–72.
Ke J, et al. Role of NF-kappaB in TNF-alpha-induced COX-2 expression in synovial fibroblasts from human TMJ. J Dent Res. 2007;86(4):363–7.
Basu A, et al. STAT3 and NF-κB are common targets for kaempferol-mediated attenuation of COX-2 expression in IL-6-induced macrophages and carrageenan-induced mouse paw edema. Biochem Biophys Rep. 2017;12:54–61.
Acknowledgements
We thank all the patients and investigators involved in the NORD-STAR study.
Funding
This work is supported by the European Union’s Horizon 2020 research project ArthritisHeal under the Marie Skłodowska-Curie grant agreement (No. 812890), the Swedish Rheumatism Association (grant no: R-931761), King Gustaf V’s 80 Years Foundation and the Swedish research council (2021–03420).
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
P-J.J., M.K. and H.I. contributed to the study design. K.L. helped with the withdrawal of samples from biobank. J.Liu and H.I. set up the analytical method and carried out the mass-spectrometry analysis. J.Liu performed statistical analysis and drafted the manuscript. R.V., J.Lampa, A.R., D.N., B.G., G.G., T.U., K-H.P., M.H., M.Ø., and M.N. were involved in NORD-STAR trial design. The manuscript was reviewed and revised by all authors. All authors have given approval of the submission of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients have signed a written informed consent. The study (NORD-STAR trial) was approved by the regional ethics board in Stockholm (d.nr. 2011/2069-31/4 and amendment 2019–05705).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Idborg, H., Korotkova, M. et al. Urinary prostanoids are elevated by anti-TNF and anti-IL6 receptor disease-modifying antirheumatic drugs but are not predictive of response to treatment in early rheumatoid arthritis. Arthritis Res Ther 26, 61 (2024). https://doi.org/10.1186/s13075-024-03295-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-024-03295-9