Abstract
Osteoarthritis (OA) is a common and prevalent degenerative joint disease characterized by degradation of the articular cartilage. However, none of disease-modifying OA drugs is approved currently. Teriparatide (PTH (1–34)) might stimulate chondrocyte proliferation and cartilage regeneration via some uncertain mechanisms. Relevant therapies of PTH (1–34) on OA with such effects have recently gained increasing interest, but have not become widespread practice. Thus, we launch this systematic review (SR) to update the latest evidence accordingly. A comprehensive literature search was conducted in PubMed, Web of Science, MEDLINE, the Cochrane Library, and Embase from their inception to February 2022. Studies investigating the effects of the PTH (1–34) on OA were obtained. The quality assessment and descriptive summary were made of all included studies. Overall, 307 records were identified, and 33 studies were included. In vivo studies (n = 22) concluded that PTH (1–34) slowed progression of OA by alleviating cartilage degeneration and aberrant remodeling of subchondral bone (SCB). Moreover, PTH (1–34) exhibited repair of cartilage and SCB, analgesic, and anti-inflammatory effects. In vitro studies (n = 11) concluded that PTH (1–34) was important for chondrocytes via increasing the proliferation and matrix synthesis but preventing apoptosis or hypertrophy. All included studies were assessed with low or unclear risk of bias in methodological quality. The SR demonstrated that PTH (1–34) could alleviate the progression of OA. Moreover, PTH (1–34) had beneficial effects on osteoporotic OA (OPOA) models, which might be a therapeutic option for OA and OPOA treatment.
Similar content being viewed by others
Background
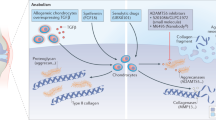
Osteoarthritis (OA) is a common musculoskeletal disorder and prevalent degenerative disease worldwide [1, 2]. Both non-load bearing and load-bearing joints are affected by multiple factors such as trauma, senility, gender, genetics, and obesity [3], which resulted in functional disability or decreased quality of life. Articular cartilage is an avascular tissue, while chondrocytes are unique cellular components and responsible for the maintenance of the extracellular matrix (ECM) via the balance of catabolism and anabolism. Type II collagen (COL II) and aggrecan (AGC) are secreted proteins, which are essential for the integrity of cartilage. Break-down of chondrocytes is one of the molecular characteristics of OA, which is characterized by progressive damage including cartilage erosion, synovitis, and subchondral bone (SCB) disturbance. The normal metabolism of cartilage is disturbed by inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), shifting to catabolism and ECM degradation [4]. Oxidative stress and apoptosis generate the decrease of chondrocytes and loss of cartilage [5]. The schematic diagram of normal and osteoarthritic joint was illustrated in Fig. 1.
Recommendation of OA treatment includes physiotherapy, pharmacological, and surgical interventions [6, 7]. Physiotherapy should be advocated due to its safety and effectiveness. However, limited choices and less effectiveness of drugs were restricted to symptom relief and accompanied by adverse effects [8]. Currently, no disease-modifying OA drugs (DMOADs) are available to alleviate the progression of OA. And therefore, strategies to protect the chondrocytes and the cartilage represent potential new therapeutic modalities.
Teriparatide (PTH (1–34)) contains 34 amino acids of parathyroid hormone, which was applied on the treatment of osteoporosis (OP) and bone fracture [9, 10] by maintaining calcium homeostasis, increasing cortical and trabecular thickness, and stimulating bone formation [11]. In addition, quantitative studies documented PTH (1–34) could mediate anabolic effects among chondrocytes [12] by enhancing chondral regeneration [13] and increasing ECM synthesis [14]. Experimental studies investigated the benefits of PTH (1–34) on OA pitiful without frequent practice or systematic review (SR). For these reasons, we reviewed the accessible research to update the effect of PTH (1–34) on OA.
Methods
Protocol
We performed this SR in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statements [15]. We recorded the study protocol on the international Prospective Register of Systematic Reviews (PROSPERO) with code CRD42022315089.
Literature search strategy
A comprehensive literature search was conducted in 5 databases (PubMed, Web of Science, Medline, the Cochrane Library, and Embase) from their inception to February 2022. The Medical Subject Headings (MeSH) terms and keywords were combined with boolean operators, “OR” or “AND”. The MeSH terms and keywords were as follows: “Teriparatide,” “hPTH (1–34),” “Human Parathyroid Hormone (1–34),” “Parathar,” “Teriparatide Acetate,” “Forteo,” “Osteoarthritis,” “Osteoarthritides,” “Osteoarthrosis,” “Osteoarthroses,” “Arthritis,” “Degenerative,” “Arthritides,” “Degenerative,” “Degenerative Arthritides,” “Degenerative Arthritis,” “Arthrosis,” “Arthroses,” “Osteoarthrosis Deformans.” In addition, the reference lists of all retrieved papers were further obtained manually. The search strategy of these five databases is provided in Additional file 1.
Inclusion and exclusion criteria
The eligible studies should meet the following criteria: (1) prospective and retrospective studies, randomized and controlled clinical trials; (2) patients or animal models with OA treated by PTH (1–34) directly or indirectly; and (3) studies published in the English language. Studies were excluded from this review if they were reviews, research protocols, abstracts only, commentaries, or editorials.
Study selection
All records of five databases were imported into the reference management software program Endnote X 9.3.3. After the removal of duplicates, two authors (GQL and SL) independently reviewed the titles and abstracts of the remaining records for relevance to the topic. Studies that potentially or completely met the inclusion criteria were kept and full texts were retrieved. The two authors (GQL and SL) independently assessed the full texts to decide whether to keep the records or not. A consensus meeting with a third reviewer (FY) was used to resolve discrepancies. The final included studies were reviewed by all authors for agreement.
Data extraction
The information of in vivo and in vitro studies was extracted in the standardized information forms: (1) first author’s surname, year of publication, and country; (2) subjects; (3) intervention; (4) dose and duration of treatment; (5) route; and (6) findings. Two investigators (GQL and SL) independently reviewed and extracted information from included studies. Disagreements were discussed with a third author (JW) to reach a consensus.
Quality assessment
The methodological quality of the in vivo studies was assessed by SYRCLE’s risk of bias tool [16] while the in vitro studies with Checklist for Reporting In-vitro Studies (CRIS) instruction [17]. Two authors (GQL and SL) independently assessed the methodological quality of the articles included, and discrepancies were resolved by discussion with a third author (FY).
Results
Identification of relevant studies
The initial literature search resulted in 296 articles from PubMed (n = 35), Web of Science (n = 26), MEDLINE (n = 90), the Cochrane Library (n = 101), and Embase (n = 44) (Fig. 2). There are 152 duplicate records that were removed, and the remaining 144 records were screened by title and 103 records were excluded. Next, 41 full-text articles were assessed for their eligibility. Nineteen were excluded for (1) review (n = 5); (2) research protocol (n = 3); (3) abstract only (n = 2); (4) commentaries or editorials (n = 4); and (5) subjects treated without PTH (1–34) (n = 5). In addition, 11 additional records were added. Finally, 33 papers were considered and included.
In vivo studies showed potential effects of PTH (1–34) on OA models
According to the inclusion criteria, 22 in vivo studies were included (Table 1). The studies were conducted in numerous countries including China (Shao et al., 2020 [18]; Shao et al., 2021 [19]; Chen et al. 2021 [20]; Chen et al. 2018 [21]; Rajalakshmanan et al. 2012 [22]; Ma et al. 2017 [23]; Zhang et al. 2022 [24]; Chang et al. 2009 [25]; Yan et al. 2014 [26]; Dai et al. 2016 [27]; Cui et al. 2019 [28]; He et al. 2021 [29]; Longo et al. 2020 [30]), Germany (Orth et al. 2014 [31]; Orth et al. 2013 [32]), and the USA (Dutra et al. 2017 [33]; Sampson et al. 2011 [34]; Brien et al. 2017 [35]; Bagi et al. 2015 [36]; Antunes et al. 2013 [37]), Spain (Lugo et al. 2012 [38]; Bellido et al. 2011 [39]).
Studies implied that PTH (1–34) exhibited protective effects on both cartilage and SCB. Shao et al. concluded similar findings among collagenase-induced OA (CIOA) mouse models in a dose-dependent manner via the JAK2/STAT3 and WNT5A/ROR2 signaling pathway [18, 40]. At the dose of 10 μg/kg/day of PTH (1–34), Orth et al. reported that PTH (1–34) could broaden the calcified cartilage layer, result in cartilage degeneration, and induce alterations in the microarchitecture of SCB to provoke early OA [31]. Moreover, PTH (1–34) would stimulate articular cartilage and SCB repair [41]. Bellido et al. suggested that PTH (1–34) could improve microstructural and remodeling parameters of SCB, which contributed to preventing cartilage damage and OA progression in OVX and ACLT rabbits [39].
PTH (1–34) would reduce the predisposing factors for OA progression. At the dose of 40 μg/kg/day, Cui et al. believed that PTH (1–34) reduced the accumulation of senescent cells in SCB by inhibiting p16 for age-related OA [28]. In addition, Sampson et al. considered that it might be useful to decelerate cartilage degeneration among meniscal ligamentous injury (MLI) mice and induce ECM regeneration among OA patients [34]. Bagi et al. concluded that PTH (1–34) would reduce joint inflammation, curb excessive bone remodeling, improve cartilage regeneration, and reduce pain in post-traumatic OA rats [36]. At the dose of 80 μg/kg/day of PTH (1–34), Dutra et al. found that it could result in mineralization and alteration of the mandibular condylar cartilage (MCC), with cartilage degeneration and abnormal remodeling of the SCB [33]. He et al. concluded that PTH (1–34) had an obvious analgesic and anti-inflammatory effect on DMM mice via the PKA and the NF-κB signaling pathways [29]. Brien et al. concluded that it would increase the differentiation and mineralization of chondrocytes as well as density of the SCB among the transgenic mice [35].
PTH (1–34) prevents cartilage damage and retards the deterioration of SCB. Yan et al. concluded that 15 μg/kg/day of PTH (1–34) protected the cartilage among guinea pigs [26]. Dai et al. found that 24 μg/kg/day of PTH (1–34) exhibited protective effects on cartilage degeneration among meniscectomy guinea pigs, which exhibited superior performance to celecoxib in both cartilage metabolism and maintenance of SCB micro-architecture [27]. Antunes et al. argued that SCB contributed to the disruption of the cartilage, but PTH (1–34) protected the destruction of the SCB [37]. Zhang et al. supposed that PTH (1–34) improved cartilage metabolism and SCB health on patellar ligament shortening SD rats [42].
Different routines would differ the effect of PTH (1–34). Eswaramoorthy et al. found that controlled-release property of PTH (1–34) via intra-articular (IA) injection suppressed early stages of OA in papain-induced OA (PIOA) rats [22]. Chen et al. suggested that PTH (1–34) improved spontaneous OA by directly affecting the cartilage rather than the SCB or metaphyseal bone [43], reduce chondrocyte terminal differentiation and apoptosis, and increase autophagy on ACLT rats via IA injection [44]. Longo et al. concluded that PTH (1–34) promoted the regenerative and chondroprotective effects of the tissue-engineered meniscus by inhibiting the differentiation of mesenchymal stem cells (BMSC) chondrogenesis and cartilage degeneration among the meniscectomy dogs [30], which represented a promising method to increase the chance of regeneration in the tissue-engineered meniscus.
In vitro studies showed potential mechanism of PTH (1–34) intracellularly
Based on the inclusion criteria, 11 in vitro investigations were included in the SR (Table 2). These studies were conducted in numerous countries including China (Chang et al. 2009 [25], Shao et al. 2022 [45]; Chang et al. 2016 [46]), Canada (Mwale et al. 2010 [47]), USA (Funk et al. 1998 [48]), Sweden (Petersson et al. 2006 [49]), Australia (Music et al. 2020 [50]), Japan (Tsukazaki et al. 1996 [51]; Dogaki et al. 2016 [52]; Hosokawa et al. 2015 [53]), and Netherlands (Rutgers et al. 2019 [54]).
As for the effects on human articular chondrocytes, PTH (1–34) influenced its differentiation and regeneration. Tsukazaki et al. concluded that PTHrP was an important autocrine and paracrine factor for chondrocyte metabolism as for cell growth and differentiation [51]. Rutgers et al. suggested that PTH (l–34) inhibited healthy human articular chondrocyte regeneration but did not influence hypertrophic differentiation [54]. Chang et al. concluded that PTH (l–34) could reverse the terminal differentiation of chondrocytes without affecting normal chondrocytes, while PTHrP prevented the chondrocyte degeneration initiated by dexamethasone [25]. Moreover, Chang et al. held that PTH (1–34) treated early OA without affecting normal chondrocytes [55]. When PTH (1–34) was applied for RA or OA chondrocytes treatment, the survival and inflammatory cytokines would be affected. Petersson et al. found that PTH (1–34) increased the proliferation of chondrocytes from human and RA patients [49]. However, Funk et al. revealed that the PTHrP could be examined in synovium and synoviocytes obtained from RA patients, which help to clarify the pathogenesis of RA to a certain extent and remain to be investigated further [48]. In addition, Lugo et al. found that PTH (1–34) ameliorated OA by improving SCB integrity, inhibiting cartilage degradation, and exerting effects on synovial changes [38]. PTH (1–34) held potential therapeutic option for synoviopathy associated with OA.
PTH (1–34) protected MSC with various effects. Shao et al. maintained that PTH (1–34) worked on MSC by increasing the migration, proliferation, ECM formation, and inhibiting proinflammatory cytokines [56]. Mwale et al. argued that PTH (1–34) helped to prevent precocious MSC hypertrophy [47]. Music et al. believed that PTH (1–34) suppressed MSC hypertrophic [50]. Dogaki et al. implied that PTH (1–34) may not have a positive effect at the fracture site because no positive effect was noticed when the fracture haematoma-derived progenitor cells were treated with PTH (1–34) [52]. Hosokawa et al. indicated that PTH (1–34) could reset the circadian rhythm of ATDC5 cells, which is expected to be useful to assess the molecular mechanisms of PTH (1–34) on chondrogenic differentiation [53]. PTH (1–34) played a significant role in chondrocytes through affecting the proliferation and ECM synthesis.
Quality assessment of included studies
Methodological quality was assessed for all 33 involved studies (Fig. 3). An unclear risk of selection bias (because of lacking data regarding randomization method: n = 16); detection bias (blinding of outcome assessment, n = 20); performance bias (because of absent data about blinding of subjects, n = 11), attrition bias (n = 17), reporting bias (n = 21), and other bias (n = 15) were found.
Discussion
To our knowledge, this is the first SR evaluating the existing papers about the effect of PTH (1–34) on OA regarding in vivo and in vitro investigations. The chondro-protective and cartilage-regenerative effects were reviewed, indicating that PTH (1–34) might be a potential preventative and therapeutic treatment for OA.
OA is the most prevalent degenerative joint disease with complicated pathogenesis characterized with damage to cartilage, narrow synovial cavity, invasion of the SCB, formation of osteophytes, and synovitis [57]. OP is a metabolic bone disease with decreased bone strength but increased fracture risk. OP and OA are common clinical conditions with high prevalence among older adults. Antiresorptive agents exhibited effects on bone mineralization and cartilage degradation for OA or OPOA [58]. However, treatments with polypharmacy for OA are limited to pain relief with less effective, which should be individualized to reduce the risk of side effects [59]. And therefore, DMOADs are highly demanded for OA or OPOA.
Quantitative studies indicated that PTH (1–34) played a significant role in calcium metabolism with an anabolic effect in the treatment of OP, fracture healing, non-union and stress fracture, augmentation of implant fixation, and chondro-protection in OA [14, 60]. In addition, PTH (1–34) could be a systemic pharmacology for OA by influencing cartilage quality such as ECM and chondrocyte contents [61]. The effects of PTH (1–34) were involved in decreasing COLX or RUNX2 but increasing AGC [34], which not only inhibited matrix metallopeptidase 13 (MMP13) or ADAM metallopeptidase with thrombospondin type 1 motif 4 (ADAMTS4), but also enhanced COLII and AGC [26, 42]. Moreover, PTH (1–34) reversed terminal differentiation towards hypertrophy and decreased apoptosis of chondrocytes [46, 47].
The anabolic effects of PTH (1–34) on both cartilage and SCB were explained by multiple mechanisms (Fig. 4). The activation of NF-κB elevated inflammatory mediators of IL-1β, TNF-α, cyclooxygenase-2 (COX2), and inducible nitric oxide synthase (iNOS), which resulted in the initiation of OA and regulated the levels of MMP13 [62]. It is well established that the parathyroid 1 receptor (PTH1R) was a key regulator to induce differentiation and endochondral ossification by inducing ECM synthesis, suppressing maturation, and inhibiting degeneration [20]. PTH (1–34) elevated the expression of PTH1R, osteoprotegerin (OPG), and receptor activator of NF-κB ligand (RANKL) via the OPG/RANKL/RANK signaling pathway [26]. The Notch pathway was activated by PTH (1–34) with increased expression of JAGGED1 [63]. The expression of TNF-α was inhabited by PTH (1–34) via the PKA signaling pathway [29]. PTH (1–34) inhibited chondrocyte differentiation towards hypertrophy via the p38 and the p-AKT signaling pathway [47]. PTH (1–34) downregulated JAK2/STAT3 and Wnt5A/ROR2 [19] but upregulated the Wnt/β-catenin through an alternative signaling pathway [64].
In addition, the attenuation of signaling pathways including oxidative stress and apoptosis had an indispensable role in OA. Autophagy was a protective mechanism in normal cartilage. PTH (1–34) alleviated OA progression by reducing terminal differentiation, reducing apoptosis, and increasing autophagy via the mechanistic target of rapamycin (mTOR) and p62 [21]. Apoptosis was reversed, while both Bcl-2 and Bax were upregulated by PTH (1–34). Moreover, PTH (1–34) might reduce the accumulation of senescent cells by inhibiting p16 [28]. Both the sustained and intermittent action of PTH (1–34) suppressed OA effectively [22, 65]. IA application would directly affect the cartilage rather than the SCB or metaphyseal bone [43]. PTH (1–34) inhibited the terminal differentiation of human chondrocytes in vitro and inhibits OA progression in rats in vivo [25]. PTHrP was up-regulated and mediated by calcium-sensing receptor in OA cartilage, which might promote both proliferation of chondrocyte and osteophyte formation [66]. Stimulation of focal osteochondral defect, enhancement of allograft bone union, and differentiation of MSCs are various effects of PTH (1–34) in tissue engineering [32, 67].
An ideal DMOAD can not only repair and regenerate cartilage, but also alleviate inflammation of synovium and pain. Healthy synovial joints are capable of maintaining extraordinary lubrication, attributed to structures as well as the cellular constitutions. However, both synovitis and OP contributed to cartilage degradation [68] but all pathology above could be suppressed by PTH (1–34) [69]. Impairment of SCB aggravated cartilage damage in early OPOA rabbits [39] and is associated with weight-bearing pain [70]. Overall, PTH (1–34) exhibited protective effects on the change of synovitis as well as pain relief.
Clinically, resorption played a significant role while PTH (1–34) was a reasonable option for OP patients [71]. Successful osteoanabolic treatment with PTH (1–34) benefited symptomatic stress concentration with completely stem tip pain-free [72]. The periprosthetic BMD was preserved after total hip arthroplasty (THA) [73] while bone ingrowth was promoted after total knee arthroplasty (TKA) [74] enforced by PTH (1–34). In addition, nonunion of periprosthetic fracture after TKA benefited from PTH (1–34) as well [75]. However, early mineralization of the MCC caused by PTH (1–34) might shift modifications of the subarticular spongiosa. Overall, we had better use the PTH (1–34) in proper situations and dosages.
There are some limitations in our current review. Firstly, the present review cannot identify the mechanisms accounting for the precious mechanism of PTH (1–34) on OA. Further research evidence is needed to deepen our current review. Secondly, although a thorough search was performed from five English databases, some pertinent studies may still have been missed. Thirdly, limited information in the current reviewed investigations is an urgent call for subsequent studies to confirm the findings based on additional information. Finally, there are only included studies published in English; thereby, some studies in other languages would be missed out.
Conclusion
In conclusion, the SR, which included both in vivo and in vitro studies, described the beneficial effects of PTH (1–34) on OA via alleviating cartilage damage progression, inhibiting the abnormal SCB remodeling, suppressing synovitis, reducing oxidative stress or apoptosis of chondrocytes, and elevating autophagy. Some of the OA or OAOP patients might benefit from PTH (1–34) as well. The present SR is a description of existing studies regarding the effectiveness of PTH (1–34) administration in OA together with mechanisms, which suggested the necessity for further clinical trials and animal investigations to achieve concise conclusions about the effects of PTH (1–34) on OA.
Availability of data and materials
Data are available from the corresponding authors upon reasonable request with the permission of Department of Bone and Joint Surgery in Peking University Shenzhen Hospital.
Abbreviations
- PTH (1–34):
-
Teriparatide
- OA:
-
Osteoarthritis
- SR:
-
Systematic review
- SCB:
-
Subchondral bone
- ECM:
-
Extracellular matrix
- COL II:
-
Type II collagen
- AGC:
-
Aggrecan
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- DMOADs:
-
Disease-modifying OA drugs
- OP:
-
Osteoporosis
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- PROSPERO:
-
Prospective Register of Systematic Reviews
- MeSH:
-
Medical Subject Headings
- CRIS:
-
Checklist for Reporting In-vitro Studies
- CIOA:
-
Collagenase-induced osteoarthritis
- OPOA:
-
Osteoporotic osteoarthritis
- OVX:
-
Ovariectomized
- ACLT:
-
Anterior cruciate ligament transection
- MLI:
-
Meniscal ligamentous injury
- MCC:
-
Mandibular condylar cartilage
- DMM:
-
Destabilization of the medial meniscus
- SD:
-
Sprague–Dawley
- nM:
-
Nmol/L
- PIOA:
-
Papain-induced osteoarthritis
- MSCs:
-
Mesenchymal stem cells
- M:
-
mol/L
- RA:
-
Rheumatoid arthritis
- PTHrP:
-
Parathyroid hormone-related protein
- COLX:
-
Type X collagen
- MMP13:
-
Matrix metallopeptidase 13
- ADAMTS4:
-
ADAM Metallopeptidase With Thrombospondin Type 1 Motif 4
- PTH1R:
-
Parathyroid 1 receptor
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor activator of NF-κB ligand
- COX2:
-
Cyclooxygenase-2
- iNOS:
-
Inducible nitric oxide synthase
- MAPK:
-
Mitogen-activated protein kinase
- mTOR:
-
Target of rapamycin
- THA:
-
Total hip arthroplasty
- TKA:
-
Total knee arthroplasty
- SC:
-
Subcutaneous injection
- IA:
-
Intra-articular
- PFJOA:
-
Patellofemoral joint osteoarthritis
- pM:
-
pmol/L
- μM:
-
μmol/L
References
Zheng S, Tu L, Cicuttini F, Zhu Z, Han W, Antony B, Wluka AE, Winzenberg T, Aitken D, Blizzard L, et al. Depression in patients with knee osteoarthritis: risk factors and associations with joint symptoms. BMC Musculoskelet Disord. 2021;22(1):40.
Wood TJ, Gazendam AM, Kabali CB: Postoperative outcomes following total hip and knee arthroplasty in patients with pain catastrophizing, anxiety, or depression. The Journal of arthroplasty 2021.
Petersen WP Jr, Teo GM, Friedlander S, Schwarzkopf R, Long WJ. The implications of aging population demographics on the delivery of primary total joint arthroplasty in a bundled payment system. The Journal of bone and joint surgery American. 2020;102(19):1679–86.
Xin PL, Jie LF, Cheng Q, Bin DY, Dan CW. Pathogenesis and function of interleukin-35 in rheumatoid arthritis. Front Pharmacol. 2021;12: 655114.
Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clinical immunology (Orlando, Fla). 2013;146(3):185–96.
2011 Osteoarthritis Research Society International World Congress, OARSI. Osteoarthritis and cartilage 2011, 19.
Cai X, Yuan S, Zeng Y, Wang C, Yu N, Ding C. New trends in pharmacological treatments for osteoarthritis. Front Pharmacol. 2021;12: 645842.
Zhang Z, Huang C, Jiang Q, Zheng Y, Liu Y, Liu S, Chen Y, Mei Y, Ding C, Chen M et al: Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition). Annals of translational medicine 2020, 8(19):1213.
Porwal K, Pal S, Bhagwati S, Siddiqi MI, Chattopadhyay N: Therapeutic potential of phosphodiesterase inhibitors in the treatment of osteoporosis: Scopes for therapeutic repurposing and discovery of new oral osteoanabolic drugs. European journal of pharmacology 2021, 899.
Ueda K, Yamanaka Y, Harada D, Yamagami E, Tanaka H, Seino Y. PTH has the potential to rescue disturbed bone growth in achondroplasia. Bone. 2007;41(1):13–8.
Tile L, Bleakney R, Tomlinson G, Khan A, Lau ANC, Ridout R, Chang J, Lakhesar J, Scher J, Hu H, et al. Teriparatide for the healing of incomplete atypical femur fractures: The TAFF Trial. J Bone Miner Res. 2020;35(SUPPL 1):23.
Rajagopal K, Ramesh S, Madhuri V: Early addition of parathyroid hormone-related peptide regulates the hypertrophic differentiation of mesenchymal stem cells. Cartilage 2021, 13(2_suppl):143s-152s.
Sondergaard BC, Klausen IJ, Sims NA, Nielsen RH, Gooi JH, Karsdal MA, Bay-Jensen AC. Osteoarthritic articular cartilage expresses the PTH receptor; PTH effects cartilage metabolism. Osteoarthritis Cartilage. 2011;19:S122–3.
Sain A, Bansal H, Pattabiraman K, Sharma V. Present and future scope of recombinant parathyroid hormone therapy in orthopaedics. Journal of clinical orthopaedics and trauma. 2021;17:54–8.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43.
Krithikadatta J, Gopikrishna V, Datta M. CRIS Guidelines (Checklist for Reporting In-vitro Studies): a concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. Journal of conservative dentistry : JCD. 2014;17(4):301–4.
Shao LT, Gou Y, Fang JK, Hu YP, Lian QQ, Zhang YY, Wang YD, Tian FM, Zhang L. Parathyroid hormone (1–34) ameliorates cartilage degeneration and subchondral bone deterioration in collagenase-induced osteoarthritis model in mice. Bone Joint Res. 2020;9(10):675–88.
Shao LT, Gou Y, Fang JK, Hu YP, Lian QQ, Yang Z, Zhang YY, Wang YD, Tian FM, Zhang L. The protective effects of parathyroid hormone (1–34) on cartilage and subchondral bone through down-regulating JAK2/STAT3 and WNT5A/ROR2 in a collagenase-induced osteoarthritis mouse model. Orthop Surg. 2021;13(5):1662–72.
Chen CH, Kang L, Chang LH, Cheng TL, Lin SY, Wu SC, Lin YS, Chuang SC, Lee TC, Chang JK, et al. Intra-articular low-dose parathyroid hormone (1–34) improves mobility and articular cartilage quality in a preclinical age-related knee osteoarthritis model. Bone & Joint Research. 2021;10(8):514–25.
Chen CH, Ho ML, Chang LH, Kang L, Lin YS, Lin SY, Wu SC, Chang JK: Parathyroid hormone-(1–34) ameliorated knee osteoarthritis in rats via autophagy. Journal of applied physiology (Bethesda, Md : 1985) 2018, 124(5):1177–1185.
Eswaramoorthy R, Chang C-C, Wu S-C, Wang G-J, Chang J-K, Ho M-L. Sustained release of PTH(1–34) from PLGA microspheres suppresses osteoarthritis progression in rats. Acta Biomater. 2012;8(6):2254–62.
Ma L, Wu J, Jin QH. The association between parathyroid hormone 1–34 and the Wnt/beta-catenin signaling pathway in a rat model of osteoarthritis. Mol Med Rep. 2017;16(6):8799–807.
Zhang C, Zhu J, Jia J, Guan Z, Sun T, Zhang W, Yuan W, Wang H, Leng H, Song C. Effect of single versus multiple fractures on systemic bone loss in mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2021;36(3):567–78.
Chang JK, Chang LH, Hung SH, Wu SC, Lee HY, Lin YS, Chen CH, Fu YC, Wang GJ, Ho ML. Parathyroid hormone 1–34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009;60(10):3049–60.
Yan JY, Tian FM, Wang WY, Cheng Y, Song HP, Zhang YZ, Zhang L. Parathyroid hormone (1–34) prevents cartilage degradation and preserves subchondral bone micro-architecture in guinea pigs with spontaneous osteoarthritis. Osteoarthritis Cartilage. 2014;22(11):1869–77.
Dai MW, Chu JG, Tian FM, Song HP, Wang Y, Zhang YZ, Zhang L. Parathyroid hormone(1–34) exhibits more comprehensive effects than celecoxib in cartilage metabolism and maintaining subchondral bone micro-architecture in meniscectomized guinea pigs. Osteoarthritis Cartilage. 2016;24(6):1103–12.
Cui C, Zheng L, Fan Y, Zhang J, Xu R, Xie J, Zhou X. Parathyroid hormone ameliorates temporomandibular joint osteoarthritic-like changes related to age. Cell Prolif. 2020;53(4): e12755.
He YJ, Liang X, Zhang XX, Li SS, Sun Y, Li TF. PTH1-34 inhibited TNF-α expression and antagonized TNF-α-induced MMP13 expression in MIO mice. Int Immunopharmacol. 2021;91: 107191.
Longo UG, Loppini M, Romeo G, Maffulli N, Denaro V. Histological scoring systems for tissue-engineered, ex vivo and degenerative meniscus. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(7):1569–76.
Orth P, Cucchiarini M, Wagenpfeil S, Menger MD, Madry H. PTH [1-34]-induced alterations of the subchondral bone provoke early osteoarthritis. Osteoarthritis Cartilage. 2014;22(6):813–21.
Orth P, Cucchiarini M, Zurakowski D, Menger MD, Kohn DM, Madry H. Parathyroid hormone [1-34] improves articular cartilage surface architecture and integration and subchondral bone reconstitution in osteochondral defects in vivo. Osteoarthritis Cartilage. 2013;21(4):614–24.
Dutra EH, O’Brien MH, Gutierrez T, Lima A, Nanda R, Yadav S. PTH [1-34]-induced alterations predispose the mandibular condylar cartilage to mineralization. Orthod Craniofac Res. 2017;20(Suppl 1):162–6.
Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, Bukata SV, O'Keefe RJ, Awad H, Puzas JE et al: Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Science translational medicine 2011, 3(101).
O' Brien MH, Dutra EH, Lima A, Nanda R, Yadav S: PTH 1–34 induced differentiation and mineralization of mandibular condylar cartilage. Scientific reports 2017, 7.
Bagi CM, Berryman E, Zakur DE, Wilkie D, Andresen CJ: Effect of antiresorptive and anabolic bone therapy on development of osteoarthritis in a posttraumatic rat model of OA. Arthritis research & therapy 2015, 17.
Antunes BP, Vainieri ML, Alini M, Monsonego-Ornan E, Grad S, Yayon A. Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in Fibrin-Hyaluronan hydrogel by multi-axial mechanical loading and FGF18. Acta Biomater. 2020;105:170–9.
Lugo L, Villalvilla A, Gómez R, Bellido M, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G, Roman-Blas JA. Effects of PTH [1-34] on synoviopathy in an experimental model of osteoarthritis preceded by osteoporosis. Osteoarthritis Cartilage. 2012;20(12):1619–30.
Bellido M, Lugo L, Roman-Blas JA, Castañeda S, Calvo E, Largo R, Herrero-Beaumont G. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthritis Cartilage. 2011;19(10):1228–36.
Shao L-T, Gou Y, Fang J-K, Hu Y-P, Lian Q-Q, Zhang Y-Y, Wang Y-D, Tian F-M, Zhang L. Parathyroid hormone (1–34) ameliorates cartilage degeneration and subchondral bone deterioration in collagenase-induced osteoarthritis model in mice. Bone & joint research. 2020;9(10):675–88.
Zhang W, Chen J, Tao J, Hu C, Chen L, Zhao H, Xu G, Heng BC, Ouyang HW. The promotion of osteochondral repair by combined intra-articular injection of parathyroid hormone-related protein and implantation of a bi-layer collagen-silk scaffold. Biomaterials. 2013;34(25):6046–57.
Zhang H, Bei M, Zheng Z, Liu N, Cao X, Xiao Y, Lian Q, Wang Y, Hou X, Tian F. Parathyroid hormone (1–34) attenuates cartilage degradation and preserves subchondral bone micro-architecture in rats with patella baja-induced-patellofemoral joint osteoarthritis. Calcif Tissue Int. 2022;111(1):87–95.
Chen CH, Kang L, Chang LH, Cheng TL, Lin SY, Wu SC, Lin YS, Chuang SC, Lee TC, Chang JK, et al. Intra-articular low-dose parathyroid hormone (1–34) improves mobility and articular cartilage quality in a preclinical age-related knee osteoarthritis model. Bone Joint Res. 2021;10(8):514–25.
Shan R, Liu N, Yan Y, Liu B. Apoptosis, autophagy and atherosclerosis: relationships and the role of Hsp27. Pharmacol Res. 2021;166: 105169.
Liu Y, Zhang L, Hu N, Shao J, Yang D, Ruan C, Huang S, Wang L, Lu WW, Zhang X, et al. An optogenetic approach for regulating human parathyroid hormone secretion. Nat Commun. 2022;13(1):771.
Chang LH, Wu SC, Chen CH, Wang GJ, Chang JK, Ho ML. Parathyroid hormone 1–34 reduces dexamethasone-induced terminal differentiation in human articular chondrocytes. Toxicology. 2016;368–369:116–28.
Mwale F, Yao G, Ouellet JA, Petit A, Antoniou J. Effect of Parathyroid Hormone on Type X and Type II Collagen Expression in Mesenchymal Stem Cells from Osteoarthritic Patients. Tissue Eng Part A. 2010;16(11):3449–55.
Funk JL, Cordaro LA, Wei H, Benjamin JB, Yocum DE: Synovium as a source of increased amino-terminal parathyroid hormone-related protein expression in rheumatoid arthritis. A possible role for locally produced parathyroid hormone-related protein in the pathogenesis of rheumatoid arthritis. The Journal of clinical investigation 1998, 101(7):1362–1371.
Petersson M, Bucht E, Granberg B, Stark A. Effects of arginine-vasopressin and parathyrold hormone-related protein (1–34) on cell proliferation and production of YKL-40 in cultured chondrocytes from patients with rheumatoid arthritis and osteoarthritis. Osteoarthritis Cartilage. 2006;14(7):652–9.
Music E, Futrega K, Palmer JS, Kinney M, Lott B, Klein TJ, Doran MR. Intermittent parathyroid hormone (1–34) supplementation of bone marrow stromal cell cultures may inhibit hypertrophy, but at the expense of chondrogenesis. Stem Cell Res Ther. 2020;11(1):321.
Tsukazaki T, Ohtsuru A, Namba H, Oda J, Motomura K, Osaki M, Kiriyama T, Iwasaki K, Yamashita S. Parathyroid hormone-related protein (PTHrP) action in rat articular chondrocytes: Comparison of PTH(1–34), PTHrP(1–34), PTHrP(1–141), PTHrP(100–114) and antisense oligonucleotides against PTHrP. J Endocrinol. 1996;150(3):359–68.
Dogaki Y, Lee SY, Niikura T, Koga T, Okumachi E, Nishida K, Kuroda R, Kurosaka M. Effects of parathyroid hormone 1–34 on osteogenic and chondrogenic differentiation of human fracture haematoma-derived cells in vitro. J Tissue Eng Regen Med. 2016;10(10):E365–71.
Hosokawa T, Tsuchiya Y, Okubo N, Kunimoto T, Minami Y, Fujiwara H, Umemura Y, Koike N, Kubo T, Yagita K. Robust circadian rhythm and parathyroid hormone-induced resetting during hypertrophic differentiation in ATDC5 chondroprogenitor cells. Acta Histochem Cytochem. 2015;48(6):165–71.
Rutgers M, Bach F, Vonk L, van Rijen M, Akrum V, van Boxtel A, Dhert W, Creemers L: PTH decreases in vitro human cartilage regeneration without affecting hypertrophic differentiation. PloS one 2019, 14(4).
Chang LH, Wu SC, Chen CH, Wang GJ, Chang JK, Ho ML. Parathyroid hormone 1–34 reduces dexamethasone-induced terminal differentiation in human articular chondrocytes. Toxicology. 2016;368:116–28.
Shao LT, Luo L, Qiu JH, Deng DYB. PTH (1–34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res Ther. 2022;24(1):96.
Reginster JY. Pharmacological management : osteoporosis and osteoarthritis, similarities and differences. Osteoporos Int. 2013;24(1 SUPPL. 1):S75–6.
Li SS, He SH, Xie PY, Li W, Zhang XX, Li TF, Li DF. Recent progresses in the treatment of osteoporosis. Front Pharmacol. 2021;12: 717065.
Apostu D, Lucaciu O, Mester A, Oltean-Dan D, Baciut M, Baciut G, Bran S, Onisor F, Piciu A, Pasca RD, et al. Systemic drugs with impact on osteoarthritis. Drug Metab Rev. 2019;51(4):498–523.
Zhang C, Song C. Combination therapy of PTH and antiresorptive drugs on osteoporosis: a review of treatment alternatives. Front Pharmacol. 2020;11: 607017.
Siebuhr AS, Gudmann NS, Musa K, Kehlet S, Hansen G, Byrjalsen I, Andersen J, Bihlet A, Christiansen C, Karsdal MA, et al. PTH stimulates cartilage formation in low turnover patients-a possible systemic anabolic treatment for OA? Ann Rheum Dis. 2015;74:919.
Chang LH, Chen CH, Wu SC, Chang JK, Ho ML. Cyclooxygenase-2 regulates PTHrP transcription in human articular chondrocytes and is involved in the pathophysiology of osteoarthritis in rats. Journal of orthopaedic translation. 2021;30:16–30.
Sampson ER, Hilton MJ, Mooney RA, Awad H, Rosier RN, Zuscik MJ. TeriparatiDe as a chondro-regenerative therapy for injury-induced knee osteoarthritis. Osteoarthritis Cartilage. 2011;19:S227.
Ma L, Wu J, Jin QH. The association between parathyroid hormone 1–34 and the Wnt/β-catenin signaling pathway in a rat model of osteoarthritis. Mol Med Rep. 2017;16(6):8799–807.
Ho M, Eswaramoorthy R, Wu S, Wang G, Chang J, Fu Y, Tzeng C, Chen H, Wang Y: Controlled release of parathyroid hormone (1–34) in a subject (human) suffering from osteoarthritis, comprises administering a hydrogel of crosslinked methacrylated-hyaluronic acid and the hormone (1–34) to joint e.g. knee joint of subject. In.: Univ Kaohsiung Medical.
Burton DW, Foster M, Johnson KA, Hiramoto M, Deftos LJ, Terkeltaub R. Chondrocyte calcium-sensing receptor expression is up-regulated in early guinea pig knee osteoarthritis and modulates PTHrP, MMP-13, and TIMP-3 expression. Osteoarthritis Cartilage. 2005;13(5):395–404.
Nishikawa M, Kaneshiro S, Takami K, Owaki H, Fuji T. Bone stock reconstruction for huge bone loss using allograft-bones, bone marrow, and teriparatide in an infected total knee arthroplasty. Journal of clinical orthopaedics and trauma. 2019;10(2):329–33.
Lugo L, Villalvilla A, Gomez R, Bellido M, Sanchez-Pernaute O, Largo R, Herrero-Beaumont G, Roman-Blas JA. Effects of PTH 1–34 on synoviopathy in an experimental model of osteoarthritis preceded by osteoporosis. Osteoarthritis Cartilage. 2012;20(12):1619–30.
Li TF, Liu S, Han L, Liu L. PTH signaling prevents osteoarthritis formation through suppressing synovitis. Int J Rheum Dis. 2016;19:136.
Aso K, Izumi M, Ikeuchi M: Effect of teriparatide on subchondral bone lesions and pain in mono-iodoacetate-induced osteoarthritis rat. Journal of Orthopaedic Research 2017, 35.
Ledin H, Good L, Johansson T, Aspenberg P. No effect of teriparatide on migration in total knee replacement: a randomized controlled trial involving 50 patients. Acta Orthop. 2017;88(3):259–62.
Mazzucchelli RA, Meier C, Wahl P. Successful osteoanabolic treatment with Teriparatide for symptomatic stress concentration at the tip of a tapered, fluted, uncemented hip arthroplasty stem: a case report (9761). Swiss Med Wkly. 2021;151(SUPPL 250):49S.
Kobayashi N, Inaba Y, Uchiyama M, Ike H, Kubota S, Saito T. Teriparatide versus alendronate for the preservation of bone mineral density after total hip arthroplasty - a randomized controlled trial. J Arthroplasty. 2016;31(1):333–8.
Kaneko T, Otani T, Kono N, Mochizuki Y, Mori T, Nango N, Ikegami H, Musha Y. Weekly injection of teriparatide for bone ingrowth after cementless total knee arthroplasty. J Orthop Surg (Hong Kong). 2016;24(1):16–21.
Ochi K, Ikari K, Naomi A, Momohara S. Administration of teriparatide treatment for a challenging case of nonunion of periprosthetic fracture after total knee arthroplasty. Arch Osteoporos. 2013;8:159.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript but do not meet the criteria for authorship are named in the Acknowledgements. We especially thank professor Yingqi Chen, Anjaneyulu Udduttula, Xiaoyan Huang, Jianchen, and Canhui Cao, who offered their kind assistance in writing, editing, reviewing, technical help, and general support in methodology.
Funding
This study was supported by grants from National Natural Science Foundation of China (No. 82172432), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515012586), Shenzhen Key Medical Subject (No. SZXK023), Shenzhen “San-Ming” Project of Medicine (No. SZSM201612092), and the Scientific Research Foundation of Peking University Shenzhen Hospital (No. KYQD2021099).
Author information
Authors and Affiliations
Contributions
GL: investigation, methodology, data curation, formal analysis, writing—original draft, writing—review, editing. SL: investigation, methodology. HX: methodology, writing—review, editing. YC: investigation, methodology. JD: investigation, methodology. AX: methodology. DW: methodology. JW: investigation, review. FY: investigation, review. LG: investigation, review. CD: investigation, review. HZ: investigation, conceptualization, supervision, funding acquisition, resources, review, editing. The authors read and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search strategy
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, G., Liu, S., Xu, H. et al. Potential effects of teriparatide (PTH (1–34)) on osteoarthritis: a systematic review. Arthritis Res Ther 25, 3 (2023). https://doi.org/10.1186/s13075-022-02981-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02981-w