Abstract
Background
There is an urgent need for cities to become more climate resilient; one of the key strategies is to include more green spaces in the urban environment. Currently, there is a worry that increasing green spaces might increase mosquito nuisance. As such, this study explores a comprehensive understanding of how mosquitoes utilise contrasting grey and green habitats at different life stages and which environmental factors could drive these distributions.
Methods
We used a setup of six paired locations, park (green) vs. residential (grey) areas in a single model city (Leiden, The Netherlands), where we sampled the abundances of different mosquito life stages (eggs, larvae, adults) and the local microclimatic conditions. In this study, we focused on Culex pipiens s.l., which is the most common and abundant mosquito species in The Netherlands.
Results
Our results show that while Cx. pipiens ovipositioning rates (number of egg rafts) and larval life stages were far more abundant in residential areas, adults were more abundant in parks. These results coincide with differences in the number of suitable larval habitats (higher in residential areas) and differences in microclimatic conditions (more amenable in parks).
Conclusions
These findings suggest that Cx. pipiens dispersal may be considerably more important than previously thought, where adult Cx. pipiens seek out the most suitable habitat for survival and breeding success. Our findings can inform more targeted and efficient strategies to mitigate and reduce mosquito nuisance while urban green spaces are increased, which make cities more climate resilient.
Graphical Abstract

Similar content being viewed by others
Background
In recent decades, the world has witnessed a remarkable increase in urbanisation, and it is estimated that in 2050 > 70% of the world’s population will live in sprawling urban areas [1]. Urbanisation and urban areas have been associated with increased mosquito abundance due to the loss of natural predators and the availability of suitable breeding habitats [2, 3]. This increase in mosquito abundance, coupled with a higher density of human population, has increased the occurrence of mosquito-borne diseases in urban areas, with suburban residential areas and areas in proximity to parks being particularly vulnerable [4,5,6]. Moreover, the temporal occurrence of these outbreaks can be linked to extreme weather events, such as heatwaves, drought and heavy erratic rainfall, which are projected to increase due to global climate change and which are generally more extreme in cities [7,8,9,10,11]. As cities continue to expand and climate change impacts become more pronounced, there is an urgent need to address the challenges posed by increasing mosquito-borne disease occurrences [7, 12].
One of the key strategies employed by cities to become more climate resilient are nature-based solutions, such as establishing green spaces, promoting biodiversity and integrating nature into the urban fabric [13,14,15]. For instance, cities are constructing green infrastructure, such as wetlands and city parks, and retrofitting buildings with green roofs, to absorb and manage stormwater and mitigate the urban heat island effect. Currently, there is a worry that (some of these) climate adaptation strategies will affect mosquito-borne disease risk, but it is unclear whether these are detrimental or beneficial for mosquitoes [7, 13, 16]. While the need for such solutions is clear, the challenge lies in implementing them while minimizing the risk of mosquito-borne diseases and mosquito-related nuisances [7, 17]. This necessitates a comprehensive understanding of the ecological factors driving mosquito distribution in urban areas. While some general patterns regarding the effects of vegetation and the availability of container breeding sites are known, there are almost no empirical data on mosquito distribution within urban environments—comparing green to grey spaces—or what role green and grey spaces in cities might play in mosquito population dynamics [18,19,20,21,22,23,24,25,26,27,28,29,30]. For this, we need to understand the distribution of the different life stages of mosquitoes, which is essential for establishing links with environmental factors, such as microclimate.
To understand how the various mosquito life stages are distributed across the urban fabric, we set out to investigate how these life stages (eggs, larvae, adults) are distributed across contrasting urban environments (city parks vs. residential areas) and how this covaries with the local microclimatic conditions. To this end, we investigated these factors in the metropolitan area of Leiden (NUTS-3 region), a typical Northwestern European urban area with a highly heterogeneous urban landscape consisting of densely populated residential areas interspersed with parks. In this study, we focused on Culex pipiens/torrentium (hereafter: Cx. pipiens), which is the most common and abundant mosquito (Culicidae) species in The Netherlands and Northwestern Europe [31,32,33]. This species is particularly abundant in urban areas, utilising a variety of habitats ranging from artificial containers to natural stagnant waterbodies for breeding [34,35,36,37]. Culex pipiens is also the primary vector for the transmission of both Usutu virus (USUV) and West Nile virus (WNV) [38,39,40,41].
Methods
Study design
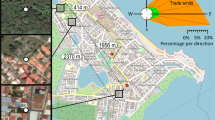
A field study was conducted from 30 May to 8 July 2022, starting 2 weeks after a period of precipitation. This time frame enabled us to assess variations in the response of mosquito life stages to rainfall between residential areas and city parks, precipitation being one of the driving factors of mosquito abundances [42, 43]. We used a setup of six paired locations, each location pair consisted of three park sites (urban green areas) and three residential sites (urban grey areas) which were selected based on differences in human population density and vegetation composition (Fig. 1). As a proxy for residential areas, schoolyards in residential areas were chosen as they are relatively uniform in terms of vegetation and size and more accessible than privately owned spaces in residential areas.
Map showing the locations of mosquito sampling in the city of Leiden, The Netherlands, in relation to the percentage tree cover (grey: artificial surfaces; green: tree cover). Black dots indicate park sites and orange dots indicate residential sites. Yellow boxes indicate the six paired locations. Maps created using QGIS (version 3.16, Hannover; Development Team, 2022) and Copernicus land cover data from 2018
Sampling the abundances of adult Cx. pipiens mosquitoes
Adult mosquitoes were trapped every week during two subsequent trapping nights at all six paired locations (6 park and 6 residential). Per location three carbon dioxide-baited BG Pro traps (Biogents GmbH, Regensburg, Germany) were placed for a total of 36 trapping sites [44]. Traps were spaced approximately 30–40 m apart in a triangular pattern to prevent competition between traps caused by intersecting CO2 plumes [45]. We attached the traps to a tree or pole at a height of ~ 1.5 m, surrounded by high vegetation to provide shelter from the wind for the carbon dioxide plume [46, 47]. Carbon dioxide was generated using a modified sugar fermentation protocol by mixing 200 g granulated sugar, 5 g dried Saccharomyces cerevisiae yeast (Fermentis, SafSpirit FD-3), 5 g store-bought tomato paste and approximately 1.5 l tap water in 4-l jerrycan bags (Packforce, Jerrycan pouch) [47, 48]. The jerrycan bags were placed in the BG Pro bags and connected to the mosquito traps through silicone tubes (Ø7 mm; RubberBV, Hilversum, The Netherlands). Collected mosquitoes were separated by sex and the female mosquitoes were identified morphologically based on the characteristics outlined in Becker et al. [17]. Culex pipiens s.l. and its sibling species Cx. torrentium are challenging to differentiate reliably based on morphological characteristics of adult females and were therefore grouped together and referred to as Cx. pipiens [17, 35, 49].
Measuring Cx. pipiens ovipositioning
Culex pipiens egg rafts were collected weekly during two subsequent trapping nights at all 36 sites within the 6 paired locations using three 5-l (202 × 171 mm) round black buckets (Dijkstra Plastics, Haaksbergen, The Netherlands), which were placed under each adult mosquito trap. To attract gravid mosquitoes and outcompete local breeding sites, we mimicked the nutrient level of a hypertrophic land puddle (100 mg/l N-total) [18, 50, 51]. To each bucket we added 4 l ditch water and dried cow manure (2.4% N; 1.5% P2O5; 3.1% K2O) to reach 100 mg/l N-total, which was then left to incubate for 1 week so that the microbial communities could stabilise [50]. To ensure that egg raft collection coincided with the adult mosquito trapping, a lid was placed on the bucket and removed during the two trapping nights. Thereafter, the egg rafts were counted and removed from the bucket to prevent any potential confounding effects of mosquito larval presence on oviposition behaviour [52].
Counting Cx. pipiens breeding habitats and measuring occupancy
Culex pipiens breeding habitats were counted and sampled for occupancy once in early June and 2 weeks after a period of precipitation. This method ensured that we only counted the breeding habitats that persisted long enough for adult Cx. pipiens to lay their eggs and for larvae to develop, which typically takes about 2 weeks under local climatic conditions [50]. For all 36 sites, we registered and sampled all potential breeding habitats within a 100-m radius using both larval dipping and eDNA collection methods. We utilised both approaches because they complement each other: larval dipping provides accurate estimates when mosquito numbers are high and habitats are small, while environmental DNA can detect mosquito presence in cases of low larval numbers and large habitats [53]. However, eDNA is also able to detect legacy mosquito presence via ‘zombie DNA’, e.g. eDNA is still present in the water or sediment after the adults emerged [54, 55]. This residual eDNA can persist for 1–4 days until it is fully degraded by microbial activity and abiotic factors such as UV-B and pH [56,57,58,59]. We defined four mosquito breeding habitat categories, lake, ditch, pond and artificial (Fig. 2), to examine the overall disparities in the number of mosquito breeding habitats between parks and residential areas. These mosquito breeding habitats were then sampled using a larval dipper (BioQuip, Compton, CA, USA) using the “simple scoop” dipping technique, during which larvae were counted [60]. Large habitats such as lakes, ditches and ponds were sampled ten times to get a representative sample, and confined artificial habitats, such as storm drains, were sampled once. For every habitat category encountered in the 100 m radius, a total of 1 l water was collected for eDNA analysis via subsampling with a pipet controller (Integra Bioscience) to improve detection probabilities [61].
Extraction of eDNA from mosquito breeding habitats
Environmental DNA was collected using a 250-ml Sartorius filtering tower (Sartorius-stedim), a mobile vacuum pump (Datura Molecular Solutions) and multiple 0.45-micron polyethersulfone (PES) filters with a diameter of 47 mm (Tisch Scientific). To prevent cross-contamination, the Sartorius filtering tower was cleaned between filtrations with tap water (to remove large particles) and then soaked for 30 min in a 10% bleach solution to degrade remaining DNA. After filtration, the filter was immediately placed in a 2-ml centrifuge tube and completely immersed in 900 μl CTAB buffer. DNA extraction was performed using a modified PCI protocol followed by a column based DNA extraction as clean-up [62,63,64,65,66,67]. The PES filters were incubated at 65 °C for 10 min. Next, chloroform-isoamylalcohol (CI 24:1) was added to the filters and vortexed until completely disintegrated. The resulting mixture was centrifuged at 15,000 × g for 15 min, and 500 μl of the aqueous layer was transferred to a new tube. To this, 1 ml 96% ethanol and 180 µl 3 M NaOAc were added, followed by thorough vortexing. The tube was then placed in the freezer at −20 °C overnight for precipitation. For DNA purification, the tubes were centrifuged again, and the liquid was removed. The pellets were air-dried until no visible liquid remained and were then resuspended in 180 μl ATL buffer. DNA extraction was continued with the DNeasy Blood & Tissue kit (Qiagen) for DNA purification using the manufacture protocol. DNA was finally eluted in 200 μl AE buffer.
Measuring Cx. pipiens eDNA traces in mosquito breeding habitats
We measured Cx. pipiens eDNA traces using Cx. pipiens s.l.-specific primers (forward: 5ʹ-CTGGAGCTTCAGTAGACT-3ʹ and reverse: 5ʹ-AGAGTAATTCCTGAAGATCG-3ʹ) in conjunction with a probe (5ʹ-[HEX]AGCAGGAATTTCATCATTTTAGGTGC[BHQ1]-3ʹ) targeting the COX1 genomic region. These primers and probe were designed and tested in silco using the Primer3 plug-in for Geneious, version R10 [68, 69]. For validation we used a mispriming library containing Aedes albopictus, Culiseta annulata and Chironomus riparius. The primers and probe were validated in vitro with a thermal gradient to select the optimal annealing temperature and test for cross amplification in the species from the mispriming library. Culex pipiens eDNA concentration was measured in triplicate using the Droplet Digital PCR system (ddPCR) with the Probe assay (Bio-Rad Laboratories, Inc.). The ddPCR reaction mix (22 μl) consisted of 11 μl of ddPCR™ Supermix for Probes, 1 μl of each primer (10 μM), 1 μl probe (5 μM), 7 μl RNase and DNase free-water, and 2 μl DNA template. To partition 20 μl of the PCR reaction mix into droplets, we used the QX200 Droplet Generator. PCR was performed in a Bio-Rad C1000 touch Thermal Cycler, using the following programme: 5 min at 95 °C, and 40 cycles of 30 s at 95 °C and 60 s at 52.3 °C with ramp rate of 2.0 °C/s, followed by 10 min at 98 °C. After amplification, the PCR plate was analysed using the QX200 Droplet Reader. We quantified the number of target copies using Bio-Rad's QuantaSoft software version 1.7.4. PCR replicates were consolidated into a single sample through QuantaSoft's merge function. The positive signal threshold was established based on a positive control sample, following QuantaSoft manual guidelines. We calculated the threshold values in test samples by determining the separation value between the threshold and the center of the negative droplet band, which was set at 1800 units above the average negative band. Droplets exceeding this threshold were counted as positive events. Blank samples containing only RNase and DNase free water were used as negative controls for the test samples. Sample count estimates were compared to the maximum confidence interval (95%) of the negative controls to ascertain whether DNA concentrations were statistically different from zero.
Assessing microclimate
The microclimate at three parks and three residential areas sites were monitored continuously using a single open-source weather station for each site [70]. The weather stations were deployed on 30 May and retrieved on 8 July 2022 and recorded the following variables at a 5-min interval: air temperature (°C; accuracy ± 1 °C), relative humidity (%; accuracy ± 3%) and wind speed (m/s; accuracy ± 0.1 m/s). These climatic variables are known to be of key importance for mosquito survival, development and behaviour [42, 43, 71, 72].
Statistical analysis
We tested overall differences in adult Cx. pipiens abundances, egg rafts, larvae counts and eDNA traces between parks and residential areas over time using generalized linear mixed models (GLMMs). We used weekly trap data, observed egg rafts, larvae counts and eDNA concentration as response variables. For these models we used site (parks vs. residential areas) and week as predictor variables, with paired locations as random effects. We employed a Poisson distribution with a log-link function and assessed predictors using an analysis of variance (ANOVA). To test for differences in microclimatic conditions between parks and residential areas, we first calculated the weekly minimum, mean and maximum values for temperature, relative humidity and wind speed. Minimum wind speed was always zero and was excluded from further analysis. A linear mixed-effect model (LMEM) was run to test for differences between parks and residential areas over time for each of the three variables, temperature, humidity and wind speed, and three levels, minimum, mean and maximum. A Bartlett test was conducted to assess the homogeneity of variances between parks and residential areas for each of the three microclimatic variables. All data analysis was conducted with RStudio (R version 4.1.0; R Core Team, 2021) [73]. All relevant assumptions were checked before the statistical tests were carried out.
Results
Differences in adult Cx. pipiens abundances between parks and residential areas
We captured a total of 10,227 adult female Cx. pipiens mosquitoes. In parks we observed almost double the number of mosquitoes (6624) compared to (3603) residential areas. We fitted a GLMM to predict the number of adult Cx. pipiens, with an R2 of 0.98, of which 0.49 is related to the fixed effects (e.g. park vs. residential area and week). When we look at the weekly number of adult Cx. pipiens per trap, we observe that this difference is overall statistically significant (Wald test, χ2 = 6.7523, df = 1, Ρ < 0.01; Fig. 3A), as is the interaction with week (Wald test, χ2 = 82.6743, df = 5, Ρ < 0.001; Fig. 3B).
Differences in Cx. pipiens oviposition between parks and residential areas
We counted a total of 2841 Cx. pipiens egg rafts. In residential areas we observed almost three times more egg rafts (2067) compared to parks (774). We fitted a GLMM to predict the number of Cx. pipiens egg rafts, with an R2 of 0.88, of which 0.73 is related to the fixed effects. When we look at the weekly number of Cx. pipiens egg rafts per ovipositioning bucket, we observe that this difference is overall statistically significant (Wald test, χ2 = 39.233, df = 1, Ρ < 0.001; Fig. 4A), as is the interaction with week (Wald test, χ2 = 86.810, df = 5, Ρ < 0.001; Fig. 4B).
Differences in number of Cx. pipiens larvae between parks and residential areas
We counted a total of 665 Cx. pipiens larvae, all observed in storm drains in residential areas. These observations were confirmed by the outcome of our eDNA measurements, which detected on average 20 times higher concentrations of Cx. pipiens eDNA traces in breeding habitats in residential areas compared to breeding areas in parks. We fitted a GLMM to predict the concentrations of Cx. pipiens eDNA traces, with an R2 of 0.80 of which 0.38 is related to the fixed effect of parks versus residential. The difference in eDNA concentrations is overall statistically significant (Wald test, χ2 = 9.0782, df = 1, Ρ < 0.01; Fig. 5B).
The microclimate in parks and residential areas showed remarkable similarity in maximum temperature, minimum relative humidity, and mean and maximum wind speed
The microclimate in parks and residential areas showed remarkable similarity in temperature (maximum), relative humidity (minimum) and wind speed (mean, maximum). Maximum temperature in parks was indistinguishable from that in residential areas (22.9 vs. 22.9 °C) (LMER, t = −0.0232, df = 4, Ρ = 0.983; Fig. 6A). In contrast, mean temperatures were 1.1 °C lower in parks compared to residential areas (17.2 vs. 18.3 °C) (LMER, t = −3.04, df = 4, Ρ < 0.05; Fig. 6A), and minimum temperatures in parks were 2.1 °C lower (13.7 vs. 15.8 °C) (LMER, t = −2.68, df = 4, Ρ = 0.0551; Fig. 6A). For humidity we found that parks maintained 10.4% higher levels of maximum relative humidity (82.9% vs. 72.5%) (LMER, t = 3.72, df = 4, Ρ < 0.05; Fig. 6B), 7.7% higher levels of mean relative humidity (72.4% vs. 64.7%) (LMER, t = 3.22, df = 4, Ρ < 0.05; Fig. 6B) and 3.4% higher levels of minimum relative humidity (55.3% vs. 51.9%) but this was not statistically significant (LMER, t = 0.447, df = 4, Ρ = 0.678; Fig. 6B). Only small differences were observed for wind speed. The maximum wind speed was slightly higher in parks compared to residential areas (2.4 m/s vs. 2.2 m/s), but this was not statistically significant (LMER, t = 0.165, df = 4, Ρ = 0.878; Fig. 6C). Mean wind speed showed a similar pattern: small non-significant differences between residential areas and parks (0.59 m/s vs. 0.52 m/s) (LMER, t = 0.131, df = 4, Ρ = 0.902; Fig. 6C). Interestingly, when examining the variance between parks and residential areas, wind speed demonstrates significantly higher variability in parks (3.82 vs. 2.46 (m/s)2) compared to residential areas (Bartlett test, K2 = 17.188, df = 1, Ρ < 0.0001). Conversely, for both temperature (Bartlett test, K2 = 0.96025, df = 1, Ρ = 0.327) and relative humidity (Bartlett test, K2 = 0.059317, df = 1, Ρ = 0.808), no significant differences in variance were observed between parks and residential areas.
Discussion
Our results indicate that while adult Cx. pipiens mosquitoes were more abundant in park sites, Cx. pipiens ovipositioning (eggs) and larval stages were far more abundant in densely populated residential areas. These results coincide with differences in the availability of artificial breeding habitats and microclimatic conditions. These results suggest that, depending on the life stage, different habitat characteristics are governing Cx. pipiens distributions. It is, however, important to realize that all results were derived from a single metropolitan area in Northwestern Europe and for a single species complex. Although Leiden is a typical European metropolitan area with residential areas interspersed with green parks, it remains to be tested whether patterns observed here reflect those occurring in other regions or climate zones and for different species. Given the fact that most cities are composed of separated grey and green spaces, our results might hold for other urban environments where Cx. pipiens is the most abundant species.
Parks are more populated by adult Cx. pipiens mosquitoes
Vegetation structure plays an important role in modifying microclimatic conditions such as temperature, humidity and wind speed by acting as a buffer, which are all linked to adult mosquito survival and development [42, 71, 72, 74]. Conversely, built-up structures, such as roads and buildings, also modify these microclimatic conditions, but then in the opposite direction, resulting in the urban heat island effect [75]. Indeed, our results show that parks were cooler and maintained higher relative humidity levels compared to residential areas, which is line with literature and promotes mosquito abundance based on their temperature preferences [74,75,76,77]. Adult mosquitoes are fragile insects that dry out quickly and are easily overwhelmed by strong gusts of wind [37, 78]. Wind may also impact host-seeking behaviour, which depends on mosquito olfactory senses, that require particles moving past them, making it necessary for mosquitoes to sense these compounds downwind [79]. For mosquitoes to navigate towards these sources, the prevailing wind speed must be lower than their average flight speed of 1 m/s [78]. Our results show that there is no difference in overall wind speed between parks and residential areas. However, we did measure higher variance in wind speed within parks. These results suggest that wind speed provides no proximate explanation to differences in adult Cx. pipiens abundance between the grey and green urban habitats. Overall, our results suggest that the distribution of adult Cx. pipiens mosquitoes is likely impacted by the observed differences in humidity and temperature between residential and green areas. However, we did not confirm the relation between microclimate and adult Cx. pipiens mosquito abundances because of restraints in our experimental design.
Residential areas are more populated by immature Cx. pipiens life stages
Residential areas generally host an abundance of artificial substrates (containers, floodwater drains, etcetera) in which temporal water bodies can form after precipitation [2, 21, 22]. In parks, characterised by permeable soils and permanent water bodies, precipitation will either infiltrate into the soil or flow towards permanent water bodies before immature mosquitoes can emerge as adults. These permanent water bodies often contain an abundance of mosquito predators such as fish, amphibians and dragonfly larvae [72,73,74,75]. Even though we only sampled publicly accessible mosquito breeding habitats in the residential environment and all breeding habitats in the parks, we only visually observed larvae in residential areas in storm drains, which are a well-known habitat for Cx. pipiens [80, 81]. These habitats were often littered with organic material and devoid of any potential mosquito predator. Interestingly, with eDNA we were also able to detect Cx. pipiens eDNA traces in almost all other sampled potential breeding habitats, albeit in far lower concentrations than in the storm drains where we also found larvae. This suggests that Cx. pipiens mosquitoes do oviposit in all available breeding habitats, but that the larvae do not reach high enough abundances to be detected with larval dipping techniques [60, 82]. This could be due to low ovipositioning rates because more attractive habitats were available and/or that mosquito larvae were eaten by predators before they could reach detectable densities [18, 83,84,85,86,87]. Nevertheless, these permanent water bodies do have a large surface area where immature mosquito larvae can occur, particularly between water bank vegetation and within small, shallow, semi-connected water bodies along the shoreline [18, 87,88,89]. It is plausible that these habitats may function as reservoirs, maintaining low densities of Cx. pipiens mosquitoes. These Cx. pipiens mosquitoes might subsequently migrate to more suitable temporary water bodies following a precipitation event [85, 86]. In summary, these findings show higher larval Cx. pipiens abundances, higher Cx. pipiens ovipositioning rates and higher availability of suitable breeding habitats in residential areas, implying a series of important pull factors for gravid Cx. pipiens mosquitoes looking for a suitable site for egg deposition.
Short-scale migration of Cx. pipiens in the urban environment?
Mosquitoes require an aquatic habitat to oviposit and for larvae to feed and develop via four successive molts into pupae from which adults can emerge [17, 18, 37]. These adult mosquitoes fly out and disperse to seek shelter against the elements, nectar to feed, a bloodmeal to develop eggs and suitable breeding habitats to oviposit their eggs [71, 78]. We observed that parks likely provide a more favourable environment for adult Cx. pipiens with their favourable microclimates. We also observed that residential areas provide a more suitable environment for the immature life stages of Cx. pipiens. As an explanation for this remarkable division, we propose that short distance Cx. pipiens mosquito migration between the various urban habitats is underlying this pattern. According to this hypothesis, the bulk of the urban Cx. pipiens emerges from artificial breeding habitats in residential areas and a fair number of them will move towards parks to rest, mate and seek a blood meal. As parks lack the required predator-free habitat for egg deposition, they (unintentionally) migrate back to residential areas to deposit their eggs. While we cannot exclude the possibility that the urban environment has two distinct populations that do not mix, this is unlikely given the previously reported dispersal distances of Cx. pipiens [71, 90].
Implications for urbanising landscapes under a changing climate
Nature-based solutions play a crucial role in enhancing the climate resilience of cities, with a significant focus on increasing biodiversity and integrating nature into the urban fabric [13,14,15]. Although our study was not designed to assess the effects of nature-based solutions, our results do offer valuable insights into how these solutions could potentially influence Cx. pipiens populations. Our results suggest that increasing green areas in the form of high woody vegetation in residential areas to reduce the urban heat island effect will not necessarily increase the total number of adult Cx. pipiens, although there are competing factors at play. Based on our results we cannot make any inference on how many Cx. pipiens that emerge from residential breeding habitats die on their way to find sheltered environments. Having more sheltered (nature-based) environments in residential areas could potentially increase adult survival, thus increasing total abundance in the urban environment. On the other hand, greening the city could potentially reduce the possible number of (artificial) temporal breeding habitats by increasing permeable surfaces like grasslands and green roofs, creating new permanent water bodies can help absorb rainwater, which may result in a reduction in stormwater runoffs. The latter set of measures will likely reduce the number of Cx. pipiens larvae via the reduction of (potential) artificial breeding habitats and increase predation in newly formed permanent water bodies that are linked to well-established ecosystems [91,92,93,94]. Our study shows that while Cx. pipiens mosquitoes do oviposit in permanent water bodies, we did not detect any Cx. pipiens larvae. This might be due to the presence of mosquito predators [18, 83,84,85]. This would imply that new permanent water bodies should be linked to existing water bodies with a well-established ecosystem, from which predator species can colonise these new habitats [18, 95].
Conclusions
Our study suggests that, based on the results from a single metropolitan area in Northwestern Europe, Cx. pipiens mosquitoes spend their adult life mostly in parks, where a variety of hosts (mainly bird and mammal species) co-occur and more suitable microclimates can be found (more humid and more buffered temperatures). In sharp contrast, we show that immature Cx. pipiens life stages are almost exclusively spent in densely populated residential areas, with their abundance of artificial habitats (temporal with eutrophic conditions). Overall, these results highlight the importance of urban landscape structure to understand mosquito distributions and suggest that Cx. pipiens dispersal may be considerably more important than previously thought, where adult Cx. pipiens mosquitoes seek out the most suitable habitat for survival and ovipositioning. Moreover, our findings suggest that enhancing the climate resilience of urban areas through nature-based solutions, while considering and mitigating the potential risks associated with their implementation, will not necessarily increase the population of adult Cx. pipiens mosquitoes.
Availability of data and materials
All data are available upon request to the corresponding author.
Abbreviations
- ANOVA :
-
Analysis of variance
- BHQ1:
-
Black Hole Quencher 1
- CI:
-
Chloroform-isoamyl alcohol
- COX1:
-
Cytochrome c Oxidase Subunit 1
- CTAB:
-
Cetyltrimethylammonium bromide
- ddPCR:
-
Digital Droplet Polymerase Chain Reaction
- df:
-
Degrees of freedom
- DNA:
-
Deoxyribonucleic acid
- DNase:
-
Deoxyribonuclease
- eDNA:
-
Environmental DNA
- G:
-
Gravitational force
- GLMM:
-
Generalized Linear Mixed Model
- HEX:
-
6-carboxyhexachlorofluorescein
- LMEM:
-
Linear mixed-effect model
- NUTS-3:
-
Nomenclature of Territorial Units for Statistics - Level 3
- PCR:
-
Polymerase Chain Reaction
- PCI:
-
Phenol-chloroform-isoamyl alcohol
- PES:
-
Polyethersulfone
- pH:
-
Potential of hydrogen
- RNase:
-
Ribonuclease
- s.l.:
-
Senso lato
- USUV:
-
Usutu virus
- WNV:
-
West Nile virus
References
United Nations Department of Economic and Social Affairs. World urbanization prospects: The 2018 revision. United Nations; 2019. https://www.un-ilibrary.org/content/books/9789210043144.
Ferraguti M, de La Puente JM, Roiz D, Ruiz S, Soriguer R, Figuerola J. Effects of landscape anthropization on mosquito community composition and abundance. Sci Rep. 2016;6(1):29002. https://doi.org/10.1038/srep29002.
Ferraguti M, Magallanes S, Ibáñez-Justicia A. Implication of human landscape transformation on mosquito populations. In: Gutiérrez-López R, Logan JG, de la Puente J, editors. Ecology and control of vector borne diseases. Wageningen Academic Publishers; 2022. p. 279–83. https://doi.org/10.3920/978-90-8686-931-2.
Kache PA, Santos-Vega M, Stewart-Ibarra AM, Cook EM, Seto KC, Diuk-Wasser MA. Bridging landscape ecology and urban science to respond to the rising threat of mosquito-borne diseases. Nat Ecol Evol. 2022;6(11):1601–16. https://doi.org/10.1038/s41559-022-01876-y.
Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–83. https://doi.org/10.1146/annurev-ento-112408-085419.
Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011;39(Supplement 4):S3–S11. https://doi.org/10.2149/tmh.2011-S05.
Ligsay A, Telle O, Paul R. Challenges to mitigating the urban health burden of mosquito-borne diseases in the face of climate change. Int J Environ Res Public Health. 2021;18(9):5035. https://doi.org/10.3390/ijerph18095035.
Caminade C, McIntyre KM, Jones AE. Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci. 2019;1436(1):157–73. https://doi.org/10.1016/S1473-3099(19)30161-6.
Franklinos LHV, Jones KE, Redding DW, Abubakar I. The effect of global change on mosquito-borne disease. Lancet Infect Dis. 2019;19(9):e302–12. https://doi.org/10.1016/S1473-3099(19)30161-6.
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, et al. Global change and the ecology of cities. Science. 2008;319(5864):756–60. https://doi.org/10.1126/science.1150195.
Landsberg HE. The urban climate. Cambridge: Academic Press; 1981.
Glidden CK, Nova N, Kain MP, Lagerstrom KM, Skinner EB, Mandle L, et al. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. Curr Biol. 2021;31(19):R1342–61. https://doi.org/10.1016/j.cub.2021.08.070.
Stead D. Urban planning, water management and climate change strategies: adaptation, mitigation and resilience narratives in the Netherlands. Int J Sustain Dev World Ecol. 2014;21(1):15–27. https://doi.org/10.1080/13504509.2013.824928.
Gill SE, Handley JF, Ennos AR, Pauleit S. Adapting cities for climate change: the role of the green infrastructure. Built Environ. 2007;33(1):115–33. https://doi.org/10.2148/benv.33.1.115.
Braubach M, Egorov A, Mudu P, Wolf T, Ward TC, Martuzzi M. Effects of Urban Green Space on Environmental Health, Equity and Resilience. In: Kabisch N, Korn H, Stadler J, Bonn A, editors. Nature-based solutions to climate change adaptation in urban areas. Theory and practice of urban sustainability transitions. Cham: Springer; 2017. p. 187–205. https://doi.org/10.1007/978-3-319-56091-5_11.
Valdelfener M, Barraud S, Sibeud E, Bacot L, Perrin Y, Jourdain F, et al. Do sustainable drainage systems favour mosquito proliferation in cities compared to stormwater networks? Urban Water J. 2019;16(6):436–43. https://doi.org/10.1080/1573062X.2018.1523442.
Becker N, Petrić D, Zgomba M, Boase C, Madon MB, Dahl C, et al. Mosquitoes: identification, ecology and control. Cham: Springer; 2020.
Rejmánková E, Grieco J, Achee N, Roberts DR. Ecology of larval habitats. In: Manguin S, editor. Anopheles mosquitoes—new insights malar vectors. IntechOpen; 2013. p. 397–446. https://doi.org/10.5772/55229.
Marcantonio M, Rizzoli A, Metz M, Rosà R, Marini G, Chadwick E, et al. Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. PLoS One. 2015;10:e0121158.
Hunt SK, Galatowitsch ML, McIntosh AR. Interactive effects of land use, temperature, and predators determine native and invasive mosquito distributions. Freshw Biol. 2017;62:1564–77. https://doi.org/10.1111/fwb.12967.
Washburn JO. Regulatory factors affecting larval mosquito populations in container and pool habitats: implications for biological control. J Am Mosq Control Assoc. 1995;11:279–83.
Ramasamy R, Surendran SN. Mosquito vectors developing in atypical anthropogenic habitats: global overview of recent observations, mechanisms and impact on disease transmission. J Vector Borne Dis. 2016;53:91–8.
Rakotoarinia MR, Blanchet FG, Gravel D, Lapen DR, Leighton PA, Ogden NH, et al. Effects of land use and weather on the presence and abundance of mosquito-borne disease vectors in an urban and agricultural landscape in Eastern Ontario, Canada. PLoS One. 2022;17:e0262376. https://doi.org/10.1371/journal.pone.0262376.
Zhao J, Tang T, Wang X. Effects of landscape composition on mosquito population in urban green spaces. Urban For Urban Green. 2020;49:1–7. https://doi.org/10.1016/j.ufug.2020.126626.
Yang Y, Ratkowsky DA, Yang J, Shi P. Effects of plant coverage on the abundance of adult mosquitos at an urban park. Plants. 2023;12:983. https://doi.org/10.3390/plants12050983.
Brown H, Diuk-Wasser M, Andreadis TG, Fish D. Remotely-sensed vegetation indices identify mosquito clusters of West Nile virus vectors in an urban landscape in the northeastern United States. Vector Borne Zoonot Dis. 2008;8:197–206. https://doi.org/10.1089/vbz.2007.0154.
Yang L, Turo KJ, Riley CB, Inocente EA, Tian J, Hoekstra NC, et al. Can urban greening increase vector abundance in cities? The impact of mowing, local vegetation, and landscape composition on adult mosquito populations. Urban Ecosyst. 2019;22:827–39. https://doi.org/10.1007/s11252-019-00857-7.
Rodríguez-González S, Portela R, Córdoba-Aguilar A. Research trends in mosquito studies in urban areas. Acta Trop. 2023;241:106888. https://doi.org/10.1016/j.actatropica.2023.106888.
Carlson J, Keating J, Mbogo CM, Kahindi S, Beier JC. Ecological limitations on aquatic mosquito predator colonization in the urban environment. J Vector Ecol. 2004;29:331–9.
Fontenille D, Fournet F, Mercat M, Moiroux N, Van Landuyt A, Simard F. Greening cities and vectorial risks for human, animal and plants. In: Proceedings of the 22nd European Society for Vector Ecology Conference. European Society for Vector Ecology; 2022. p. 96–97.
Ibañez-Justicia A, Stroo A, Dik M, Beeuwkes J, Scholte EJ. National mosquito (Diptera: Culicidae) survey in The Netherlands 2010–2013. J Med Entomol. 2015;52:185–98. https://doi.org/10.1093/jme/tju058.
Van Bortel WV, Versteirt V, Dekoninck W, Hance T, Brosens D, Hendrickx G. MODIRISK: mosquito vectors of disease, collection, monitoring and longitudinal data from Belgium. GigaByte. 2022. https://doi.org/10.46471/gigabyte.58.
Schäfer M. A survey of the German mosquito fauna (Diptera: Culicidae) and identification of associated pathogens. Rheinische Friedrich-Wilhelms-Universität Bonn; 2015. https://hdl.handle.net/20.500.11811/6418.
Vinogradova EB. Culex pipiens pipiens mosquitoes: taxonomy, distribution, ecology, physiology, genetic, applied importance and control. Sofia: Pensoft Publishers; 2000.
Becker N, Jöst A, Weitzel T. The Culex pipiens complex in Europe. J Am Mosq Control Assoc. 2012;28:53–67. https://doi.org/10.2987/8756-971X-28.4s.53.
Gangoso L, Aragonés D, Martínez-de la Puente J, Lucientes J, Delacour-Estrella S, Estrada Peña R, et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Environ Res. 2020;188:109837. https://doi.org/10.1016/j.envres.2020.109837.
Wilkerson RC, Linton Y-M, Strickman D. Mosquitoes of the world. Vol 1. Baltimore: Johns Hopkins University Press; 2021. https://doi.org/10.1353/book.79680.
Vogels CBF. The role of Culex pipiens mosquitoes in transmission of West Nile virus in Europe. Wageningen University; 2017. https://doi.org/10.18174/418217.
Nikolay B. A review of West Nile and Usutu virus co-circulation in Europe: how much do transmission cycles overlap? Trans R Soc Trop Med Hyg. 2015;109:609–18. https://doi.org/10.1093/trstmh/trv066.
Vilibic-Cavlek T, Savic V, Petrovic T, Toplak I, Barbic L, Petric D, et al. Emerging trends in the epidemiology of West Nile and Usutu virus infections in Southern Europe. Front Vet Sci. 2019;6:437. https://doi.org/10.3389/fvets.2019.00437.
Vogels CBF, Göertz GP, Pijlman GP, Koenraadt CJM. Vector competence of European mosquitoes for West Nile virus. Emerg Microbes Infect. 2017;6:1–13. https://doi.org/10.1038/emi.2017.82.
Carrieri M, Fariselli P, Maccagnani B, Angelini P, Calzolari M, Bellini R. Weather factors influencing the population dynamics of Culex pipiens (Diptera: Culicidae) in the Po Plain Valley, Italy (1997–2011). Environ Entomol. 2014;43:482–90. https://doi.org/10.1603/EN13173.
Wang J, Ogden NH, Zhu H. The impact of weather conditions on Culex pipiens and Culex restuans (Diptera: Culicidae) abundance: a case study in peel region. J Med Entomol. 2011;48:468–75. https://doi.org/10.1603/ME10117.
Bidlingmayer WL. The measurement of adult mosquito population changes–some considerations. J Am Mosq Control Assoc. 1985;1:328–48.
Gillies MT, Wilkes TJ. A comparison of the range of attraction of animal baits and of carbon dioxide for some West African mosquitoes. Bull Entomol Res. 1969;59:441–56. https://doi.org/10.1017/s0007485300003412.
Bellini R, Veronesi R, Draghetti S, Carrieri M. Study on the flying height of Aedes caspius and Culex pipiens females in the Po Delta area. Italy J Am Mosq Control Assoc. 1997;13:356–60.
Silver JB. Mosquito ecology: field sampling methods. Berlin: Springer Science & Business Media; 2007.
Saitoh Y, Hattori J, Chinone S, Nihei N, Tsuda Y, Kurahashi H, et al. Yeast-generated CO2 as a convenient source of carbon dioxide for adult mosquito sampling. J Am Mosq Control Assoc. 2004;20:261–4.
Harbach RE. Culex pipiens: species versus species complex—taxonomic history and perspective. J Am Mosq Control Assoc. 2012;28:10–23. https://doi.org/10.2987/8756-971X-28.4.10.
Boerlijst SP, Johnston ES, Ummels A, Krol L, Boelee E, van Bodegom PM, et al. Biting the hand that feeds: anthropogenic drivers interactively make mosquitoes thrive. Sci Total Environ. 2023;858:159716. https://doi.org/10.1016/j.scitotenv.2022.159716.
Loeb R, Verdonschot PFM. Complexiteit van nutriëntenlimitaties in oppervlaktewateren. Wageningen: Wettelijke Onderzoekstaken Natuur & Milieu; 2009. https://edepot.wur.nl/5464.
Faierstein GB, Lu W, Sena AKLS, Barbosa RMR, Leal WS. Conspecific and allospecific larval extracts entice mosquitoes to lay eggs and may be used in attract-and-kill control strategy. Sci Rep. 2019;9:13747. https://doi.org/10.1038/s41598-019-50274-1.
Barnes MA, Turner CR. The ecology of environmental DNA and implications for conservation genetics. Conserv Genet. 2016;17:1–17.
Dejean T, Valentini A, Duparc A, Pellier-Cuit S, Pompanon F, Taberlet P, et al. Persistence of environmental DNA in freshwater ecosystems. PLoS One. 2011;6:e23398. https://doi.org/10.1371/journal.pone.0023398.
Boerlijst SP, Trimbos KB, Van der Beek JG, Dijkstra KDB, Van der Hoorn BB, Schrama M. Field evaluation of DNA based biodiversity monitoring of Caribbean mosquitoes. Front Ecol Evol. 2019;7:240. https://doi.org/10.3389/fevo.2019.00240.
Barnes MA, Turner CR, Jerde CL, Renshaw MA, Chadderton WL, Lodge DM. Environmental conditions influence eDNA persistence in aquatic systems. Environ Sci Technol. 2014;48:1819–27. https://doi.org/10.1021/es404734p.
Strickler KM, Fremier AK, Goldberg CS. Quantifying effects of UV-B, temperature, and pH on eDNA degradation in aquatic microcosms. Biol Conserv. 2015;183:85–92. https://doi.org/10.1016/j.biocon.2014.11.038.
Odero J, Gomes B, Fillinger U, Weetman D. Detection and quantification of Anopheles gambiae sensu lato mosquito larvae in experimental aquatic habitats using environmental DNA (eDNA). Wellcome Open Res. 2018;3:26. https://doi.org/10.12688/wellcomeopenres.14193.1.
Zhao B, van Bodegom PM, Trimbos KB. Bacterial abundance and pH associate with eDNA degradation in water from various aquatic ecosystems in a laboratory setting. Front Environ Sci. 2023;11. https://doi.org/10.3389/fenvs.2023.1025105.
O’Malley C. Guidelines for larval surveillance. In: Proceedings of the Seventy-Sixth Annual Meeting of the New Jersey Mosquito Control Association Inc. 1989. p. 44–55.
Turner CR, Barnes MA, Xu CC, Jones SE, Jerde CL, Lodge DM. Particle size distribution and optimal capture of aqueous macrobial eDNA. Methods Ecol Evol. 2014;5:676–84. https://doi.org/10.1111/2041-210X.12206.
Dempster EL, Pryor KV, Francis D, Young JE, Rogers HJ. Rapid DNA extraction from ferns for PCR–based analyses. Biotechniques. 1999;27:66–8. https://doi.org/10.2144/99271bm13.
Coyne KJ, Craig CS. Molecular approaches to the investigation of viable dinoflagellate cysts in natural sediments from estuarine environments. J Eukaryot Microbiol. 2005;52:90–4. https://doi.org/10.1111/j.1550-7408.2005.05202001.x.
Coyne KJ, Hutchins DA, Hare CE, Cary SC. Assessing temporal and spatial variability in Pfiesteria piscicida distributions using molecular probing techniques. Aquat Microb Ecol. 2001;24:275–85. https://doi.org/10.3354/ame024275.
Deiner K, Walser J-C, Mächler E, Altermatt F. Choice of capture and extraction methods affect detection of freshwater biodiversity from environmental DNA. Biol Conserv. 2015;183:53–63. https://doi.org/10.1016/j.biocon.2014.11.018.
Goldberg CS, Turner CR, Deiner K, Klymus KE, Thomsen PF, Murphy MA, et al. Critical considerations for the application of environmental DNA methods to detect aquatic species. Methods Ecol Evol. 2016;7:1299–307. https://doi.org/10.1111/2041-210X.12595.
Zhou YJ, Estes MK, Jiang X, Metcalf TG. Concentration and detection of hepatitis A virus and rotavirus from shellfish by hybridization tests. Appl Environ Microbiol. 1991;57:2963–8. https://doi.org/10.1128/aem.57.10.2963-2968.1991.
Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Misener S, Krawetz SA, editors. Bioinformatics methods and protocols. Totowa: Humana Press; 1999. p. 365–86. https://doi.org/10.1385/1-59259-192-2:365.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9. https://doi.org/10.1093/bioinformatics/bts199.
Boerlijst S. SamBoerlijst/WHIMSY: v0.2.5-alpha. Zenodo; 2023. https://doi.org/10.5281/zenodo.7924736
Verdonschot PFM, Besse-Lototskaya AA. Flight distance of mosquitoes (Culicidae): a metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica. 2014;45:69–79. https://doi.org/10.1016/j.limno.2013.11.002.
Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD. The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol. 2014;51:55–62. https://doi.org/10.1603/me13003.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
Stoutjesdijk PH, Barkman JJ. Microclimate, vegetation & fauna. 2nd ed. Leiden: Brill; 2015.
Yang S, Wang LL, Stathopoulos T, Marey AM. Urban microclimate and its impact on built environment–a review. Build Environ. 2023;238:110334. https://doi.org/10.1016/j.buildenv.2023.110334.
Hug DO, Stegmayer RI, Blanckenhorn WU, Verhulst NO. Thermal preference of adult mosquitoes (Culicidae) and biting midges (Ceratopogonidae) at different altitudes in Switzerland. Med Vet Entomol. 2023;37:562–73. https://doi.org/10.1111/mve.12653.
Wimberly MC, Davis JK, Evans MV, Hess A, Newberry PM, Solano-Asamoah N, et al. Land cover affects microclimate and temperature suitability for arbovirus transmission in an urban landscape. PLoS Negl Trop Dis. 2020;14:e0008614. https://doi.org/10.1371/journal.pntd.0008614.
Clements AN. The sources of energy for flight in mosquitoes. J Exp Biol. 1955;32:547–54. https://doi.org/10.1242/jeb.32.3.547.
Clements AN. The biology of mosquitoes. Volume 2: sensory reception and behaviour. 1st ed. CABI Publishing; 1999.
Geery P, Holub R. Seasonal abundance and control of Culex spp. in catch basins in Illinois. J Am Mosq Control Assoc. 1989;5:537–40.
Rydzanicz K, Jawień P, Lonc E, Modelska M. Assessment of productivity of Culex spp. larvae (Diptera: Culicidae) in urban storm water catch basin system in Wroc\law (SW Poland). Parasitol Res. 2016;115:1711–20. https://doi.org/10.1007/s00436-016-4912-x.
Low M, Tsegaye AT, Ignell R, Hill S, Elleby R, Feltelius V, et al. The importance of accounting for larval detectability in mosquito habitat-association studies. Malar J. 2016;15:1–9. https://doi.org/10.1186/s12936-016-1308-4.
Garcia R. Mosquito management: ecological approaches. Environ Manage. 1983;7:73–8. https://doi.org/10.1007/BF01867044.
Dambach P. The use of aquatic predators for larval control of mosquito disease vectors: opportunities and limitations. Biol Control. 2020;150:104357. https://doi.org/10.1016/j.biocontrol.2020.104357.
Vonesh JR, Blaustein L. Predator-induced shifts in mosquito oviposition site selection: a meta-analysis and implications for vector control. Isr J Ecol Evol. 2010;56:263–79. https://doi.org/10.1560/IJEE.56.3-4.263.
Boerlijst SP, Johnston ES, Ummels A, Krol L, Boelee E, Bodegom PM, et al. Biting the hand that feeds: anthropogenic drivers interactively make mosquitoes thrive. Sci Total Environ. 2023;858:159716. https://doi.org/10.1016/j.scitotenv.2022.159716.
Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu Rev Entomol. 2009;54:37–56. https://doi.org/10.1146/annurev.ento.54.110807.090611.
Grech MG, Manzo LM, Epele LB, Laurito M, Claverie AÑ, Ludueña-Almeida FF, et al. Mosquito (Diptera: Culicidae) larval ecology in natural habitats in the cold temperate Patagonia region of Argentina. Parasit Vectors. 2019;12:214. https://doi.org/10.1186/s13071-019-3459-y.
Dale P, Knight J. Wetlands and mosquitoes: a review. Wetl Ecol Manag. 2008;16:255–76. https://doi.org/10.1007/s11273-008-9098-2.
Ciota AT, Drummond CL, Ruby MA, Drobnack J, Ebel GD, Kramer LD. Dispersal of Culex mosquitoes (Diptera: Culicidae) from a wastewater treatment facility. J Med Entomol. 2012;49:35–42. https://doi.org/10.1603/me11077.
Krol L, Blom R, Dellar M, van der Beek JG, Stroo AC, van Bodegom PM, et al. Interactive effects of climate, land use and soil type on Culex pipiens/torrentium abundance. One Health. 2023;17:100589. https://doi.org/10.1016/j.onehlt.2023.100589.
Hassall C. The ecology and biodiversity of urban ponds. Wiley Interdiscip Rev Water. 2014;1:187–206. https://doi.org/10.1002/wat2.1014.
Hassall C, Anderson S. Stormwater ponds can contain comparable biodiversity to unmanaged wetlands in urban areas. Hydrobiologia. 2015;745:137–49. https://doi.org/10.1007/s10750-014-2100-5.
Guilland C, Maron P-A, Damas O, Ranjard L. Biodiversity of urban soils for sustainable cities. Environ Chem Lett. 2018;16:1267–82. https://doi.org/10.1007/s10311-018-0751-6.
Willott E. Restoring nature, without mosquitoes? Restor Ecol. 2004;12:147–53. https://doi.org/10.1111/j.1061-2971.2004.00392.x.
Acknowledgements
We express our gratitude to the administrators of the research sites for granting us permission to carry out our fieldwork. Sam P. Boerlijst is thanked for his invaluable contribution to the design and building of the weather stations.
Funding
This publication is part of the project 'Preparing for vector-borne virus outbreaks in a changing world: a One Health Approach' (NWA.1160.1S.210) which is (partly) financed by the Dutch Research Council (NWO).
Author information
Authors and Affiliations
Contributions
LK: conceptualization, investigation, data curation, formal analysis, visualization, writing—original draft; ML: investigation; LB: investigation; DW: investigation; ED: investigation; KT: writing—review & editing; MD: writing—review & editing; PB: writing—review & editing, supervision; GG: writing—review & editing, supervision; MS: conceptualization, writing—review & editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Krol, L., Langezaal, M., Budidarma, L. et al. Distribution of Culex pipiens life stages across urban green and grey spaces in Leiden, The Netherlands. Parasites Vectors 17, 37 (2024). https://doi.org/10.1186/s13071-024-06120-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06120-z