Abstract
Background
The effects of bone marrow mesenchymal stem cells (BMSCs) during the treatment of cartilage damage have been proven to be attributed to paracrine mechanisms, particularly the effect of exosomes. Exosomes from different batches are inhomogeneous, and different treatment effects are observed between samples. The purpose of this research was to find more effective and homogeneous exosomes for the repair of chondrocytes in osteoarthritis (OA). We observed the potential effects and possible mechanisms of exosomes derived from parathyroid hormone (PTH) (1-34)-preconditioned BMSCs (ExoPTH) in the alleviation of OA.
Materials and methods
Exosomes derived from BMSCs (ExoBMSC) and ExoPTH were isolated by differential centrifugation. Primary rat chondrocytes were used to establish the OA model by interleukin 1 beta (IL-1β) in vitro. The effects of these two types of exosomes on OA chondrocyte proliferation, migration, apoptosis, and extracellular matrix formation were measured and compared. We observed changes in IL-2, TNF-α, and IL-6 levels via Western blotting (WB), and quantitative real-time PCR (qRT–PCR).
Results
We successfully extracted ExoBMSC and ExoPTH and established an IL-1β-induced OA model in primary chondrocytes from rats. Our study showed that IL-2, TNF-α, and IL-6 levels increased significantly in OA chondrocytes; however, both ExoBMSC and ExoPTH reduced the levels of IL-2, TNF-α, and IL-6. In addition, ExoPTH exhibited stronger anti-inflammatory effects. ExoPTH had a more marked effect on proliferation, migration, and production of the extracellular matrix (Col-II) in OA chondrocytes than ExoBMSC at 24 h.
Conclusion
ExoPTH increased the migration, proliferation, and chondral matrix formation of OA chondrocytes in vitro. In OA chondrocyte therapy, the potential mechanism of ExoPTH might involve the inhibition of production of proinflammatory cytokines. Although the two types of exosomes had some similar effects, most effects of ExoPTH were better than those of ExoBMSC, so ExoPTH may have a better ability to alleviate OA.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is the most prevalent disease and the leading cause of physical disability [1]. OA affects approximately 265 million people worldwide, and the prevalence of OA is rising due to population aging and the increasing prevalence of obesity [2]. Articular cartilage consists of chondrocytes and extracellular matrix components, which have limited self-regenerative capacity due to the lack of a vasculature and neurons [3]. Currently, there are no effective treatments for patients with OA.
In recent years, bone marrow mesenchymal stem cells (BMSCs) have been a widely applied and effective method for regenerating damaged cartilage [4,5]. However, many challenges are persistent, such as the sources and variation in the quality of BMSCs, complex transplant surgery, and tumor formation [6]. In addition, cell state influence such effects of treatment [7]. Hence, exploration of noncellular agents is urgently needed to protect chondrocytes and delay the progression of OA.
Exosomes have attracted great interest because of their potential value in OA therapy. BMSCs play an important role in the inflammatory response through paracrine mechanisms [4], particularly the effect of exosomes. Exosomes derived from BMSCs (ExoBMSC) contain large amounts of active substances, such as DNA, RNA, and proteins [8,9]. Compared with BMSCs therapy, treatment with ExoBMSC exhibits more advantages, including targeted delivery, excellent tissue penetration, outstanding long-term cycling performance, better homing capacity, stable chemical properties, and a substantial capacity for tissue repair and regeneration [10,11,12,13]. Recent studies have demonstrated that OA progression can be ameliorated by ExoBMSC promoting cartilage repair in vivo and vitro [5,7,10]. ExoBMSC protect cartilage by promoting chondrocyte proliferation and maintaining expression of the cartilage matrix [14].
However, the application of ExoBMSC does result in some unwanted reactions [15]. During cartilage regeneration, ExoBMSC can lead to opposing effects, including hypertrophic differentiation and subsequent calcification [7]. Therefore, the identification of more effective and homogeneous exosomes to repair OA chondrocyte lesions is very important. It was reported that parathyroid hormone (PTH) (1-34) enhances the therapeutic effect of BMSCs in cartilage repair [16]. It was previously reported that BMSCs influenced chondrocytes primarily via paracrine mechanisms, particularly exosomes. Therefore, it was also speculated that PTH (1-34) could stimulate BMSCs to produce potent exosomes to regulate chondrocyte metabolism and proliferation.
Thus, we investigated whether exosomes derived from PTH-preconditioned BMSCs (ExoPTH) influenced OA-associated chondrocyte damage in vitro.
Materials and methods
Characterization of BMSCs and chondrocytes
BMSCs were purchased from Shanghai GuanDao Biological Engineering Co., Ltd. Using flow cytometry, we analyzed BMSC-specific surface markers (CD44, CD90, and CD45) [17]. Briefly, the cell concentration (one million cells/mL) was determined by a cell counting instrument (CountStar®). Then, the cells were incubated with 1% bovine serum albumin (BSA; Gibco) for 30 min. Next, antibodies (CD44, CD90, and CD45) were added to the samples. After incubation for 30 min, the samples were centrifuged at 1000×g. The precipitates were washed in PBS and repeated three times. Finally, the samples were identified by flow cytometry. In addition, We also performed the STR testing. Chondrocytes were purchased from Wuhan Procell Life Sci&Tech Co., Ltd. Chondrocytes were confirmed by immunofluorescence (IF) staining for type II collagen (Col-II).
Extraction and identification of exosomes
First, BMSCs were pretreated with PTH(1-34) for 6 h, then the medium was replaced again, and the medium was collected after 48 h. To harvest exosomes, 200-ml cell culture media were collected. Cells debris was removed from the cell culture supernatant by centrifugation at 300g and 2000g, and each rotational speed was for 10 min. To remove non-exosome vesicles and any possible apoptotic bodies, the supernatants were then spun at 10,000g for 40min. Finally, exosomes were obtained at 120,000 g for 70 min. All centrifugations were done at 4 °C. Exosomes were used directly or stored at − 80 °C for follow-up experiments.
Exosomes were analyzed by an HT7700 transmission electron microscope(TEM) (HITACHI, Japan). Protein markers of exosomes were assessed by WB analysis. Antibodies against TSG101 and CD9 (abCAM, USA) were used. The size distribution was detected by nanoparticle tracking analysis (NTA) after dilution 100 times. Primary chondrocytes were treated with exosomes (10 μg/mL) for 24 h, as previously described [18,19].
IF staining assays
IF staining was performed, as it was previously reported with slight modifications [20]. In brief, chondrocytes were fixed in 4% paraformaldehyde and washed with PBS. Subsequently, the chondrocytes were treated with 0.5% Triton X-100, blocked with 1% bovine serum albumin (BSA) for 30 min, and incubated with primary antibodies overnight at 4 °C. Then, the cells were washed with PBS and incubated with secondary antibodies for 1 h at room temperature. Finally, nuclear counterstaining was performed using DAPI.
Western blot (WB) assays
WB assays were performed as previously reported with slight modifications [21]. Briefly, samples were lysed in cell lysis buffer, incubated on ice for 5 min, and scraped from the plates. Cell debris was removed by centrifugation. The protein concentrations were measured by a microplate reader. Protein was electrophoresed on a 10% gradient gel at 180 V for approximately 30 min. Then, the proteins were electrically transferred to PVDF membranes. After doing so, the membranes were blocked for 1 h with 5% skim milk at room temperature and subsequently incubated with specific primary antibodies overnight at 4 °C. Following 5×7 min washes in PBS, the membranes were blotted with secondary antibodies for approximately 1 h. The labeled proteins were analyzed with the LAS-3000 Luminescent Image Analyzer (FujiFilm, Tokyo, Japan).
Cell proliferation assay
EdU staining was measured with an EdU flow cytometry kit. Briefly, the cells were incubated, fixed, and permeabilized following the manufacturer’s protocol. After washing, the EdU-positive cells were detected by flow cytometry.
Cell proliferation was measured via a CCK-8 assay according to the manufacturer’s instructions. Briefly, chondrocytes were seeded onto 96-well plates (2×103 cells/well) and cultured at 37 °C. Chondrocytes were incubated for 4 h before CCK-8 reagents were instilled. Next, 10 μl of CCK-8 solution was added to the 96-well plates. The absorbance was measured with a microplate reader at 450 nm after incubation at 37°C for 1 h.
Cell migration assay
Cell migration was performed using the scratch assay. When the Cells filled 90–100% confluence in the wells, cells were treated with serum-free DMEM medium for 24 h and a linear scratch was made by a 100-μl sterile pipette tip. Finally, images were acquired using a microscope.
Apoptosis assay
For the apoptosis assays, 3×105cells were collected from each sample, rinsed with PBS, and resuspended in 200μl 1× Binding Buffer. After doing so, Annexin 5μl Annexin V-FITC and 5 μl Propidium Iodide (PI) solution was added for 10 min at room temperature. Finally, the cells were analyzed on a flow cytometer.
Cytokine antibody array
Cytokine expression in chondrocytes was detected using Quantibody® Rat Inflammation Array (RayBiotech, China) and quantified following the manufacturer’s instructions. Briefly, the cells were incubated with a standard protein cocktail, biotinylated antibody cocktail, and Cy3 equivalent dye labeled-streptavidin. Finally, scan and perform data extraction and analysis.
Total RNA extraction and quantitative real-time PCR (qRT-PCR)
In short, total RNA was extracted using RNA-Quick Purification Kit (Shanghai Yishan Biotechnology Co, Ltd, China). First-strand cDNA was synthesized using HiScript II One Step RT-PCR Kit (Tiangen, China). The primers used to amplify the indicated genes are listed in Supplementary Table 1. Finally, using ChamQ SYBR qPCR Master Mix (Tiangen, China) performed qRT-PCR.
Results
Characterization of BMSCs and chondrocytes
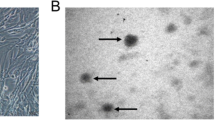
The BMSCs were observed by an inverted microscope (Figure Supplementary 1A). Most BMSCs were negative for CD45 and positive for CD44 and CD90 (Figures Supplementary 1B-D). These features were observed by flow cytometry analysis. STR genotyping was performed by Shanghai GuanDao Biological Engineering Co., Ltd. (Figure Supplementary 1E). Chondrocytes were purchased from Wuhan Procell Life SCI&TECH Co., Ltd. Chondrocytes were identified by Col-II (IF) (Figure Supplementary 1F).
Identification of ExoBMSC and ExoPTH
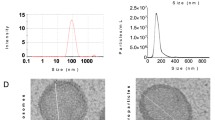
The ExoBMSC and ExoPTH particles were mostly in the 60 to 150 nm range (Fig. 1A), as indicated by NTA. For SEM observation, the results showed that ExoBMSC and ExoPTH exhibited a saucer or cup shape, which is a typical morphology for exosomes (Fig. 1B). The results of CD9 and TSG101 are specifically expressed by WB analysis in the exosomes (Fig. 1C). Exosomes labeled with Dil are shown in dotted red. We observed that the distribution of exosomes was in the cytosolic domain of the chondrocytes (Fig. 1D), indicating the successful uptake of ExoBMSC and ExoPTH by chondrocytes.
Characterization of ExoBMSC and ExoPTH. A. Size distribution of ExoBMSC and ExoPTH is indicated by nanoparticle tracking analysis. B Representative transmission electron microscopy images of ExoBMSC and ExoPTH (scale bar = 100 nm). C Positive staining for the exosome markers CD9 and TSG101 was observed for ExoBMSCs and ExoPTH, as determined by Western blotting. D Dil-labeled exosomes (red fluorescence) were used to observe exosome uptake by chondrocytes. ExoBMSC: Exosomes derived from BMSCs; ExoPTH: exosomes derived from PTH-preconditioned BMSCs; BMSCs: bone marrow mesenchymal stem cells
Selection of the concentration of IL-1β / PTH (1-34)
The migration ratio of chondrocytes was significantly inhibited by IL-1β at concentrations of 20 and 50 ng/ml. Compared with the control, 1 ng/ml group, and 5 ng/ml group, 20 ng/ml group significantly decreased (78.97%, 77.76%, and 72.71% respectively), and 50 ng/ml significantly decreased (84.21%, 97.02%, and 79.51% respectively). Compared with the control and 1 ng/ml groups, the migration ratio of the 10 ng/ml group was significantly decreased (76.35% and 74.99%). However, there was no difference between the 10, 20, and 50 ng/ml groups. Therefore, we chose 20 ng/ml as the concentration of IL-1β (Figure Supplementary 2A, D). Chondrocytes and BMSCs were cultured with various concentrations of PTH (1-34) and compared with the controls. The migration ratio of the PTH (1-34) 10 nM/ml group in chondrocytes and BMSCs was significantly higher than that in the other groups. Compared with the control, 1 nM/ml, 5 nM/ml, 20 nM/ml and 50 nM/ml groups, the migration ratio of the PTH (1-34) 10 nM/ml group in BMSCs was significantly increased (28.02%, 26.55%, 28.95%, 21.23%, and 54.88% respectively) (Figures Supplementary 2B, E). Compared with the control, 1 nM/ml, 5 nM/ml, 20 nM/ml and 50 nM/ml groups, the migration ratio of the PTH (1-34) 10 nM/ml group in chondrocytes was significantly increased (46.95%, 48.14%, 42.42%, 79.66%, and 111.72% respectively) (Figures Supplementary 2C, F).
The effects of ExoBMSC and ExoPTH on chondrocytes
We observed the cell viability of chondrocytes treated with ExoBMSC and ExoPTH via the scratch and CCK-8 assay in vitro. As shown in Figures Supplementary 3A-B, the scratch assay data showed that the migration ratio of chondrocytes was promoted by ExoBMSC and ExoPTH. Compared with the ExoBMSC and ExoPTH groups, the migration ratio of the control group significantly decreased (46.45% and 61.07%). Moreover, the ExoPTH treatment showed stronger promotion than the ExoBMSC treatment. Compared with the ExoBMSC group, the ExoPTH group significantly increased by 37.56%. The proliferation of chondrocytes is shown in Figure Supplementary 3C, after 48 h, both ExoBMSC and ExoPTH promoted chondrocyte proliferation, and ExoPTH showed stronger promotion than ExoBMSC.
The effects of ExoBMSC and ExoPTH on OA chondrocyte migration and proliferation
OA chondrocyte proliferation was assessed by flow cytometry (Fig. 2A). Compared with the ExoBMSC and ExoPTH groups, the rate of OA chondrocyte proliferation decreased significantly by 27.06% and 50.73%. Compared with the ExoBMSC group, the ExoPTH group significantly increased by 48.06%.
The effect of ExoBMSC and ExoPTH on OA chondrocyte proliferation was assayed by a flow cytometry, a scratch migration assay, and CCK-8 assay. A Flow cytometry was used to explore the cell proliferation of the OA, ExoBMSC, and ExoPTH groups by the EdU flow cytometry kit. B A scratch wound-healing migration assay was performed with control, OA, ExoBMSC, and ExoPTH treatments. C Statistical results of the scratch wound-healing migration assay. D The cell proliferation ability of the control, OA, ExoBMSC, and ExoPTH groups was detected by a CCK-8 assay. Data are presented as the mean ± SD, *p < 0.05 versus OA group and #p < 0.05 versus ExoBMSC group. Ctrl, control; OA, osteoarthritis; ExoBMSC, exosomes derived from BMSCs; ExoPTH, exosomes derived from PTH-preconditioned BMSCs; BMSCs, bone marrow mesenchymal stem cells
As shown in Fig. 2B, C, the migration ratio of chondrocytes was significantly inhibited in the OA group. Compared with the ExoBMSC and ExoPTH groups, the migration ratio of OA chondrocytes decreased significantly (59.52% and 70.82%). Moreover, the ExoPTH treatment was more effective than the ExoBMSC treatment. Compared with the ExoBMSC group, the ExoPTH group significantly increased by 39.96%.
We observed the viability of OA chondrocytes treated with ExoBMSC and ExoPTH via a CCK-8 assay (Fig. 2D). Both ExoBMSC and ExoPTH promoted OA chondrocyte proliferation after 24 h, 48 h, and 72 h. Moreover, ExoPTH was more effective than ExoBMSC.
The Ki67 index (percentage of Ki67-positive cells/total cells) was calculated (Fig. 3). Compared with the control, ExoBMSC, and ExoPTH groups, the percentage of Ki67-positive cells was significantly decreased in the OA group (70.44%, 30.03%, and 64.51%, respectively). The percentage of Ki67-positive cells is significantly increased by 97.17% in the ExoPTH group than in the ExoBMSC group.
The effect of ExoBMSC and ExoPTH on OA chondrocyte proliferation was assayed by immunofluorescence staining for ki67 and PCNA. A Immunofluorescence staining for ki67 and PCNA. B The positive expression ratio of ki67. C The positive expression ratio of PCNA. Data are presented as the mean ± SD, *p < 0.05 versus OA group and #p < 0.05 versus ExoBMSC group. Bars = 50 μm. Ctrl, control; OA, osteoarthritis; ExoBMSC, exosomes derived from BMSCs; ExoPTH, exosomes derived from PTH-preconditioned BMSCs; BMSCs, bone marrow mesenchymal stem cells
The PCNA index (percentage of PCNA-positive cells/total cells) was calculated (Fig. 3). Compared with the control, ExoBMSC, and ExoPTH groups, the percentage of PCNA-positive cells was significantly decreased in the OA group. Compared with the control, ExoBMSC, and ExoPTH groups, the percentage of PCNA-positive cells was significantly decreased in the OA group (74.34%, 40.46%, and 68.74%, respectively). The percentage of PCNA-positive cell was significantly lower in the ExoBMSC group than the ExoPTH group (ExoPTH group significantly increased by 47.50%)
The apoptotic effects of ExoBMSC and ExoPTH on OA chondrocytes
OA chondrocyte apoptosis was assessed by flow cytometry (Fig. 4A). The rate of OA chondrocyte apoptosis increased significantly in the ExoBMSC and ExoPTH groups, but no significant differences were detected in the ExoBMSC and ExoPTH groups. Compared with the ExoBMSC and ExoPTH groups, the rate of OA chondrocyte apoptosis increased significantly by 90.44% and 176.59%. However, there were no significant differences between the ExoPTH group and the ExoBMSC group.
The effect of ExoBMSC and ExoPTH on OA chondrocyte apoptosis was determined by flow cytometry and Western blotting. A Cells were processed by flow cytometry using Annexin V-FITC/PI staining. The percentage of the Annexin V-positive population indicates apoptosis induction in each group. B Cell lysates were prepared and analyzed by Western blotting for Bax and Caspase-3. β-actin was used as a loading control. Band intensities were quantified by IPP and normalized to β-actin. Data are presented as the mean ± SD, *p < 0.05 versus the OA group. Ctrl, control; OA, osteoarthritis; ExoBMSC, exosomes derived from BMSCs; ExoPTH, exosomes derived from PTH-preconditioned BMSCs; BMSCs, bone marrow mesenchymal stem cells
Conformational changes were analyzed by WB using Bax and Caspase-3 antibodies (Fig. 4B). In the OA group, Bax and Caspase-3 expression levels were significantly higher than those in the ExoPTH group and the ExoBMSC group (the ExoPTH group significantly decreased [78.98% and 89.78%]; the ExoBMSC group significantly decreased [87.08% and 89.39%]), but no significant differences were detected in the Bax and Caspase-3 in the ExoBMSC and ExoPTH groups.
The effects of ExoBMSC and ExoPTH on the extracellular matrix in OA chondrocytes
Col-II and MMP-13 expression were observed by WB and IF, as shown in Figure Supplementary 4. By WB analysis, compared with the OA group, Col-II expression of control (significantly increased [92.54%]) and ExoPTH groups (significantly increased [91.75%]) were significantly upregulated. Col-II expression was significantly downregulated in the OA group compared with the other three groups by IF analyses (OA vs control group, OA group significantly decreased [91.79%]; OA vs ExoBMSC group, OA group significantly decreased [86.10%]; OA vs ExoPTH group, OA group significantly decreased [96.14%]). The ExoPTH group displayed significantly higher Col-II expression than the ExoBMSC group (ExoPTH vs ExoBMSC group, ExoPTH group significantly increased [260.29%]). By WB analysis, compared with the OA group, MMP-13 expression of control (significantly decreased [77.42%] and ExoPTH groups (significantly decreased [85.56%]) were significantly downregulated. By IF analysis, compared with the OA group, MMP-13 expression of control (significantly decreased [89.22%] and ExoPTH groups (significantly decreased [89.48%]) were significantly downregulated. However, no significant difference was observed between the OA and ExoBMSC groups.
The effects of ExoBMSC and ExoPTH on inflammatory cytokines in OA chondrocytes
Using Rat Inflammation Cytokine Antibody Array, we detected the expression level of inflammatory factors in both the ExoBMSC group and the ExoPTH group (Fig. 5). IL-2, TNF-α, and IL-6 expression levels were significantly lower in the ExoPTH group than in the ExoBMSC group (ExoPTH group significantly decreased [88.04%, 74.71%, and 58.06%, respectively]). However, IL-1a, IL-1b, IL-4, IL-13, MCP-1, and IFNg were not different between the two groups.
The effects of ExoBMSC and ExoPTH on inflammatory cytokines in OA chondrocytes were detected by an inflammation array. A Gene-level exploratory analysis and heatmaps of differentially expressed genes in the ExoBMSC and ExoPTH groups; (M1–M4): ExoBMSC group; (M5–M8): ExoPTH group. B KEGG functional enrichment in KEGG pathways was analyzed. C Statistical results of the inflammation array. Data are presented as the mean ± SD, *p < 0.05 (ExoPTH versus ExoBMSC group). ExoBMSC, Exosomes derived from BMSCs; ExoPTH, exosomes derived from PTH-preconditioned BMSCs; BMSCs, bone marrow mesenchymal stem cells
IL-2, TNF-α, and IL-6 expression were detected by WB (Fig. 6A, B). By WB analysis, compared with the OA group, IL-2 expression of the control (significantly decreased [90.95%]), ExoBMSC (significantly decreased [52.67%]), and ExoPTH groups (significantly decreased [72.93%]) were significantly downregulated. The TNF-α expression levels were significantly lower in the control, ExoBMSC, and ExoPTH than in the OA group (control, ExoBMSC, and ExoPTH group significantly decreased [92.84%, 70.14%, and 91.99%, respectively]). The IL-6 expression levels were significantly lower in the control, ExoBMSC, and ExoPTH than in the OA group (control, ExoBMSC, and ExoPTH group significantly decreased [92.22%, 56.42%, and 86.67%, respectively]). The ExoPTH group displayed significantly lower IL-2, TNF-α, and IL-6 expression than the ExoBMSC group (ExoPTH group significantly decreased [43.69%, 73.16%, and 69.43%, respectively]).
IL-2, TNF-α, and IL-6 expression were detected by qRT-PCR (Fig. 6C). By qRT-PCR analysis, compared with the OA group, IL-2 expression of the control (significantly decreased [35.08%]), ExoBMSC (significantly decreased [2.79%]), and ExoPTH groups (significantly decreased [15.02%]) were significantly downregulated. The TNF-α expression levels were significantly lower in the control, ExoBMSC, and ExoPTH than in the OA group (control, ExoBMSC, and ExoPTH group significantly decreased [33.75%, 6.07%, and 17.80%, respectively]). The IL-6 expression levels were significantly lower in the control, ExoBMSC, and ExoPTH than in the OA group (control, ExoBMSC, and ExoPTH group significantly decreased [47.53%, 7.58%, and 11.75%, respectively]). The ExoPTH group displayed significantly lower IL-2, TNF-α, and IL-6 expressions than the ExoBMSC group (ExoPTH group significantly decreased [12.58%, 12.49%, and 4.52%, respectively]).
Discussion
In the present study, we found that both ExoBMSC and ExoPTH enhanced the chondrogenic phenotype of chondrocytes, but ExoPTH appeared to have better performance.
OA, which seriously influences the quality of life of patients, is influenced by many factors. The most important feature of OA is articular cartilage degeneration, which leads to the inhibition of cell proliferation, migration, and matrix production [22]. OA therapies are of widespread interest, but OA therapy is limited to symptomatic treatment at present. Due to the complexity of OA pathogenesis, the transplantation of BMSCs may be the best therapeutic option to date [23,24]. However, many questions remain open, such as the variable sources and quality of BMSCs, the complexity of transplant surgery, and the risk of tumor formation [6]. Currently, there is a widely accepted point of view that ExoBMSC have a positive impact on OA, but ExoBMSC suffer from low efficiency as well as concerns about heterogeneity [7]. PTH induces BMSC differentiation into chondrocytes and enhances repair after articular cartilage injury [16,25,26,27]. Thus, we investigated whether the effect of ExoBMSC could be improved by extrinsic PTH (1-34) in our study.
To induce a model of OA in primary chondrocytes, we treated the cells with topical IL-1β, which has become a widely accepted method [28,29]. According to a cell migration experiment and the available literature [30], 20 ng/ml IL-1β was selected as the optimal concentration. The proliferation and migration of chondrocytes decreased significantly, and the apoptosis of chondrocytes obviously increased after stimulation with IL-1β. This finding was subsequently confirmed by many experiments [31,32]. As we observed in the present study, IL-2, TNF-α, and IL-6 were increased in OA chondrocyte, which is consistent with some previous studies from other groups [33,34]. These results demonstrate that the in vitro model of OA chondrocytes used in the following experiments was established successfully, which is consistent with previous studies.
In recent years, many studies have shown that exosomes can arrest the progression of cartilage destruction in OA. Exosomes regulate various biological processes by transferring microRNAs, proteins, and other nucleic acids to other cells. Lei He et al. provided evidence that ExoBMSC treatment significantly attenuated the inhibitory effect of IL-1β on the proliferation and migration of chondrocytes and IL-1β-induced downregulation of Col-II and upregulation of MMP-13 [10,35]. These effects of ExoBMSC on OA chondrocytes are compatible with our findings. More importantly, we discovered that ExoPTH has a more marked effect on the proliferation, migration, and production of extracellular matrix (Col-II) than ExoBMSC in OA chondrocytes. Unfortunately, in comparison to ExoBMSC, ExoPTH does not significantly inhibit apoptosis or MMP-13 expression in OA chondrocytes. This is likely due to two main factors. A limitation is that our testing was performed at only one time point, and another limitation is that we used only one concentration of ExoPTH. Therefore, our results reveal that ExoPTH may play an important role in preventing OA progression by promoting proliferation, migration, and production of the extracellular matrix (Col-II) in OA chondrocytes but not by inhibiting apoptosis and MMP-13 expression.
A number of factors can influence OA chondrocytes. ExoBMSC and ExoPTH could alter the state of OA chondrocytes, but the mechanism of this process remains poorly understood. In many studies, the proinflammatory cytokine (IL-2, TNF-α, and IL-6) has been identified as playing an important role in inflammatory effects by controlling the proliferation and apoptosis of chondrocytes [36,37,38,39]. It has been reported that chondrocytes are impaired because of excess IL-2, TNF-α, and IL-6 leading to disruption of homeostasis between anabolism and catabolism of the extracellular matrix [40,41,42,43]. In the present study, compared with that in the OA group, IL-2, TNF-α, and IL-6 expression was decreased in the ExoBMSC and ExoPTH groups. Moreover, ExoPTH has a stronger ability to inhibit IL-2, TNF-α, and IL-6 production. Thus, ExoPTH may exert stronger effects on proliferation, migration, and extracellular matrix production by decreasing IL-2, TNF-α, and IL-6 expression in OA chondrocytes. The possible mechanism of ExoPTH on OA chondrocytes is shown in Fig. 7.
Although our study showed that ExoPTH plays an important role in OA chondrocyte repair and chondral matrix formation, there are still several limitations that are worth noting. Exosome contents, which contain microRNAs, mRNAs, lncRNAs, and even various proteins, play a major role in their regulatory function. A large number of proteomic and RNA cargos in ExoBMSC and ExoPTH are unknown. Second, the interval for joint injections and optimum concentration of ExoPTH need to be explored. Third, the possible mechanisms which may affect cartilage homeostasis are numerous [24], we only studied the changes of some inflammatory factors. Despite several limitations of this study, several nanomedicines based on the results of previous studies are potentially useful in the treatment of arthritis [44,45,46]. Therefore, we went on to explore potential mechanisms of ExoPTH in the future, which may provide a novel idea for the early treatment of OA.
Conclusion
In the present study, we found that ExoPTH could enhance the proliferation, migration, and chondral matrix formation of OA chondrocytes induced by IL-1β. Moreover, the potential mechanism of ExoPTH in OA therapy might involve the inhibition of the production of proinflammatory factors (IL-2, TNF-α, and IL-6). Although these types of exosomes have some similar effects, ExoPTH had better effects than ExoBMSC, so ExoPTH may play a more important role in the alleviation of OA. In conclusion, we conclude that ExoPTH may be effective in OA treatment, and investigation of intra-articular injections of ExoPTH could shed new light on their clinical application.
Availability of data and materials
All data supporting our findings within the manuscript
References
Zhu X, Lee CW, Xu H, Wang YF, Yung PSH, Jiang Y, et al. Phenotypic alteration of macrophages during osteoarthritis: a systematic review. Arthritis Res Ther. 2021;23(1):110.
Reichenbach S, Felson DT, Hincapié CA, Heldner S, Bütikofer L, Lenz A, et al. Effect of biomechanical footwear on knee pain in people with knee osteoarthritis: the BIOTOK randomized clinical trial. Jama. 2020;323(18):1802–12.
Hu H, Dong L, Bu Z, Shen Y, Luo J, Zhang H, et al. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesic. 2020;9(1):1778883.
Zhang R, Ma J, Han J, Zhang W, Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11(10):6275–89.
Zhou X, Liang H, Hu X, An J, Ding S, Yu S, et al. BMSC-derived exosomes from congenital polydactyly tissue alleviate osteoarthritis by promoting chondrocyte proliferation. Cell Death Dis. 2020;6(1):142.
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–59.
Liu C, Li Y, Yang Z, Zhou Z, Lou Z, Zhang Q. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine (London, England). 2020;15(3):273–88.
Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020;22(1):75.
He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–55.
He L, He T, Xing J, Zhou Q, Fan L, Liu C, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11(1):276.
Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, et al. Chondrocyte-targeted MicroRNA delivery by engineered Exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–47.
Bari E, Perteghella S, Catenacci L, Sorlini M, Croce S, Mantelli M, et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (London, England). 2019;14(6):753–65.
Chen X, Shi Y, Xue P, Ma X, Li J, Zhang J. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res Ther. 2020;22(1):256.
Wang X, Li Z, Cui Y, Cui X, Chen C, Wang Z. Exosomes isolated from bone marrow mesenchymal stem cells exert a protective effect on osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front Cell Dev Biol. 2021;9:644380.
Qiao L, Hu S, Liu S, Zhang H, Ma H, Huang K, et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest. 2019;129(6):2237–50.
Zhao W, Zou T, Cui H, Lv Y, Gao D, Ruan C, et al. Parathyroid hormone (1-34) promotes the effects of 3D printed scaffold-seeded bone marrow mesenchymal stem cells on meniscus regeneration. Stem Cell Res Ther. 2020;11(1):328.
Zhang S, Hu P, Liu T, Li Z, Huang Y, Liao J, et al. Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration. Theranostics. 2019;9(24):7108–21.
Xu F, Xiang Q, Huang J, Chen Q, Yu N, Long X, et al. Exosomal miR-423-5p mediates the proangiogenic activity of human adipose-derived stem cells by targeting Sufu. Stem Cell Res Ther. 2019;10(1):106.
Liang L, Wang L, Zhou S, Li J, Meng L, Zhang H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells repair injured endometrial epithelial cells. J Assist Reprod Genet. 2020;37(2):395–403.
Chapuis J, Hot D, Hansmannel F, Kerdraon O, Ferreira S, Hubans C, et al. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer's disease. Mol Psychiatry. 2009;14(11):1004–16.
Pagliari M, Munari F, Toffoletto M, Lonardi S, Chemello F, Codolo G, et al. Helicobacter pylori affects the antigen presentation activity of macrophages modulating the expression of the immune receptor CD300E through miR-4270. Front Immunol. 2017;8:1288.
Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475(22):3629–38.
Han Y, Li X, Zhang Y. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886.
Le H, Xu W, Zhuang X, Chang F, Wang Y, Ding J. Mesenchymal stem cells for cartilage regeneration. J Tissue Eng. 2020;26(11):2041731420943839.
Chen Y, Chen Y, Zhang S, Du X, Bai B. Parathyroid hormone-induced bone marrow Mesenchymal stem cell Chondrogenic differentiation and its repair of articular cartilage injury in rabbits. Med Sci Monit Basic Res. 2016;22:132–45.
Wang J, Wang X, Holz JD, Rutkowski T, Wang Y, Zhu Z, et al. Runx1 is critical for PTH-induced onset of mesenchymal progenitor cell chondrogenic differentiation. PLoS One. 2013;8(9):e74255.
Shao LT, Gou Y, Fang JK, Hu YP, Lian QQ, Zhang YY, et al. Parathyroid hormone (1-34) ameliorates cartilage degeneration and subchondral bone deterioration in collagenase-induced osteoarthritis model in mice. Bone Joint Res. 2020;9(10):675–88.
Zwerina J, Redlich K, Polzer K, Joosten L, Krönke G, Distler J, et al. TNF-induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci U S A. 2007;104(28):11742–7.
Liu X, Wang L, Ma C, Wang G, Zhang Y, Sun S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J Orthop Surg Res. 2019;14(1):470.
Alquraini A, Jamal M, Zhang L, Schmidt T, Jay GD, Elsaid KA. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther. 2017;19(1):89.
Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47.
Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother= Biomedecine & pharmacotherapie. 2019;109:1586–92.
Liang Y, Chen S, Yang Y, Lan C, Zhang G, Ji Z, et al. Vasoactive intestinal peptide alleviates osteoarthritis effectively via inhibiting NF-κB signaling pathway. J Biomed Sci. 2018;25(1):25.
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183–90.
Qi H, Liu DP, Xiao DW, Tian DC, Su YW, Jin SF. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell Dev Biol Anim. 2019;55(3):203–10.
Dokić J, Tomić S, Cerović S, Todorović V, Rudolf R, Colić M. Characterization and immunosuppressive properties of mesenchymal stem cells from periapical lesions. J Clin Periodontol. 2012;39(9):807–16.
Bocelli-Tyndall C, Bracci L, Schaeren S, Feder-Mengus C, Barbero A, Tyndall A, et al. Human bone marrow mesenchymal stem cells and chondrocytes promote and/or suppress the in vitro proliferation of lymphocytes stimulated by interleukins 2, 7 and 15. Ann Rheum Dis. 2009;68(8):1352–9.
Liu Q, Wu Z, Hu D, Zhang L, Wang L, Liu G. Low dose of indomethacin and hedgehog signaling inhibitor administration synergistically attenuates cartilage damage in osteoarthritis by controlling chondrocytes pyroptosis. Gene. 2019;712:143959.
Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50.
Lu J, Zhang H, Pan J, Hu Z, Liu L, Liu Y, et al. Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways. Arthritis Res Ther. 2021;23(1):142.
Buhrmann C, Shayan P, Aggarwal BB, Shakibaei M. Evidence that TNF-β (lymphotoxin α) can activate the inflammatory environment in human chondrocytes. Arthritis Res Ther. 2013;15(6):R202.
Kovács B, Vajda E, Nagy EE. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage Interface in osteoarthritis. Int J Mol Sci. 2019;20(18):4653.
Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082–92.
Feng X, Xu W, Li Z, Song W, Ding J. Immunomodulatory Nanosystems. Adv Sci. 2019;6(17):1900101.
Su T, Feng X, Yang J, Xu W, Liu T, Zhang M, et al. MPolymer nanotherapeutics to correct autoimmunity. J Control Release. 2022;343:152–74.
Feng X, Liu J, Xu W, Li G, Ding J. Tackling autoimmunity with nanomedicines. Nanomedicine (London). 2020;15(16):1585–97.
Acknowledgements
All authors thank Yu Xia, Qi Xu, Lu Ding, Ming Shi, Xian-long Li, Quan Gan, and Zhi-guang Chang for providing technical support in the study.
Funding
The study was supported by the National Natural Science Foundation of China (NSFC JCYJ20210324123001003) and the Postdoctoral start-up fund (ZSQYRSFPD0037).
Author information
Authors and Affiliations
Contributions
Conception and design: Litao Shao, David Y.B Deng. Collection of data: Litao Shao, Jie-hong Qiu. Drafting of the article: Litao Shao, David Y.B Deng. Paper revision: Litao Shao, Liang Luo. Approved the final manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiment was approved by the Institutional Animal Care and Use Committee.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure Supplementary 1.
BMSCs and chondrocytes were identified. (A). Morphology of BMSCs observed by inverted microscopy. (B-D). BMSCs were authenticated by flow cytometry. (E). BMSCs were authenticated by STR DNA profiling. (F) Immunofluorescence staining was used to identify chondrocytes. BMSCs: bone marrow mesenchymal stem cells. Ch: chondrocytes.
Additional file 2: Figure Supplementary 2.
The concentrations of IL-1β and PTH (1-34) were chosen. (A). A series of IL-1β concentrations were investigated in chondrocytes. (B). Different concentrations of PTH (1-34) were investigated in BMSCs. (C). Different concentrations of PTH (1-34) were investigated in chondrocytes. (D). Statistical results of IL-1β concentrations. *p < 0.05 versus the control group, #p < 0.05 versus the 1 ng/ml group and &p < 0.05 versus the 5 ng/ml group. (E). Statistical results of different concentrations of PTH (1-34) acted on BMSCs. *p < 0.05 versus 10 nM/ml group and #p < 0.05 versus 50 nM/ml group. (F). Statistical results showing the effects of different concentrations of PTH (1-34) acted on chondrocytes. *p < 0.05 versus the 10 nM/ml group and #p < 0.05 versus 50 nM/ml group. IL-1β: interleukin-1β; PTH: parathyroid hormone; OA: osteoarthritis; BMSCs: bone marrow mesenchymal stem cells.
Additional file 3: Figure Supplementary 3.
The effect of ExoBMSC and ExoPTH on chondrocyte proliferation was assayed by the scratch wound-healing migration assay and CCK-8. (A). The scratch wound-healing migration assay was performed with control, ExoBMSC, and ExoPTH groups. (B). Statistical results of the migration ratio. (C). The cell proliferation ability of the control, ExoBMSC and ExoPTH groups was detected by a CCK-8 assay. Data are presented as the mean ± SD, *p < 0.05 versus the control group and #p < 0.05 versus the ExoBMSC group. ExoBMSC: exosomes derived from BMSCs; ExoPTH: exosomes derived from PTH-preconditioned BMSCs.
Additional file 4: Figure Supplementary 4:
The effect of ExoBMSC and ExoPTH on the OA chondrocyte extracellular matrix was detected by Western blot and immunofluorescence. (A). Western blot assay for collagen-II (Col-II) and matrix metalloproteinase-13 (MMP-13) in each group. (B). Immunofluorescence assay for Col-II and MMP-13 in each group. Data are presented as the mean ± SD, *p < 0.05 versus the OA group and #p < 0.05 versus the ExoBMSC group. Bars = 50 μm; OA: osteoarthritis; ExoBMSC: exosomes derived from BMSCs; ExoPTH: exosomes derived from PTH-preconditioned BMSCs; BMSCs: bone marrow mesenchymal stem cells.
Additional file 5: Table 1.
List of the three PCR Primer Pairs chosen for in vitro PCR validation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shao, Lt., Luo, L., Qiu, Jh. et al. PTH (1-34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res Ther 24, 96 (2022). https://doi.org/10.1186/s13075-022-02778-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02778-x