Abstract
This review brings together information on mosquitoes, the diseases they transmit and the wetlands that provide habitats for the immature stages (eggs and larvae). Wetland values are mentioned, though the main literature on this does not generally overlap the mosquito issue. Mosquito management is overviewed to include: the use of larvicides, source reduction in intertidal wetlands and management in freshwater systems. There is not a great deal of information on mosquitoes and freshwater systems, except for constructed wetlands and they are considered separately. We then consider restoration mainly in the context of wetlands that have been the subject of habitat modification for mosquito control. Land use and climate change, as they affect mosquitoes and the diseases they transmit, are also reviewed, as this will affect wetlands via management activities. Finally the review addresses the critical issue of balancing health, both human and environmental, in an adaptive framework. It concludes that there is a need to ensure that both mosquito and wetland management communicate and integrate to sustain wetland and human health.
Similar content being viewed by others
Introduction
Wetlands are indisputably important ecosystems, as evidenced by a wealth of literature spanning several decades. Many conferences, books and papers have been devoted to the subject of wetlands: their importance, threats to their integrity and management issues. There is also an extensive literature on mosquito-borne disease, mosquitoes and their management. The immature stages of mosquitoes need water and so there is a coincidence of wetlands and mosquito-borne disease vectors at both the global and local scales. To illustrate this at the global scale Fig. 1 shows the world distribution of wetlands of various kinds and the distribution of some of the major mosquito-borne diseases and their vectors: malaria, yellow fever, dengue, filariasis and Japanese encephalitis. At the scale presented, details are not shown for the salt-affected or inland wetlands nor are the large areas of permafrost in the northern hemisphere shown, though global warming could reduce the extent of permafrost and replace it with other wetlands.
World distribution of wetlands and some significant mosquito-borne human diseases (Adapted from the following sources: World wetlands map http://www.soils.usda.gov/use/worldsoils/mapindex/wetlands.html. Accessed 25th October 2007; other diseases: World Health Organization (WHO) http://www.ciesin.columbia.edu/docs/001-613/001-613.html. Accessed 28th September 2007
This review focuses on the issue of wetlands, mosquitoes and mosquito-borne disease. It will first provide an overview of mosquito ecology, focussing on the habitats of the immature stages. It will then very briefly consider the wetlands of the world and their values, as these values may be impacted by mosquito management. The major mosquito-borne diseases will be reviewed including references to the wetland habitats that are important to them. The review will cover mosquito management and its impact on a range of wetlands, impacts of change (especially of climate change), and adaptive approaches to balancing the environment and human health issues. It will also highlight gaps in knowledge that need to be filled in order to understand the issues and optimise wetland and disease-vector mosquito management.
Approach
The topic of wetlands and mosquitoes is a large one. In order to obtain an overview of the field, as well as key references, we carried out a broad preliminary analysis of the literature. The database was compiled in Endnote v 9 and comprised searches on title, keywords and abstract content, to provide a broad view. The wide search was used to inform specific aspect of the review but frequency counts were restricted to title content, for focussed information. Databases included Amed and Healthstar (both via OVID), Pubmed, Web of Science and the results were combined to include the authors’ personal Endnote records. Preliminary sorting removed duplicates and items that, although important to the area were not the focus of the current review. These included inter alia genetic analyses of mosquitoes (though not if also related to management), pathogens, vaccine development and clinical case studies. A database of over 3000 references resulted and was sorted by categories, such as ‘mosquito’ AND ‘wetland’, ‘disease’ AND ‘mosquito’. We tried to include as many review papers as possible since they represent a synthesis that helps cover at least indirectly the breadth of the topic area. In the following sections we first outline results showing how the literature has changed since 1983 and how focus has shifted within sub-categories. We chose 1983 as it is convenient for searching electronic databases and, in any case, important earlier papers are referenced in the papers that we have cited here.
Mosquitoes and wetlands
The post 1983 literature shows a recent large increase in interest in mosquitoes and wetlands and highlights the increasing recognition that both aspects are important. Around 10% of the references included as terms both mosquito and wetlands of various types (e.g., salt marsh (in its various forms), mangrove, swamp etc.). Of these 21% were published recently, between 2004 and 2007. The following section summarises mosquito ecology with emphasis on the role of wetlands as mosquito habitats.
Mosquito ecology overview
Mosquitoes are arthropods (in the Phylum Arthropoda). The virus diseases they can transmit are often referred to as arboviruses (arthropod-borne viruses). Mosquitoes belong to the Class Insecta, the Order Diptera (flies) and, within the order, all mosquitoes belong to the Family group called Culicidae. Within the family there are Sub families and then Genera. Mosquito genera important to human disease transmission include Aedes, Culex and Anopheles. Each has distinct habitat requirements, and there are differences between species. The general pattern of mosquito development is from egg to hatch through four larval instars, to pupation and then emergence as adults. The timeframe for a complete cycle may be as short as five days in tropical and subtropical environments and there are differences between species in their development times.
All mosquitoes have an intimate relationship with wetlands. Water is an essential requirement for the larval stages. There are many reference works on mosquito ecology. An early reference work is that of Lounibos et al. (1985) covering community and population dynamics, ecology, epidemiology and also the role of genetics in the life strategies of the insect. The major reference text remains that of Service (1993) who reviewed the literature on the topic of the ecology, sampling and modelling of mosquito populations. There is also a literature that is used by mosquito control agencies, on a national or regional basis, such as the work for south east Australia by Russell (1993) that provides a concise overview of the ecology of and key to the common mosquitoes and their typical habitat characteristics.
The eggs are laid in water in rafts of multiple eggs (Culex spp.), on water singly (Anopheles spp.) or singly on damp substrate that will later be flooded (Aedes spp.). In water, the eggs hatch into larvae that go through four instar stages. During this stage they feed on small organisms or decaying material. They breathe air through a siphon that is at or protrudes through the water surface, or, in some species, they attach to plant stems and obtain oxygen directly from the plant tissue. After the fourth instar they pupate and then emerge as adult flying insects. In some species the newly emerged adult female may be able to lay fertile eggs but generally a blood meal is required for protein to produce eggs. For disease vectors the pathogen is picked up during the blood meal and, if it replicates within the mosquito, it may be transmitted later to a victim during another blood meal. In some cases diseases may be passed on directly from the adult female via the egg to the larva and hence to the emerging adult. This is known as vertical transmission. From a wetland perspective it is important to identify the habitats of the immature stages (eggs and larvae) as these habitats are usually wetlands and the focus of larval, and hence wetland, management.
Oviposition sites (egg laying)
Although the egg stage determines generally the area that larvae will start their cycle, information is not detailed for all vectors of disease. Knowing where oviposition sites are is an aid to identifying larval habitats as larval survey may miss some of these, such as ephemeral sites when they are dry. Mosquitoes that have received much attention include the aedine nuisance species or vectors of viruses, such as Aedes taeniorhynchus (Wiedemann) in Florida and Aedes vigilax (Skuse) in Australia. That is, in part, because these species lay eggs on the ground surface and hence can be sampled directly (as compared to setting oviposition traps). Eggshells of Aedes spp. are good indicators of oviposition and can be used to sample at times when larvae are not abundant or are absent, as eggshells are relatively stable both spatially and temporally (Ritchie 1994). Eggshell studies have been carried out for open vegetation such as salt marsh, mainly in Australia (Dale et al. 1986; Kay and Jorgensen 1986; Ritchie 1994; Ritchie and Jennings 1994; Gislason and Russell 1997; Turner and Streever 1997; Dale et al. 2008) but there has been relatively little research on the more complex mangrove forest systems (Ritchie and Johnson 1991a, b; Ritchie and Addison 1992).
Mosquito larval habitats
The element common to all mosquito species is the need for water for the larval and pupal stages. Knowing the association between wetlands and mosquitoes is important for mosquito-borne disease as it helps to focus management. Rodriguez et al. (1996) found associations between landscape features including elevation and vegetation (mangroves) and malaria vectors in Mexico. Mercer et al. (2005) found significant relationships between immature mosquitoes and habitat characteristics such as water quality, in wetlands in Iowa (USA). They also explored the literature to further identify microhabitats associated with mosquitoes and used these to estimate the risk of disease transmission. Most of the published research on larval habitats of Aedes taeniorhunchus in mangrove forests has been by Ritchie and colleagues, for example Ritchie and Addison (1992). Although not focussing on larvae, there have been compilations of mosquito species recorded, for instance, in Indian mangrove systems (Rajavel et al. 2005a, b; Rajavel and Natarajan 2006).
At a detailed level, Sogoba et al. (2007) in Mali, for malaria vectors, found that dry season habitats provided a refuge for mosquito populations and suggested that focusing larval control on those would reduce the wet season populations. Miller et al. (2007) showed that Anopheles gambiae s.l. larvae can survive in wet mud as well as in water and that this explained their survival in an uncertain environment (with implications for management).
Detailed local studies of larval habitats highlight the need to efficiently identify them so as to focus management (Balling and Resh 1983). Field survey is important to confirm the larval habitat status of a site but Remote Sensing (RS) and Geographic Information Systems (GIS) can speed up the process and survey large areas cost effectively. Dale et al. (1998) reviewed some examples for disease vector mosquitoes in Australia. Other research includes Dale and Morris (1996) who used aerial photographs to identify ephemeral wetlands in urban subtropical Australia; Mushinzimana et al. (2006) who used 1 m spatial resolution satellite imagery for African highlands in Kenya and Bian et al. (2006) who used GIS to estimate the spatial distribution of anopheline larval habitats in the highlands of West Kenya, taking into account terrain, landuse and surface water. Several studies have mapped the larval habitats of vectors of West Nile virus (WNV) in USA. For example, Diuk-Wasser et al. (2006) developed a spatial model showing that various mosquito vectors had clear habitat preferences and Zou et al. (2006) were able to distinguish larval habitats in Wyoming (USA) using Landsat imagery.
Information gaps
Detailed information on the immature stage habitats is relatively sparse in the refereed literature. Most of the local information is held, if at all, by vector control agencies. Management needs to have information at a scale relevant to the organism and that is at a fine level of detail. This information gap was noted by Vezzani et al. (2006) who saw a need to identify larval habitats of filariasis vector mosquitoes so as to focus management on relevant areas. In particular, there is lack of research on the immature stages of mosquitoes in forested wetlands, both freshwater and intertidal. Such research is inhibited by survey difficulty but there is potential to use novel RS technology (e.g., Dale et al. 2005b), though resources may limit this.
Wetlands of the world and their values
That wetlands are important and valuable is widely acknowledged. Schuyt and Brander (2004) have documented wetland types and their economic values and this is summarised at a global scale in Table 1.
According to Schuyt and Brander, North America and Latin America contain significant proportions of the world’s wetlands (36% and 19% repectively) as well as having serious mosquito-borne diseases.
That wetlands are threatened by change both natural and anthropogenic has been recognised in books and compilations such as that of Mitsch (1998) who noted the importance of the world’s wetlands and the processes that threaten them. A recent article in Science articulated the concern that mangrove losses threaten the ecosystem services they provide and thus the resource for future generations (Duke et al. 2007). A significant global advance in documenting the world’s wetlands of international significance was made by the Ramsar convention in 1971. There was a recent overview of this and comprehensive inventory of listed wetlands as of March 2008, when 160,158,832 ha of wetlands in 1722 sites were protected by the convention (Secretariat of the Ramsar Convention 2008). The Ramsar aim is to develop and extend a global list of wetlands that are ecologically significant for maintaining biodiversity, ecological functions and for sustaining systems upon which much life depends.
In the overlap between mosquitoes and wetlands, wetland value is not often mentioned and mosquito value is itself a novel concept. Exceptions include Mercer et al. (2005) who stressed the value of wetlands and the ecological services they provide, measuring the abundance of mosquitoes as well as other insects and recording other environmental variables such as water quality and vegetation cover in freshwater reconstructed wetlands in Iowa USA. Mosquito value was also addressed by Schafer (2004) who considered the contribution that mosquitoes make to wetland biodiversity in a range of wetland types from forest to meadow to shallow water. Schafer’s research is relevant to addressing a frequently asked question: What use are mosquitoes? Mokany (2007) suggested that mosquitoes, being abundant in ephemeral wetlands, can have a significant effect on ecosystem processes and functions and, by implication, wetland value.
A great deal of the wetland literature is in the area of ecology, hydrology and ecohydrology, with the latter as an important area that brings the first two disciplines together. However, relatively few papers also overlap with mosquitoes or their habitats although most wetlands have some potential as mosquito habitat. To manage the mosquito problem the ecohydrology of their environment needs to be understood. References that are useful in this respect for the intertidal systems in general include Elliott et al. (2007). For specific systems, the book by Mazda et al. (2007) bring together a literature that addresses mangrove processes in detail in order help conserve the systems. The review of salt marsh management by Dale and Hulsman (1990) addressed both salt marsh values and mosquito management issues. A specific study demonstrating the application of an hydrological approach is that of (Shaman et al. 2002) who used a dynamic hydrology model, based on meteorological data, topography, soils and vegetation to model wetness and thus to predict mosquitoes in New Jersey, USA. Freshwater wetlands are covered to some extent by the constructed wetland literature (reviewed below) but there appears to be little information on freshwater forested wetlands.
Information gaps
Wetland studies that consider wetland values, from a human perspective, tend to focus on the positive values and do not take much account of the negative ones. This needs to be addressed through research to inform a balanced approach to management. Having made that point, there is also an argument that intrinsic value should include the fauna in its entirety, including mosquitoes, as contributing to value, demonstrated, for example, by Schafer (2004). This again needs more research to investigate the nature of the values.
Mosquito transmitted diseases
There has been a growing literature on mosquitoes and disease, especially for WNV, after its introduction into the USA in 1999, affecting both humans and animals. Of over 1000 references to WNV in our database, 96% were dated 1999 or later. The spread of WNV prompted the review of host-vector disease models by Wonham et al. (2006). That 78% of all the references relate to WNV and diseases such as Ross River virus also highlights the preponderance of research into diseases of the developed areas of the world that have much less serious consequences for human health than, for example, malaria or filariasis. There are also general reviews for arboviruses in particular regions, such as the review of a wide range of arboviruses in western Europe by Lundstrom (1999). Vector competence (the ability to transmit disease) may be used to prioritise mosquito and wetland management and so is briefly introduced in the next section.
Vector competence
Mosquitoes may be a nuisance, but where they are also capable of transmitting disease to human (or animals), the case for direct or indirect management becomes important. Although there may be a correlation between the presence of specific mosquitoes and mosquito-borne disease, vector competence research is needed to confirm the relationship, usually by means of laboratory experiments. There is a large amount of research on this area, some of which reports correlations between specific diseases such as WNV and mosquito species (e.g., Dennett et al. 2007). Most vector competence research focuses on a specific mosquito or on a specific disease. A few recent examples are given here. For a mosquito species Aedes aegypti (L) has received much attention: as a vector of filariasis (Tiawsirisup and Nithiuthai 2006); of yellow fever (Johnson et al. 2002; Jupp and Kemp 2002) and of dengue (Knox et al. 2003). For a specific disease, WNV, the vector competence of several species has been explored, for example, of Culex spp. (Weng et al. 2000; Vaidyanathan and Scott 2007; Balenghien et al. 2007; Reisen et al. 2006), of Culiseta and Culex spp. (Reisen et al. 2006) and of Californian species including Culex spp., Ochlerotatus spp. and Aedes vexans (Meigen) (Goddard 2003; Chen et al. 2000). The next section provides an overview of the major diseases shown in Fig. 1 (and some others) and the wetland environments that support their vectors.
Malaria
Malaria in humans is a serious disease, endemic to the tropics, whose pathogen (Plasmodium spp.) is transmitted by mosquitoes of the genus Anopheles. The mosquitoes occupy a wide range of habitats from freshwater to saline and these may be subdivided according to elevation, landform or land use. The literature includes area-specific research on the Anopheles spp. habitats. Some, that also focus on disease, are shown in Table 2 and others that focus on the mosquitoes include Stoops et al. (2007) who found general associations with higher elevation and lower tree canopy coverage, higher water temperatures, and shallow water as well as more species specific associations in Indonesia. Muturi et al. (2006) found relationships between land use and mosquito abundance (Anopheles and also Culex spp.) in Kenya. Climate, especially temperature, is important as the pathogen does not thrive in low (<16°C) or high (>32°C) temperatures. This has implications for climate change and its potential impacts on malaria distribution.
Malaria in Sub-Sahara Africa has received a great deal of attention, as it is one of the most significant diseases there. It is also significant in other places and a review of research conducted in six areas in Indonesia that examined the relationships between malaria and environment, including socio-economic variables, found spatial and temporal heterogeneity in most variables, indicating the need for locally specific information for management (Dale et al. 2005a).
Some of the genetic research also has relevance to the distribution of the disease and hence control of wetland vectors. For example a recent paper by Yawson et al. (2007) concluded that ecological barriers are more important to gene flow in Anopheles gambiae than geographical distance. Different genotypes may have different vector competencies, resulting in different disease incidences and hence priorities for mosquito or wetland management.
Yellow fever
Yellow fever is not well covered in the research literature, at least for material that includes wetlands. Much research appears to be in the area of genetics of its major vector Aedes aegypti and hence is not covered here. As well, the vectors are mainly container breeders and natural wetlands are not necessarily the preferred habitat. A useful review is that of Bourgeade and Marchou (2003) who describe the epidemiology of yellow fever in Africa and South America.
Filariasis
Filariasis is vectored by a range of mosquito species both aedine, anopheline and culicine and infects both humans and dogs. It occurs widely (Fig. 1) but is not much reported in terms of the focus of this review. Vezzani et al. (2006) reviewed its history in Argentina. Its vectors in Italy were reviewed by Cancrini et al. (2006).
Japanese encephalitis
Japanese encephalitis (JE) is a virus transmitted by Culex spp. and often associated with pigs close to human habitation. It may be included in discussion of WNV, though JE is a more serious disease, often resulting in death or permanent impairment. It is only found in Asia in a rough triangle from Pakistan to Indonesia and to Siberia (Spira 2007) though its vectors are more widespread (Fig. 1). Bourgeade and Marchou (2003) showed three zones of the disease: epidemic, to the north (e.g., Korea, Japan, Nepal, northern India), endemic to the south (Papua-New Guinea, southern India, Indonesia) and a transition zone between. There are many papers on aspects of the disease itself that also include the term mosquito but few refer to wetlands. A study by Hemmerter et al. (2007) is an example of genetic research relevant to this review. They suggested that the lack of JE in Australia may be related to a different lineage of Culex annulirostris (the major vector in south east Asia), that may not be a very competent vector.
West Nile virus
Since the arrival of WNV in USA in 1999 it has generated a great deal of interest. Initially it was not regarded as a very serious disease but it is now recognised as causing death, especially in the elderly (Bourgeade and Marchou 2003). Kramer et al. (2007) reviewed the disease comprehensively and included information on its ecology. Petersen and Roehrig (2001) suggested that WNV was a re-emerging global pathogen and that theme has been developed by others, including, for example, Boyer et al. (2002) who viewed WNV as the first pandemic of the twenty-first century. Others include Gerhardt (2006), Glaser (2004), Hayes et al. (2005) and Hayes and Gubler (2006).
Concern over the spread of WNV was reflected by Hubalek (2000), who reviewed its epidemiology in Europe with the aim of informing what might happen in USA after the initial detection of the virus in 1999. There have been several reviews of aspects of WNV such as its introduction and subsequent spread in USA (Gould and Fikrig 2004), its biology and potential to spread to South America, citing mosquitoes in the genus Culex as the major vector (Granwehr et al. 2004). Jourdain et al. (2007) referred to risk in the Camargue in southern France and Kaptoul et al. (2007) noted the first case in Spain. Risk of WNV has been assessed for the British Isles by Medlock et al. (2005), based on the ecology of the mosquito vectors.
Many of the references relate to birds: their deaths and their potential to spread the disease e.g., Medica et al. (2007), Koenig et al. (2007) and Ladeau et al. (2007). Hubalek (2000) noted that wetlands are implicated in the transmission cycle via the role of birds.
As well as indicating mosquito vectors some papers also provide information on their habitat characteristics, landscape and climate. Pecoraro et al. (2007) found relationships between WNV and landscape and climate (this is also relevant to climate change concerns, to be discussed later).
Ross River and Barmah Forest viruses
Ross River virus is important in the Asia Pacific and especially in Australia. It has been reviewed by several authors including Russell (2002) and Gatton et al. (2004). It is widely distributed, as there are several vectors whose habitats cover a range of wetland type from intertidal to freshwater and from permanent to ephemeral water bodies (Kelly-Hope et al. 2004). Muhar et al. (2000) found that risk of Ross River virus in Brisbane, Australia was significantly related to several wetland habitats including intertidal and freshwater ones associated with floodplains. More recently, Barmah Forest virus has become a public health issue in Australia, with symptoms similar to Ross River virus and the two diseases have some mosquito vectors in common (Doggett et al. 1999; Quinn et al. 2005).
Dengue
Dengue is mainly vectored by peridomestic mosquitoes, including Aedes aegypti (and more recently Aedes albopictus (Skuse)) so it is not a major focus for wetland issues. Nevertheless Aedes albopictus vectors other arboviruses (La Crosse, WNV), is also found in natural wetlands and has the ability to spread rapidly, as noted by Benedict et al. (2007).
Information gaps
What emerges from a review of mosquitoes and disease is that relatively little emphasis is placed on the wetland habitats in which the vectors spend the critical larval stage of development and on which management often concentrates. The focus tends to be on the species that transmit disease and the relationship between vector and host (including intermediate hosts).
In practice, mosquito-borne disease management includes a range of actions from health education and clinical practice to wetland management with a focus on larval habitats. The next section focuses on larval management in wetlands.
Mosquito management and its impacts on wetlands
While this section focuses on mosquito management, the other side of the coin needs to be kept in mind: wetland management and how this interacts with mosquito control. An Australian perspective was provided by Dale and Morris (1994). In the present review 32% of the 430 ‘mosquito’ AND ‘management’ OR ‘control’ references referred to wetlands and, of these, 17% related to constructed wetlands (discussed below). Reducing the risk of mosquito problems is most effectively done at the larval stages when the larvae are spatially concentrated. Adulticiding may be carried out but, by the time the adult flying stage is reached, dispersal is often several kilometres and may be up to 50 km for some species. It is important to note that larvae per se are not a human disease or nuisance problem. The problem is when they emerge as adults ready to take a blood meal and both larviciding and source reduction aim to prevent adult emergence.
There seems to be a trend away from attempting to annihilate mosquitoes or destroy the environment. Had we looked further back in time we would find that early mosquito agencies in USA often had the term ‘extermination’ in their name. For example, the New Jersey Mosquito Extermination Association was created in 1914 and later changed ‘Extermination’ to ‘Control’, as it now is. There is a large grey literature, including manuals for mosquito management published by various mosquito control organisations but these are not reviewed here. Some were referenced in Dale and Hulsman (1990) in the salt marsh context. They noted an evolution from an approach that may destroy the intertidal wetland as well as its mosquito population to a more benign approach that seeks to minimally alter the environment, based on the premise that relatively subtle changes may affect the mosquito without destroying wetland function.
Mosquito management methods have been developed over a lengthy period, especially in USA. Patterson (2004) reviewed the development of mosquito control in USA in his book “Mosquito Wars”. In it he described the evolution from reliance on chemicals such as DDT to source reduction (habitat modification of wetlands) and to the chemicals that are currently used and that are believed to be less injurious to the environment than those used in earlier decades. Floore (2006) reviewed the history specifically of larval control in USA from the 1900s, when water bodies were treated with kerosene or an arsenical product, to today’s methods of larviciding and source reduction.
In the context of disease control there is also research being conducted on ways to reduce the vector competence of mosquitoes, such as discussed in Joardar (2005), or to develop methods based on genetics, an approach reviewed by Tu and Coates (2004) and reviewed and explored by Linser et al. (2007) or to use radiation (e.g., Helinski et al. 2006). If successful and adopted they would minimise the direct impact of mosquito management on wetlands. However, none of these are yet in common use and so are not considered in the following sections.
The following will provide a general overview of current larval management methods and their impact on wetlands, to include: source reduction in intertidal wetlands (salt marsh and mangroves), freshwater and forested wetlands (not mangroves) and constructed wetlands.
Larvicides and their impacts on wetlands
Larvicides have a role to play in disease reduction. For example, Walker and Lynch (2007), in their review of larvicide use in tropical Africa, noted its effectiveness in reducing malaria, especially when used in conjunction with other methods (e.g., bednets, source reduction).
Larvicides are generally highly effective at killing mosquitoes, though resistance may become a problem. It is beyond the scope of this review to review the products used to control/manage larval populations but some general comments can be made. There is a range of chemical and biorational larvicides currently in use. The main chemical categories include, for example, some organophosphates, surface agents such as monomolecular surface films and oils as well as products that are termed biorationals. Floore (2006) listed products that are generally no longer used in USA and reflecting the growing concern for environmental impacts. Those products included various oils, organophosphate and chlorinated hydrocarbons, though specific products varied by state. For biorationals, Floore (2007) edited a 330 page supplement to the Journal of the American Mosquito Control Association dedicated to this topic. The publication contains reviews on a wide range of biorational control agents, a few of which are referenced here. They include the widely used synthetic bacterial agents such as Bacillus thuringiensis var israelensis (B.t.i.), Bacillus sphaericus (Lacey 2007) and the insect growth regulator methoprene (Henrick 2007).
Forays into the field of biological control agents have had variable results. Other insects that are predators of mosquito larvae are reviewed by Quiroz-Martinez and Rodriguez-Castro (2007). Copepods are an example of biological organisms that predate on mosquito larvae (Marten and Reid 2007). Fish may be effective in some circumstances, though the use of Gambusia may have adverse effects on native fish (Walton 2007). Recent experiments with a mould (Lagenidum) were encouraging, including its safety for non-target organisms (Vyas et al. 2007).
Because of resistance problems there is a wealth of literature on testing new products or variants of existing ones. For example Park et al. (2005) developed a new strain of B.t.i. effective against vectors of WNV. In the present review over 10% of papers referred to efficacy testing of mosquito control agents but few of these also considered impacts on non-target organisms, an important aspect for wetland health. Exceptions include Vyas et al. (2007) noted above, for a mould, and Merritt et al. (2005) for B. sphaericus, noting no detrimental effects on, for example, taxa richness and abundance of non-target invertebrates, for six applications of insecticide over a three-year study.
Impacts
Dale and Hulsman (1990) in the context of salt marsh mosquito management noted that research on the impacts of chemicals on non-target organisms generally did not assess long-term or chronic impacts. Papers that assess the impacts of larvicides on non-target organisms may include field components associated with laboratory assessment, but long-term effects and effects on food webs are still not well understood. A study of the sub-lethal effects of selected larvicides on a native fish species was investigated by Hurst et al. (2007) and found no significant impacts for microbial larvicides and insect growth regulators, but did note effects of some organophosphate compounds. All were tested at concentrations greater than those used for mosquito management.
Source reduction and its impact on intertidal wetlands
Source reduction simply means reducing the source of mosquito populations. This does not necessarily mean destroying the wetland, as relatively small changes may adversely impact the mosquito life cycle and prevent adult emergence. In the intertidal environment the general group of source reduction methods currently in use in the USA include tidal recirculation, reported by Resh and Balling (1983) for California, Open Marsh Water Management (OMWM), first developed in New Jersey in the late 1960s (Ferrigno and Jobbins 1968), and developed on east coast USA intertidal wetlands, usually for those that had been ditched. By the 1980s, the OMWM method was widely used and guidelines for its implementation were developed and accepted by the relevant federal agencies (US Corp of Engineers and the US Fish and Wildlife Service). Other guidelines include those of Bruder (1980), Meredith et al. (1985) and Hruby and Montgomery (1986). Open Marsh Water Management was reviewed by Wolfe (1996) and its much smaller Australian version, runnelling, was reported in Hulsman et al. (1989).
In Florida, a state that was extensively impounded for mosquito control in the 1950s and 1960s, the recognition of wetland values led to the Rotational Impoundment Management (RIM) concept whereby tidal circulation is allowed during the winter when mosquitoes are less of a problem, and the impoundments are flooded up in spring to reduce the summer habitats of the immature stages (Carlson and Carroll 1983; Carlson and Vigliano 1985; Carlson 1986). The RIM method works by keeping water levels high and consistent and so the damp-drying-wetting conditions needed by aedine mosquitoes for oviposition, egg conditioning and hatch are not satisfied. Schmalzer (1995) reviewed the history of this aspect of the Florida marshes.
Impacts
Impacts of the various methods of source reduction have been assessed, generally over the short term (<3 years). Examples include, for OMWM, early papers reported in the reviews by Dale and Hulsman (1990) and Wolfe (1996) and in the assessment by Meredith et al. (1983). Much of the early work was published in conference or meeting proceedings and is not easily accessed (e.g., Romanowski and Risch 1986). Research was at that time in response to the growing concerns about coastal wetlands, their values and threats to them or to their wildlife, such as birds (Brush et al. 1986). More recent research/monitoring is in reports such as the comprehensive assessment for Suffolk County (Wertheim Demonstration Project) north east USA (Cashin Associates 2007) and theses (Latchford 1997; Breitfuss 2003), and is generally relatively short term. For a longer term study the 20 year monitoring of runnelling in Australia is relevant (Dale 2007). Some innovative multivariate impact assesment techniques have been developed for that research examining effects on process rather than simply on the state of the system (Dale et al. 2002; Dale and Dale 2002).
The impacts of impounding have been discussed in the reviews referred to above (e.g., Dale and Hulsman 1990; Patterson 2004). Florida (USA) features largely in this area of research with impacts assessed for a variety of organisms (e.g., for fish, Harrington and Harrington 1982). Brockmeyer et al. (1997) in their paper on rehabilitation of impounded estuarine wetlands noted that impounding had had “a profoundly negative impact” on the wetlands (p. 96). A response has been to develop the RIM method (referred to above) that restores or partly restores tidal circulation to impoundments. Impact assessment of RIM has focussed on the extent to which it restores flora and fauna, but a major objective is also to manage mosquitoes (see Carlson and Vigliano 1985). One difficulty is that, after being impopunded for some decades, the marsh elevation may not have evolved in line with natural marshes, and may be relatively lower in some impoundments (Parkinson et al. 2006). They suggested that restoring tidal flooding, unless this is done very gradually, may result in water to depths that inhibit plant colonisation and hence jeopardise restoration success.
Freshwater wetlands
Research on freshwater wetland communities may note mosquitoes, but may not mention disease risk or suggest management. For example, Reese and Batzer (2007), studying the floodplain of the unregulated Altamah river in south east USA, found a diverse assemblage of aquatic fauna and mentioned rapidly developing organisms including mosquitoes associated with the upper reaches. Ephemeral wetlands may be important for mosquito populations. Certain species (such as Culex annulirostris in Australia) are known to rapidly colonise ephemeral wetlands. In their work on the wetlands of Iowa (USA), Mercer et al. (2005) found that the greatest risk of disease transmission was associated with ephemeral pools, an observation supported by others such as Dale and Morris (1996). Also in an ephemeral pond context, Mokany (2007) considered the impact of mosquito larvae (and tadpoles) on the invertebrate community, although without reference to potential human health impacts of the mosquitoes after emergence from the wetland.
Natural freshwater wetlands include both forested wetlands (swamps) and marshes and have been a neglected area of mosquito-specific research. Forested wetlands include habitats for mosquito vectors of disease. For example Hachiya et al. (2007) noted that the areas sampled for the Eastern Equine Encephalitis vector (Culiseta melanura (Coquillett)) were in forested wetlands. Eastern Equine Encephalitis can also infect humans. Schafer and Lunstrom (2001) compared mosquito species trapped in four forested wetlands in Sweden. There is a literature on managing forested wetlands, which, although not specifically for mosquito control, may be relevant to mosquito issues. An example is the review by Sun et al. (2001) on the hydrologic impacts of common forest uses, including drainage. Drainage has, particularly in the past, also been used as a source reduction technique for mosquito management. However the research also showed effects such as raised water tables following harvest in some situations, for example in Cypress and other forested swamps and, incidentally, a habitat of Culiseta melanura (Hachiya et al. 2007). These are very relevent to the creation of, or increase in, mosquito larval habitats, although not surprisingly this is not mentioned in specifically forest-focussed research.
One method of managing freshwater mosquitoes, apart from larviciding, is source reduction and this may involve draining or filling the larval habitats as described by Shililu et al. (2007). Their work was on malaria vectors in a semiarid part of Africa (Eritrea) and, because of the severe health risk, local communities were enlisted to monitor and fill or drain ephemeral wetlands, though this would destroy wetland function. In that case the human health risk was a paramount consideration. Gu et al. (2006) showed theoretically that source reduction, by reducing oviposition sites, could lead to a longer mosquito life cycle (mosquitoes have to search for longer to find suitable sites to lay eggs) and that this could have important implications for reducing disease incidence (in that case malaria).
Constructed wetlands are a specific category of freshwater wetlands and these have generated a great deal of interest as discussed below.
Constructed wetlands
Constructed wetlands are increasingly being used for water treatment (stormwater, secondary treated effluent) and also contribute wetland values to the environment. Schafer et al. (2004) assessed biodiversity in natural and constructed wetlands in temperate Sweden. They found mosquito abundance and species richness higher in the natural wetlands than in the constructed ones, although adult nuisance mosquitoes were associated with all the wetlands. However constructed wetlands may also create or contribute to a mosquito problem. Russell (1999) cautioned that they provided mosquito habitats, and potential for recurrence of diseases that had been eliminated in Australia (e.g., malaria, filariasis). There has been a body of research conducted since then showing that mosquito larvae may be found in the wetlands. For example, Gingrich et al. (2006) found a range of species, including vectors of WNV, in constructed wetlands in Delaware USA. Other researches have focused on management strategies to minimise larval habitats by, for example, vegetation management (Thullen et al. 2002; Jiannino and Walton 2004; Walton and Jiannino 2005). Conversely, flooding felled and dried vegetation, especially Typha spp., may increase mosquito abundance (Walton and Jiannino 2005). Marsh design (Walton and Workman 1998; Diemont 2006) or activities such as periodic draining (Mayhew et al. 2004; Dale et al. 2007) indicate that design and maintenance are important for managing mosquito larvae to inhibit adult emergence.
Information gaps
In the area of impacts of mosquito management activites there are knowledge gaps that include: the role of mosquitoes in wetland ecology; long-term impacts on non-target organisms of larvicides; long-term impacts of habitat management on wetlands and, for forested wetlands, the need to identify the nature of larval habitats, suggest management options and evaluate impacts.
Restoration
There is a growing literature on restoring damaged wetlands. This is a complex problem and needs an integrated interdisciplinary approach such as in Wassen and Grootjans (1996), who described a key ecohydrology approach in the Netherlands. Keddy (1999), recognising that restoration tended to be somewhat ad hoc and based on individual cases, proposed a scheme to unify the process, using assembly rules and indicators of ecosystem integrity, applicable across the range of wetlands from intertidal to fresh. A recent review by Elliott et al. (2007) for estuaries, coastal and marine systems that have been damaged by human land uses, highlighted topics that are poorly understood, such as the ecological goods and services provided by wetlands and they proposed a clarified nomenclature. Zedler and Nelson (2001) wrote a handbook for an adaptive management approach to restoring tidal wetlands (mainly salt marshes), that detailed the practical work involved, for a wide range of physio-chemical and biological variables. Although not necessarily referring to mosquitoes the concepts noted above are useful guides for restoration.
Restoring wetlands that have been affected by mosquito control activities or other disturbance, usually related to modifications such as ditching or impounding, is often in intertidal wetland areas and usually involves restoring tidal circulation. An early approach is exemplified by Broome et al. (1988). Although it does not specifically refer to mosquito-related disturbance, it does discuss water management and that could include mosquito management activities. However, Broome et al. do not consider the interruption to marsh evolution that may have resulted from disturbance over a lengthy period. This is addressed, for impounded marshes in Florida (USA), by Parkinson et al. (2006, cited above).
Restoration may also specifically include mosquito management. Much of the research on this topic has been conducted in USA. For ditched marshes restoration usually re-introduces tidal flushing by the OMWM approach introduced in the 1960s in New Jersey (USA) and mentioned above (Ferrigno and Jobbins 1968). Tidal flushing has also been restored, or partly restored, to impounded marshes in many areas. A temperate example is in Connecticut (USA), where Swamy et al. (2002) reported a long-term study and noted that recovery may take decades. Recovery may be more rapid in tropical and subtropical areas and research on RIM has been conducted on Florida (USA) marshes that were impounded in the 1950s and 1960s. The method takes into account the need for mosquito management especially during the summer and the need to conserve wetland values. It thus restores tidal access during autumn and winter, and floods the marshes in spring and summer, using floodgates and pumps if needed (Carlson and Carroll 1983; O’Bryan et al. 1990; Brockmeyer et al. 1997). Lewis and Gilmore (2007) provided a very useful review of mangrove forest restoration in Florida east coast wetlands that had been impounded for mosquito control. They suggested strategies for restoration but did not discuss the potential to also restore the mosquito populations that led to impoundmnet in the first place.
Restoring wetland function and benefitting wildlife may also have the unintended effect of creating mosquito habitat, though this may be mitigated by vegetation management (removal) (Lawler et al. 2007). An example from Australia that involved restoring tidal flushing to a wetland showed not only changes to the vegetation with colonisation by salt marsh and mangrove species (intended) but also increased oviposition by disease vector mosquitoes (unintended) (Turner and Streever 1999).
Information gaps
There is a need for a better understanding of how to restore wetland function without also creating or enhancing mosquito habitat. This requires interdisciplinary approaches, as integrating research from several areas is relevant to the complex issue of mosquitoes and wetlands. Because ecosystem time frames may be long ones it will require long-term research to assess the effectiveness of restoration as well as monitoring to check for developing mosquito larval habitats.
Impacts of change
While climate change is currently a focus of interest, there are also considerable direct and indirect impacts of human land use decisions on vector-borne disease and mosquitoes and some of these may be indirectly related to climate change. Patz et al. (2004) reviewed the effects of land use changes on a variety of mosquito-borne diseases. They also referred to the Millennium Ecosystem Assessment (noted by Pongsiri and Roman (2007), see below).
Specific land use changes such as deforestation can lead to environmental change conducive to mosquito success. Walsh et al. (1993) considered the effects of deforestation on a range of diseases. Previously cited, Yasuoka and Levins (2007) did the same more recently for malaria in Africa and Asia and Vittor et al. (2006) found large increases in anopheline mosquito biting rates in an area that had been deforested in the Peruvian Amazon. Land use change can have unexpected impacts on mosquito populations. Thus Lindsay et al. (2007), reviewing inland Western Australia, reported an increase in dryland salinity, possibly related to clearing for agriculture, that appeared to have led to the incursion of the salt marsh mosquito (Aedes camptorhynchus (Thomson)), a vector of Ross River virus. Derraik and Slaney (2007) reviewed the literature on anthropogenic change (referring also to climate change) and the potential effect on mosquitoes and mosquito-borne disease in New Zealand. Climate change is explored in the next section.
Climate change
In the last quarter of the twentieth century climate change was not widely accepted as a fact. With the fourth report of the IPCC (2007) this has changed and climate change and its health impacts are now receiving much attention. The literature that refers specifically to the effect of climate change on mosquito-borne disease goes back to the 1980s when the scenario was uncertain. One of the earliest publications was that of Liehne (1988) who considered the effects in Australia. On the simple expectation, as it then was, that rainfall and temperature would increase, Liehne predicted that malaria risk would increase in the tropical areas and the breeding season of Culex annulirostris would extend further south and that this could lead to an increased incidence of polyarthritis diseases such as Ross River virus. He also predicted an increase in the range and frequency of Australian encephalitis disease.
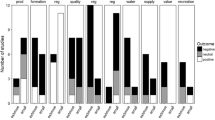
As interest in and concern about climate change and health has increased there has been an increasing number of papers on the topic since the mid 1990s (Fig. 2).
In a relatively early paper Patz and Balbus (1996) foresaw the need to model climate change scenarios and advocated the use of RS and GIS to assess risk related to climate change. Mellor and Leake (2000) reviewed the climatic factors important for vector-borne disease in order to predict climate change effects. Githeko et al. (2000) reviewed the implications of climate change on a regional basis. In summary they expected that the greatest impacts would be felt at the extremes of the temperature ranges at which transmission occurs, via effects on incubation rates and mosquito survival. For specific areas they expected particular outcomes and these are summarised in Table 3 below. They concluded for all areas that the effects of climate change are likely to be variable.
Reiter (2001) took a more conservative approach in his review of the history of the major mosquito-borne diseases (malaria, yellow fever and dengue). He noted that “ the natural history of mosquito-borne diseases is complex, and the interplay of climate, ecology, vector biology and many other factors defies simplistic analysis.” (p. 158). Githeko et al. (2000) and Reiter (2001) both noted that the effects of climate-induced disease risk may be offset by high standards of living. Hunter (2003) reviewed the potential impacts of increased rainfall and higher temperatures on vector-borne disease (and water borne disease). Kuhn et al. (2007) reviewed the use of climate to predict infectious diseases including mosquito-borne disease, in the light of climate variability and change. That was published prior to the most recent IPCC predictions, but is still relevant to developing early warning systems that would assist disease management and have potential wetland consequences.
Recent papers by Watson et al. (2005), Patz et al. (2005) and Sunyer and Grimalt (2006) discussed the likely impacts of climate change, on vector-borne disease. Watson et al. (2005), in their critical review, noted the need for very broad environmental monitoring and that this should also include responses of biological systems to climate change. A recent paper by McMichael et al. (2006) reviewed the literature on climate change and health at a global level and included a figure that summarised the pathways whereby climate change may affect human health, including the impact on natural systems (and this would include wetlands).
The use of RS, GIS and other climate change modelling approaches is growing. Some predictive modelling has been done on disease risk, based on vector ecology and likely climate changes. An example is the Hotspots models of de Wet et al. (2005) that assessed the risk of Australian disease vector mosquitoes arriving in or dispersing in New Zealand. Zou et al. (2007) used a relatively simple GIS to estimate the risk of WNV; Eisele et al. (2003) focused on malaria in Africa, as did Ceccato et al. (2005). Thomson et al. (1996, 2005) showed that satellite RS, even at low resolution, could assist in predicting malaria outbreaks in Africa, using the Normalised Difference Vegetation Index (NDVI) as an indicator of antecedent rainfall in larval habitats and a precursor to adult mosquito emergence. Other modeling that is relevant includes research on the relationship between mosquitoes and other variables that are likely to be affected by climate change. An example is research into Ross River virus in Queensland, Australia whereby relationships are shown between the disease and climate (especially rainfall) and tides (sea level) (Kelly-Hope et al. 2004; Tong et al. 2005), climatic variability (Tong and Hu 2002; Tong et al. 2004) or all of these (Naish et al. 2006).
Crane et al. (2005), in the context of chemical control of insects, developed a risk model for climate change and its impacts on insect-borne disease. While it focused on using chemicals to control insects it did so with the objective of protecting aquatic life and hence the approach is relevant to wetland conservation. As with many others, Crane et al. noted the lack of sufficient information about environmental impacts of climate change and the systems that may be affected.
Information gaps
There is information on the likely effects of climate change on mosquitoes and on the diseases they transmit but little that also addresses whole system impacts and management (and by implication wetland issues). As wetlands are likely to be directly and indirectly impacted by climate change there is a need to integrate that information with information on mosquito management.
Balancing the environment and human health issues: adaptive approaches
The issue of human health and biodiversity has been addressed by Pongsiri and Roman (2007), including specific reference to mosquito-borne disease. They reported a Millennium Ecosystem Assessment that showed the need for understanding biodiversity and its relationship with health. Can we manage both for mosquitoes and also sustain the environment? This question was addressed in Dale (1993), with the view that minimal modification of suitable habitats (salt marshes) is one acceptable solution. Others have also addressed the issue, for example O’Bryan et al. (1990) and Carlson et al. (1999). There are policy documents that outline management from the environmental perspective, for example the recent draft policy for mosquito management in wildlife refuges in USA that acknowledged both the environmental values and the health risks (Department of the Interior 2007).
Adaptive management of wetlands is the subject of research that may be relevant to the interaction between mosquito management and wetland management. McWilliams et al. (2007) used an adaptive approach to impact assessment but also stressed the need to include on-going monitoring, as simple manipulations may have complex results. Olsson et al. (2004) recounted an adaptive management process for managing wetlands in southern Sweden that involved all stakeholders. This is a model that could be used to reconcile what are often seen as conflicting priorities between mosquito control and habitat management. It is similar to the approach of Carlson (1986) describing the Florida Technical Subcommittee on Managed Salt Marshes that was established in 1983 (renamed in 1985) and that resulted in collaboration not confrontation. Other references to adaptive management in a wetland context include La Peyre et al. (2001) and Dale et al. (2006).
There is a risk that wetland management may inadvertently increase mosquito populations. In constructed wetlands the potential is acknowledged and can be avoided by design and maintenance principles. However management in other areas such as in wetland restoration projects may have the unexpected effect of creating conditions suitable for mosquitoes. For example, managing water levels may favour aedine mosquitoes. An example of water level management without apparent reference to mosquitoes is reported in Palliason et al. (2006) who discuss the effect of water level management to control water lilies in west France and its effect on their species of interest: populations of whiskered tern (Chlidonias hybridus). For other examples of conservation focused wetland management research whose results may have mosquito implications, though these are not the subject of the research, see Self (2005) and Connor and Gabor (2006).
Information gaps
In the area of balancing health and wetland interests there is not much that covers both. There has been, and is, a wealth of literature on wetland management that recognises the range of issues that need to be addressed and approaches that can be used (see for example Gosselink et al. 1990; Doust and Doust 1995; Zacharias et al. 2005) including an emphasis on the importance of hydrology, as reviewed in Gilvear and Bradley (2000). Information about mosquitoes and wetlands needs to be integrated into management to help achieve a balance between what may be conflicting interests.
Conclusion
To summarise, wetlands have high intrinsic value and also provide habitats for the immature stages of mosquitoes. Mosquitoes are perceived to be a nuisance and demonstrably transmit diseases. Management of the insect often focuses on the habitats of the immature stages and so wetlands are impacted by mosquito management activities. Information on local mosquito habitats may be held by mosquito managers but little is published in the refereed scientific literature. Because wetland values are important, mosquito management activities that impact them need to be integrated into overall management plans. There needs to be communication and cooperation between mosquito management and wetland management in order to balance the interests of each and to sustain both wetland and human health, especially in the context of changing land use and climatic environments. As the interaction between wetlands, mosquitoes and mosquito-borne disease is complex there is also a need for interdisciplinary research to provide information that is useful to management.
Finally, a limitation of this review is that it is largely restricted to peer reviewed publications in the English language and thus may have missed important information published in other languages. This is an aspect that could perhaps be explored in the future by an international, multilingual team.
References
Balenghien T, Vazeille M, Reiter P, Schaffner F, Zeller H, Bicout DJ (2007) Evidence of laboratory vector competence of Culex modestus for West Nile virus. J Am Mosq Control Assoc 23:233–236
Balling SS, Resh VH (1983) Mosquito control and salt-marsh management—factors influencing the presence of Aedes larvae. Mosq News 43:212–218
Benedict MQ, Levine RS, Hawley WA, Lounibos LP (2007) Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis 7:76–85
Bian L, Li L, Yan GY (2006) Combining global and local estimates for spatial distribution of mosquito larval habitats. GISci Remote Sens 43:128–141
Bosch I, Herrera F, Navarro JC, Lentino M, Dupuis A, Maffei J, Jones M, Fernandez E, Perez N, Perez-Eman J, Guimaraes AE, Barrera R, Valero N, Ruiz J, Velasquez G, Martinez J, Comach G, Komar N, Spielman A, Kramer L (2007) West Nile virus, Venezuela. Emerg Infect Dis 13:651–653
Bourgeade A, Marchou B (2003) Yellow fever, Dengue, Japanese encephalitis and West Nile virus infection: four major arbovirus diseases. Med Mal Infect 33:385–395
Boyer J, File T, Franks W (2002) West Nile Virus: the first pandemic of the twenty-first century. Ohio J Sci 102:98–101
Breitfuss MJ (2003) The effects of physical habitat modification for mosquito control, runnelling, on selected non-target saltmarsh resources. PhD thesis, Griffith University, Brisbane, Australia, 151 pp
Brockmeyer RE, Rey JR, Virnstein RW, Gilmore RG, Earnest L (1997) Rehabilitation of impounded estuarine wetlands by hydrolic reconnection to the Indian River Lagoon, Florida (USA). Wetl Ecol Manag 4:93–109
Broome SW, Seneca ED, Woodhouse WW Jr (1988) Tidal salt marsh restoration. Aquat Bot 32:1–22
Bruder KW (1980) The establishment of unified open marsh water management standards in New Jersey. Proc N J Mosq Control Assoc 67:72–76
Brush T, Lent RA, Hruby T, Harrington BA, Marshall RM, Montgomery WG (1986) Habitat use by salt marsh birds and response to open marsh water management. Colon Waterbirds 9:189–195
Cancrini G, Magi M, Gabrielli S, Arispici M, Tolari F, Dell’Omodarme M, Prati MC (2006) Natural vectors of dirofilariasis in rural and urban areas of the Tuscan region, central Italy. J Med Entomol 43:574–579
Carlson DB (1986) Salt marsh impoundment management along Florida’s Indian River Lagoon: historical perspectives and current implementation trends. In: Waterfowl and wetlands symposium. Wilmington, Delaware, pp 358–371
Carlson DB, Carroll JD Jr (1983) Developing and implementating impoundment management methods benefitting mosquito control, fish and wildlife: a two year progress report about the Technical Subcommittee on Mosquito Impoundments. Fla Anti-Mosq Assoc 56:24–32
Carlson DB, Vigliano RR (1985) The effects of 2 different water management regimes on flooding and mosquito production in a salt-marsh impoundment. J Am Mosq Control Assoc 1:203–211
Carlson DB, O’Bryan PD, Rey JR (1999) Florida’s salt-marsh management issues. J Am Mosq Control Assoc 15:186–193
Cashin Associates (2007) Suffolk county vector control and wetlands management long term plan and generic environmental impact statement. Suffolk Count Department of Health Services, Suffolk County, New York State, 83 pp
Ceccato P, Connor SJ, Jeanne I, Thomson MC (2005) Application of geographical information systems and remote sensing technologies for assessing and monitoring malaria risk. Parassitologia 47:81–96
Chen WJ, Dong CF, Chiou LY, Chuang WL (2000) Potential role of Armigeres subalbatus (Diptera: Culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-chiu islet, Taiwan. J Med Entomol 37:108–113
Connor KJ, Gabor S (2006) Breeding waterbird wetland habitat availability and response to water-level management in Saint John River floodplain wetlands, New Brunswick. Hydrobiologia 567:169–181
Cooper RD, Waterson DGE, Frances SP, Beebe NW, Sweeney AW (2006) The anopheline fauna of Papua New Guinea. J Am Mosq Control Assoc 22:213–221
Crane M, Whitehouse P, Comber S, Ellis J, Wilby R (2005) Climate change influences on environmental and human health chemical standards. Hum Ecol Risk Assess 11:289–318
Dale P (1993) Australian wetlands and mosquito control—contain the pest and sustain the environment? Wetlands (Australia) 12:1–11
Dale PER (2007) Assessing impacts of habitat modification on a subtropical salt marsh: 20 years of monitoring. Wetl Ecol Manag 16:77–87
Dale PER, Dale MB (2002) Optimal classification to describe environmental change: pictures from the expositions. Commun Ecol 3:19–29
Dale PER, Hulsman K (1990) A critical-review of salt-marsh management methods for mosquito-control. Crit Rev Aquat Sci 3:281–311
Dale PER, Morris CD (1994) An Australian perspective on coastal wetland management and vector borne disease. In: Mitsch WJ (ed) Wetlands of the World, Part V, Section II. Elsevier, Utrecht, pp 771–781
Dale PER, Morris CD (1996) Culex annulirostris breeding sites in urban areas: using remote sensing and digital image analysis to develop a rapid predictor of potential breeding areas. J Am Mosq Control Assoc 12:316–320
Dale PER, Hulsman K, Harrison D, Congdon B (1986) Distribution of the immature stages of Aedes vigilax on a coastal salt-marsh in South East Queensland. Aust J Ecol 11:269–278
Dale PER, Ritchie SA, Territo BM, Morris CD, Muhar A, Kay BH (1998) An overview of remote sensing and GIS for surveillance of mosquito vector habitats and risk assessment. J Vector Ecol 23:54–61
Dale MB, Dale PER, Li C, Biswas G (2002) Assessing impacts of small perturbations using a model-based approach. Ecol Model 156:185–199
Dale PER, Sipe N, Anto S, Hutajulu B, Ndoen E, Papayungan M, Saikhu A, Prabowa YT (2005a) Malaria in Indonesia: a synthesis of recent research into its environmental relationships. Southeast Asian J Trop Med Public Health 36:36–137
Dale PER, Knight J, Ritchie SA, Kay BH (2005b) A practical tool to identify water bodies with potential for mosquito habitat under mangrove canopy: large scale airborne scanning in the thermal band 8–13 μm. Wetl Ecol Manag 13:389–394
Dale PER, Dale MB, Anorov J, Knight J, Minno MC, Powell B, Raynie RC, Visser JM (2006) Aspects of adaptive management of coastal wetlands: case studies of processes, conservation, restoration, impacts and assessment. In: Verhoeven JTA, Beltman B, Bobbink R, Whigham DF (eds) Wetlands: functioning, biodiversity conservation and restoration, vol 2. Springer, Berlin, pp 197–222
Dale PER, Greenway M, Chapman H, Breitfuss MJ (2007) Constructed wetlands for sewage effluent treatment and mosquito larvae at two sites in subtropical Australia. J Am Mosq Control Assoc 23:109–116
Dale PER, Knight J, Kay BH, Chapman H, Ritchie SA, Brown MD (2008) Habitat characteristics and eggshell distribution of the salt marsh mosquito Aedes vigilax in marshes in subtropical eastern Australia. J Insect Sci 8:24. Available online: http://www.insectscience.org/8.24
de Wet N, Slaney D, Ye W, Warrick R, Hales S (2005) Hotspots: exotic mosquito risk profiles for New Zealand. International Global Change Institute, University of Waikato, Hamilton, New Zealand and Ecology and Health Research Centre, Wellington School of Medicine and Health Sciences, University of Otago, Wellington, New Zealand
Dennett JA, Bala A, Wuithiranyagool T, Randle Y, Sargent CB, Guzman H, Surin M, Hassan HK, Reyna-Nava M, Unnasch TR, Tesh RB, Parsons RE, Bueno R (2007) Associations between two mosquito populations and West Nile virus in Harris County, Texas, 2003–06. J Am Mosq Control Assoc 23:264–275
Department of the Interior (2007) Draft mosquito and mosquito borne disease management policy pursuant to the National Wildlife Refuge System Improvement Act of 1997. Federal Register USA http://www.fws.gov/policy/library/E7-20236.pdf. Accessed 21 February 2008
Derraik JGB, Slaney D (2007) Anthropogenic environmental change, mosquito-borne diseases and human health in New Zealand. Ecohealth 4:72–81
Diemont SAW (2006) Mosquito larvae density and pollutant removal in tropical wetland treatment systems in Honduras. Environ Int 32:332–341
Diuk-Wasser MA, Brown HE, Andreadis TG, Fish D (2006) Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut, USA. Vector Borne Zoonotic Dis 6:283–295
Doggett SL, Russell RC, Clancy J, Haniotis J, Cloonan MJ (1999) Barmah Forest virus epidemic on the South Coast of New South Wales, Australia, 1994–1995: viruses, vectors, human cases, and environmental factors. J Med Entomol 36:861–868
Doust LL, Doust JL (1995) Wetland Management and Conservation of Rare Species. Can J Bot 73:1019–1028
Duke NC, Meynecke JO, Dittmann S, Ellison AM, Anger K, Berger U, Cannicci S, Diele K, Ewel KC, Field CD, Koedam N, Lee SY, Marchand C, Nordhaus I, Dahdouh-Guebas F (2007) A world without mangroves? Science 317:41–42
Eisele TP, Keating J, Swalm C, Mbogo CM, Githeko AK, Regens JL, Githurem JI, Andrews L, Beier JC (2003) Linking field based ecological data with remotely sensed data using a geographic information system in two malaria endemic urban areas of Kenya. Malaria J 2:44. doi:10.1186/1475-2875-2-44
Elliott M, Burdon D, Hemingway KL, Apitz SE (2007) Estuarine, coastal and marine ecosystem restoration: confusing management and science—a revision of concepts. Estuar Coast Shelf Sci 74:349–366
Ferrigno F, Jobbins DM (1968) Open marsh water management. In: 55th Annual meeting of New Jersey mosquito extermination association, pp 104–115
Floore TG (2006) Mosquito larval control practices: past and present. J Am Mosq Control Assoc 22:527–533
Floore TG (ed) (2007) Biorational control of mosquitoes bulletin no.7. J Am Mosq Control Assoc Suppl 23(2):1–329
Gatton ML, Kelly-Hope LA, Kay BH, Ryan PA (2004) Spatial-temporal analysis of Ross River virus disease patterns in Queensland, Australia. Am J Trop Med Hyg 71:629–635
Gerhardt R (2006) West Nile Virus in the United States (1999–2005). J Am Anim Hosp Assoc 42:170–177
Gilvear DJ, Bradley C (2000) Hydrological monitoring and surveillance for wetland conservation and management; a UK perspective. Phys Chem Earth Part B 25:571–588
Gingrich JB, Anderson RD, Williams GM, O’Connor L, Harkins K (2006) Stormwater ponds, constructed wetlands, and other best management practices as potential breeding sites for West Nile virus vectors in Delaware during 2004. J Am Mosq Control Assoc 22:282–291
Gislason GM, Russell RC (1997) Oviposition sites of the saltmarsh mosquito, Aedes vigilax (Skuse) (Diptera: Culicidae), at Homebush Bay, Sydney, NSW—a preliminary investigation. Aust J Entomol 36:97–100
Githeko AK, Lindsay SW, Confalonieri UE, Patz JA (2000) Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ 78:1136–1147
Githeko AK, Ayisi JM, Odada PK, Atieli FK, Ndenga BA, Githure JI, Yan GY (2006) Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malaria J 5:107. doi:10.1186/1475-2875-5-107
Glaser A (2004) West Nile virus and North America: an unfolding story. Rev Sci Tech Off Int Epizoot 23:557–568
Goddard LB (2003) Vector competence of California mosquitoes for West Nile virus (vol 8, p 1387, 2002). Emerg Infect Dis 9:406
Gosselink JG, Shaffer GP, Lee LC, Burdick DM, Childers DL, Leibowitz NC, Hamilton SC, Boumans R, Cushman D, Fields S, Koch M, Visser JM (1990) Landscape conservation in a forested wetland watershed—can we manage cumulative impacts? Bioscience 40:588–600
Gould LH, Fikrig E (2004) West Nile virus: a growing concern? J Clin Invest 113:1102–1107
Granwehr BP, Lillibridge KM, Higgs S, Mason PW, Aronson JF, Campbell GA, Barrett ADT (2004) West Nile virus: where are we now? Lancet Infect Dis 4:547–556
Gu WD, Regens JL, Beier JC, Novak RJ (2006) Source reduction of mosquito larval habitats has unexpected consequences on malaria transmission. Proc Natl Acad Sci USA 103:17560–17563
Hachiya M, Osborne M, Stinson C, Werner BG (2007) Human eastern equine encephalitis in Massachusetts: predictive indicators from mosquitoes collected at 10 long-term trap sites, 1979–2004. Am J Trop Med Hyg 76:285–292
Harrington RW Jr, Harrington ES (1982) Effects on fishes and their forage organisms of impounding a Florida saltmarsh to prevent breeding by saltmarsh mosquitos. Bull Mar Sci 32:523–531
Hay SI, Myers MF, Burke DS, Vaughn DW, Endy T, Ananda N, Shanks GD, Snow RW, Rogers DJ (2000) Etiology of interepidemic periods of mosquito-borne disease. Proc Natl Acad Sci USA 97:9335–9339
Hayes EB, Gubler DJ (2006) West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med 57:181–194
Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL (2005) Epidemiology and transmission dynamics of West Nile Virus disease. Emerg Infect Dis 11:1167–1173
Helinski MEH, Parker AG, Knols BGJ (2006) Radiation-induced sterility for pupal and adult stages of the malaria mosquito Anopheles arabiensis. Malaria J 5:41. doi:10.1186/1475-2875-5-41
Hemmerter S, Slapeta J, van den Hurk AF, Cooper RD, Whelan PI, Russell RC, Johansen CA, Beebe NW (2007) A curious coincidence: mosquito biodiversity and the limits of the Japanese encephalitis virus in Australasia. BMC Evol Biol 7:100. doi:10.1186/1471-2148-7-100
Henrick CA (2007) Methoprene. J Am Mosq Control Assoc 23:225–239
Hruby T, Montgomery WG (1986) Open marsh water management for open tidal marshes in the northeast. A manual of methods. Mass Audubon Soc, Lincoln Massachusetts
Hubalek Z (2000) European experience with the West Nile virus ecology and epidemiology: could it be relevant for the new world? Viral Immunol 13:415–426
Hulsman K, Dale PER, Kay BH (1989) The runnelling method of habitat modification—an environment-focused tool for salt-marsh mosquito management. J Am Mosq Control Assoc 5:226–234
Hunter PR (2003) Climate change and waterborne and vector-borne disease. J Appl Microbiol 94:37S–46S
Hurst TP, Kay BH, Ryan PA, Brown MD (2007) Sublethal effects of mosquito larvicides on swimming performance of larvivorous fish Melanotaenia duboulayi (Atheriniformes: Melanotaeniidae). J Econ Entomol 100:61–65
Intergovernmental Panel on Climate Change (IPCC) (2007) Fourth assessment report. World Meteorlogical Organization (WMO) and United Nations Environment Programme (UNEP) http://www.ipcc.ch/ipccreports/assessments-reports.htm. Accessed 10 April 2008
Jiannino JA, Walton WE (2004) Evaluation of vegetation management strategies for controlling mosquitoes in a southern California constructed wetland. J Am Mosq Control Assoc 20:18–26
Joardar GK (2005) Molecular entomology: a new promising tool for malaria control. Indian J Public Health 49:231–234
Johnson BW, Chambers TV, Crabtree MB, Filippis AM, Vilarinhos PT, Resende MC, Macoris Mde L, Miller BR (2002) Vector competence of Brazilian Aedes aegypti and Ae. albopictus for a Brazilian yellow fever virus isolate. Trans R Soc Trop Med Hyg 96:611–613
Jourdain E, Toussaint Y, Leblond A, Bicout DJ, Sabatier P, Gauthier-Clerc M (2007) Bird species potentially involved in introduction, amplification, and spread of West Nile virus in a Mediterranean wetland, the Camargue (Southern France). Vector Borne Zoonotic Dis 7:15–33
Jupp PG (2001) The ecology of West Nile virus in South Africa and the occurrence of outbreaks in humans. Ann NY Acad Sci 951:143–152
Jupp PG, Kemp A (2002) Laboratory vector competence experiments with yellow fever virus and five South African mosquito species including Aedes aegypti. Trans R Soc Trop Med Hyg 96:493–498
Kaptoul D, Viladrich PF, Domingo C, Niubo J, Martinez-Yelamos S, De Ory F, Tenorio A (2007) West Nile virus in Spain: report of the first diagnosed case (in Spain) in a human with aseptic meningitis. Scand J Infect Dis 39:70–71
Kay BH, Jorgensen WK (1986) Eggs of Aedes vigilax (Skuse) and their distribution on plants and soil in southeast Queensland saltmarsh. J Aust Entomol Soc 25:267–272
Keddy P (1999) Wetland restoration: the potential for assembly rules in the service of conservation. Wetlands 19:716–732
Kelly-Hope LA, Purdie DM, Kay BH (2004) Ross River virus disease in Australia, 1886–1998, with analysis of risk factors associated with outbreaks. J Med Entomol 41:133–150
Klapsing P, MacLean JD, Glaze S, McClean KL, Drebot MA, Lanciotti RS, Campbell GL (2005) Ross River virus disease re-emergence, Fiji, 2003–2004. Emerg Infect Dis 11:613–615
Knox TB, Kay BH, Hall RA, Ryan PA (2003) Enhanced vector competence of Aedes aegypti (Diptera: Culicidae) from the Torres Strait compared with mainland Australia for dengue 2 and 4 viruses. J Med Entomol 40:950–956
Koenig WD, Marcus L, Scott TW, Dickinson JL (2007) West Nile virus and California breeding bird declines. Ecohealth 4:18–24
Kramer LD, Li J, Shi PY (2007) West Nile virus. Lancet Neurol 6:171–181
Kuhn K, Campbell-Lendrum D, Haines A, Cox J (2007) Using climate to predict infectious disease epidemics. World Health Organization, Geneva, 54 pp
La Peyre MK, Reams MA, Mendelssohn IA (2001) Linking actions to outcomes in wetland management: an overview of US state wetland management. Wetlands 21:66–74
Lacey LA (2007) Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc 23:133–163
Ladeau SL, Kilpatrick AM, Marra PP (2007) West Nile virus emergence and large-scale declines of North American bird populations. Nature 447:710–713
Latchford JA (1997) The effectiveness and environmental impacts of runnelling, a mosquito control technique. PhD thesis, Murdoch University, Perth, Western Australia, Australia, 263 pp
Lawler SP, Reimer L, Thiemann T, Fritz J, Parise K, Feliz D, Elnaiem DE (2007) Effects of vegetation control on mosquitoes in seasonal freshwater wetlands. J Am Mosq Control Assoc 23:66–70
Lewis RRI, Gilmore RG (2007) Important considerations to achieve successful mangrove forest restoration with optimum fish habitat. Bull Mar Sci 80:823–837
Liehne PFS (1988) Climatic influences on mosquito-borne diseases in Australia. In: Pearman GI (ed) Greenhouse planning for climate change. CSIRO Division of Atmospheric Research, EJ Brill, Leiden, pp 624–638
Lien JC (1991) Anopheline mosquitoes and malaria parasites in Taiwan. Gaoxiong Yi Xue Ke Xue Za Zhi 7:207–223
Lindsay MDA, Jardine A, Johansen CA, Wright AE, Harrington SA, Weinstein P (2007) Mosquito (Diptera : Culicidae) fauna in inland areas of south-west Western Australia. Aust J Entomol 46:60–64
Linser PJ, Boudko DY, Corena MDP, Harvey WR, Seron TJ (2007) The molecular genetics of larval mosquito biology: a path to new strategies for control. J Am Mosq Control Assoc 23:283–293
Lounibos LP, Rey JR, Frank JH (1985) Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory, Vero Beach, Florida, 579 pp
Lundstrom JO (1999) Mosquito-borne viruses in western Europe: a review. J Vector Ecol 24:1–39
Marten GG, Reid JW (2007) Cyclopoid copepods. J Am Mosq Control Assoc 23:65–92
Mayhew CR, Raman DR, Gerhardt RR, Burns RT, Younger MS (2004) Periodic draining reduces mosquito emergence from free-water surface constructed wetlands. Trans ASAE (Am Soc Agric Eng) 47:567–573
Mazda Y, Wolanski E, Ridd PV (2007) The role of physical processes in mangrove environments, manual for the preservation and utilization of mangrove ecosystems. Terrapub, Tokyo
McMichael AJ, Woodruff RE, Hales S (2006) Climate change and human health: present and future risks. Lancet 367:859–869
McWilliams SR, Sloat T, Toft CA, Hatch D (2007) Effects of prescribed fall burning on a wetland plant community, with implications for management of plants and herbivores. West N Am Nat 67:299–317
Medica DL, Clauser R, Bildstein K (2007) Prevalence of West Nile virus antibodies in a breeding population of American Kestrels (Falco sparverius) in Pennsylvania. J Wildl Dis 43:538–541
Medlock JM, Snow KR, Leach S (2005) Potential transmission of West Nile virus in the British Isles: an ecological review of candidate mosquito bridge vectors. Med Vet Entomol 19:2–21
Mellor PS, Leake CJ (2000) Climatic and geographic influences on arboviral infections and vectors. Rev Sci Tech Off Int Epizoot 19:41–54
Mendez F, Barreto M, Arias JF, Rengifo G, Munoz J, Burbano ME, Parra B (2006) Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am J Trop Med Hyg 74:678–683
Mercer DR, Sheeley SL, Brown EJ (2005) Mosquito (Diptera : Culicidae) development within microhabitats of an Iowa wetland. J Med Entomol 42:685–693
Meredith WH, Saveikis DE, Stachecki CJ (1983) Studies of the environmental-effects of open marsh water management, a salt-marsh mosquito-control technique. Estuaries 6:270–270
Meredith WH, Saviekis DE, Stachecki CJ (1985) Guidelines for “Open mash water management” in Delaware’s salt marshes—objectives, system designs, and installation procedures. Wetlands 5:119–133
Merritt RW, Lessard JL, Wessell KJ, Hernandez O, Berg MB, Wallace JR, Novak JA, Ryan J, Merritt BW (2005) Lack of effects of Bacillus sphaericus (Vectolex (R)) on nontarget organisms in a mosquito-control program in southeastern Wisconsin: a 3-year study. J Am Mosq Control Assoc 21:201–212
Miller JR, Huang J, Vulule J, Walker ED (2007) Life on the edge: African malaria mosquito (Anopheles gambiae s.l.) larvae are amphibious. Naturwissenschaften 94:195–199
Mitsch WJ (1998) Protecting the world’s wetlands: threats and opportunities in the 21st century. In: McComb AJ, Davis JA (eds) Wetlands for the future. Gleneagles Publishing, Adelaide, pp 19–33
Mokany A (2007) Impact of tadpoles and mosquito larvae on ephemeral pond structure and processes. Mar Freshw Res 58:436–444
Muhar A, Thalib L, Dale P, Arito E (2000) The spatial distribution of Ross River virus infections in Brisbane: significance of residential location and relationships with vegetation types. Environ Health Prev Med 4:184–189
Mushinzimana E, Munga S, Minakawa N, Li L, Feng CC, Bian L, Kitron U, Schmidt C, Beck L, Zhou GF, Githeko AK, Yan GY (2006) Landscape determinants and remote sensing of anopheline mosquito larval habitats in the western Kenya highlands. Malaria J 5:13. doi:10.1186/1475-2875-5-13
Muturi EJ, Shililu J, Jacob B, Gu WD, Githure J, Novak R (2006) Mosquito species diversity and abundance in relation to land use in a riceland agroecosystem in Mwea, Kenya. J Vector Ecol 31:129–137
Naish S, Hu W, Nicholls N, Mackenzie JS, McMichael AJ, Dale P, Tong S (2006) Weather variability, tides, and Barmah Forest virus disease in the Gladstone region, Australia. Environ Health Perspect 114:678–683
O’Bryan PD, Carlson DB, Gilmore RG (1990) Salt marsh mitigation: an example of the process of balancing mosquito control, natural resources, and development interests. Biol Sci 53:189–203
Olsson P, Folke C, Hahn T (2004) Social-ecological transformation for ecosystem management: the development of adaptive co-management of a wetland landscape in southern Sweden. Ecol Soc 9(4):2 [online] URL:http://www.ecologyandsociety.org/vol9/iss4/art2/
Paillisson JM, Reeber S, Carpentier A, Marion L (2006) Plant-water regime management in a wetland: consequences for a floating vegetation-nesting bird, whiskered tern Chlidonias hybridus. Biodivers Conserv 15:3469–3480
Paramasivan R, Mishra AC, Mourya DT (2003) West Nile virus: the Indian scenario. Indian J Med Res 118:101–108
Park HW, Bideshi DK, Wirth MC, Johnson J, Walton WE, Federici BA (2005) Recombinant larvicidal bacteria with markedly improved efficacy against Culex vectors of West Nile Virus. Am J Trop Med Hyg 72:732–738
Parkinson RW, DeLaune RR, Hutcherson CT, Stewart J (2006) Tuning surface water management and wetland restoration programs with historic sediment accumulation rates: Merritt Island National Wildlife Refuge, East-Central Florida, USA. J Coast Res 22:1268–1277
Partridge J, Ghimire P, Sedai T, Bista MB, Banerjee M (2007) Endemic Japanese encephalitis in the Kathmandu valley, Nepal. Am J Trop Med Hyg 77:1146–1149
Patterson G (2004) The Mosquito Wars: a history of mosquito control in Florida. University Press of Florida, Gainesville
Patz JA, Balbus JM (1996) Methods for assessing public health vulnerability to global climate change. Clim Res 6:113–125
Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, Bradley DJ (2004) Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect 112:1092–1098
Patz JA, Campbell-Lendrum D, Holloway T, Foley JA (2005) Impact of regional climate change on human health. Nature 438:310–317
Pecoraro HL, Day HL, Reineke R, Stevens N, Withey JC, Marzluff JM, Meschke JS (2007) Climatic and landscape correlates for potential West Nile virus mosquito vectors in the Seattle region. J Vector Ecol 32:22–28
Petersen LR, Roehrig JT (2001) West Nile virus: a reemerging global pathogen. Emerg Infect Dis 7:611–614
Pongsiri MJ, Roman J (2007) Examining the links between Biodiversity and human health: an interdisciplinary research initiative at the US Environmental Protection Agency. Ecohealth 4:82–85
Quinn HE, Gatton ML, Hall G, Young M, Ryan PA (2005) Analysis of Barmah forest virus disease activity in Queensland, Australia, 1993–2003: identification of a large, isolated outbreak of disease. J Med Entomol 42:882–890
Quiroz-Martinez H, Rodriguez-Castro A (2007) Aquatic insects as predators of mosquito larvae. J Am Mosq Control Assoc 23:110–117
Rajavel AR, Natarajan R (2006) Mosquitoes of the mangrove forests of India: part 3—Andaman and Nicobar Islands, including an update on the mosquito fauna of the islands. J Am Mosq Control Assoc 22:366–377
Rajavel AR, Natarajan R, Vaidyanathan K (2005a) Mosquitoes of the mangrove forests of India: part 1—Bhitarkanika, Orissa. J Am Mosq Control Assoc 21:131–135
Rajavel AR, Natarajan R, Vaidyanathan K (2005b) Mosquitoes of the mangrove forests of India: part 2—Sundarbans, West Bengal. J Am Mosq Control Assoc 21:136–138
Reese EG, Batzer DP (2007) Do invertebrate communities in floodplains change predictably along a river’s length? Freshw Biol 52:226–239
Reisen WK, Fang Y, Martinez VM (2006) Vector competence of Culiseta incidens and Culex thriambus for West Nile virus. J Am Mosq Control Assoc 22:662–665
Reiter P (2001) Climate change and mosquito-borne disease. Environ Health Perspect 109:141–161
Resh VH, Balling SS (1983) Tidal circulation alteration for salt-marsh mosquito-control. Environ Manag 7:79–84
Ritchie SA (1994) Spatial stability of Aedes vigilax (Diptera: Culicidae) eggshells in South East Queensland salt marshes. J Med Entomol 31:920–922
Ritchie SA, Addison DS (1992) Oviposition preferences of Aedes taeniorhynchus (Diptera: Culicidae) in Florida mangrove forests. Env Entomol 21:737–744
Ritchie SA, Jennings CD (1994) Dispersion and sampling of Aedes vigilax eggshells in southeast Queensland, Australia. J Am Mosq Control Assoc 10:181–185
Ritchie SA, Johnson ES (1991a) Aedes taeniorhynchus (Diptera: Culicidae) Oviposition Patterns in a Florida Mangrove Forest. J Med Entomol 28:496–500
Ritchie SA, Johnson ES (1991b) Distribution and sampling of Aedes taeniorhynchus (Diptera: Culicidae) eggs in a Florida mangrove forest. J Med Entomol 28:270–274
Rodriguez AD, Rodriguez MH, Hernandez JE, Dister SW, Beck LR, Rejmankova E, Roberts DR (1996) Landscape surrounding human settlements and Anopheles albimanus (Diptera: Culicidae) abundance in southern Chiapas, Mexico. J Med Entomol 33:39–48
Romanowski M, Risch T (1986) Preliminary data on the effects of open marsh water management on small mammal populations. In: 73rd Annual Meeting New Jersey Mosquito Control Association, Inc., Cape May, New Jersey, pp 105–112
Russell R (1993) Mosquitoes and mosquito borne disease in southeastern Australia. Department of Medical Entomology, Westmead Hospital, Westmead, Australia
Russell RC (1999) Constructed wetlands and mosquitoes: health hazards and management options—an Australian perspective. Ecol Eng 12:107–124
Russell RC (2002) Ross River virus: ecology and distribution. Annu Rev Entomol 47:1–31
Schafer M (2004) Mosquitoes as part of wetland biodiversity (summary). Faculty of Science and Technology, University of Uppsala, Uppsala, 63 pp
Schafer M, Lundstrom JO (2001) Comparison of mosquito (Diptera:Culicidae) fauna characteristics of forested wetlands in Sweden. Ann Entomol Soc Am 94:576–582
Schafer ML, Lundstrom JO, Pfeffer M, Lundkvist E, Landin J (2004) Biological diversity versus risk for mosquito nuisance and disease transmission in constructed wetlands in southern Sweden. Med Vet Entomol 18:256–267
Schmalzer PA (1995) Biodiversity of saline and brackish marshes of the Indian-River Lagoon—historic and current patterns. Bull Mar Sci 57:37–48
Schuyt K, Brander L (2004) Living waters: conservng the source of life. In The economic values of the worlds wetlands. World Wildlife Fund, Amsterdam, 29 pp
Secretariat of the Ramsar Convention (2008) The list of Wetlands of International Importance. http://www.ramsar.org/key_sitelist.htm. Accessed 21 March 2008
Self M (2005) A review of management for fish and bitterns, Botaurus stellaris, in wetland reserves. Fish Manag Ecol 12:387–394
Service MW (1993) Mosquito ecology: field sampling methods, 2nd edn. Elsevier Applied Science, London
Shaman J, Stieglitz M, Stark C, Le Blancq S, Cane M (2002) Using a dynamic hydrology model to predict mosquito abundances in flood and swamp water. Emerg Infect Dis 8:6–13
Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R (2007) Mosquito larval habitats in a semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. Am J Trop Med Hyg 76:103–110
Sogoba N, Doumbia S, Vounatsou P, Baber I, Keita M, Maiga M, Traore SF, Toure A, Dolo G, Smith T, Ribeiro JMC (2007) Monitoring of larval habitats and mosquito densities in the Sudan savanna of Mali: implications for malaria vector control. Am J Trop Med Hyg 77:82–88
Spira AM (2007) Japanese encephalitis: a review of trends and preventive measures. Infect Med 24:72
Stoops CA, Gionar YR, Shinta, Sismadi P, Elyazar IRF, Bangs MJ, Sukowati S (2007) Environmental factors associated with spatial and temporal distribution of Anopheles (Diptera: Culicidae) larvae in Sukabumi, West Java, Indonesia. J Med Entomol 44:543–553
Sun G, McNulty SG, Shepard JP, Amatya DM, Riekerk H, Comerford NB, Skaggs W, Swift L (2001) Effects of timber management on the hydrology of wetland forests in the southern United States. For Ecol Manag 143:227–236
Sunyer J, Grimalt J (2006) Global climate change, widening health inequalities, and epidemiology. Int J Epidemiol 35:213–216
Swamy V, Fell PE, Body M, Keaney MB, Nyaku MK, McIlvain EC, Keen AL (2002) Macroinvertebrate and fish populations in a restored impounded salt marsh 21 years after the reestablishment of tidal flooding. Environ Manag 29:516–530
Thomson MC, Connor SJ, Milligan PJM, Flasse SP (1996) The ecology of malaria—as seen from Earth-observation satellites. Ann Trop Med Parasitol 90:243–364
Thomson MC, Mason SJ, Phindela T, Connor SJ (2005) Use of rainfall and sea surface temperature monitoring for Malaria early warning in Botswana. Am J Trop Med Hyg 73:214–221
Thullen JS, Sartoris JJ, Walton WE (2002) Effects of vegetation management in constructed wetland treatment cells on water quality and mosquito production. Ecol Eng 18:441–457
Tiawsirisup S, Nithiuthai S (2006) Vector competence of Aedes aegypti (L.) and Culex quinquefasciatus (Say) for Dirofilaria immitis (Leidy). Southeast Asian J Trop Med Public Health 37(Suppl 3):110–114
Tong S, Hu W (2002) Different responses of Ross River virus to climate variability between coastline and inland cities in Queensland, Australia. Occup Environ Med 59:739–744
Tong SL, Hu WB, McMichael AJ (2004) Climate variability and Ross River virus transmission in Townsville region, Australia, 1985–1996. Trop Med Int Health 9:298–304
Tong S, Hu W, Nicholls N, Dale P, MacKenzie JS, Patz J, McMichael AJ (2005) Climatic, high tide and vector variables and the transmission of Ross River virus. Intern Med J 35:677–680
Tu Z, Coates C (2004) Mosquito transposable elements. Insect Biochem Mol Biol 34:631–644
Turner PA, Streever WJ (1997) The relationship between the density of Aedes vigilax (Diptera: Culicidae) eggshells and environmental factors on Kooragang Island, New South Wales, Australia. J Am Mosq Control Assoc 13:361–367
Turner PA, Streever WJ (1999) Changes in productivity of the saltmarsh mosquito, Aedes vigilax (Diptera: Culicidae), and vegetation cover following culvert removal. Aust J Ecol 24:240–248
Tyler KL (2004) West Nile virus infection in the United States. Arch Neurol 61:1190–1195
Vaidyanathan R, Scott TW (2007) Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector Borne Zoonotic Dis 7:193–198
Vezzani D, Eiras DF, Wisnivesky C (2006) Dirofilariasis in Argentina: historical review and first report of Dirofilaria immitis in a natural mosquito population. Vet Parasitol 136:259–273
Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA (2006) The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg 74:3–11
Vyas N, Dua KK, Prakash S (2007) Efficacy of Lagenidium giganteum metabolites on mosquito larvae with reference to nontarget organisms. Parasitol Res 101:385–390
Walker K, Lynch M (2007) Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol 21:2–21
Walsh JF, Molyneux DH, Birley MH (1993) Deforestation—effects on vector-borne disease. Parasitology 106:S55–S75
Walton WE (2007) Larvivorous fish including Gambusia. J Am Mosq Control Assoc 23:184–220
Walton WE, Jiannino JA (2005) Vegetation management to stimulate denitrification increases mosquito abundance in multipurpose constructed treatment wetlands. J Am Mosq Control Assoc 21:22–27
Walton WE, Workman PD (1998) Effect of marsh design on the abundance of mosquitoes in experimental constructed wetlands in southern California. J Am Mosq Control Assoc 14:95–107
Wassen MJ, Grootjans AP (1996) Ecohydrology: an interdisciplinary approach for wetland management and restoration. Vegetation 126:1–4
Watson RT, Patz J, Gubler DJ, Parson EA, Vincent JH (2005) Environmental health implications of global climate change. J Environ Monit 7:834–843
Weng MH, Lien JC, Lin CC, Yao CW (2000) Vector competence of Culex pipiens molestus (Diptera: Culicidae) from Taiwan for a sympatric strain of Japanese encephalitis virus. J Med Entomol 37:780–783
Wolfe RJ (1996) Effects of open marsh water management on selected tidal marsh resources: a review. J Am Mosq Control Assoc 12:701–712
Wonham MJ, Lewis MA, Renclawowicz J, Van den Driessche P (2006) Transmission assumptions generate conflicting predictions in host-vector disease models: a case study in West Nile virus. Ecol Lett 9:706–725
Yasuoka J, Levins R (2007) Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg 76:450–460
Yawson AE, Weetman D, Wilson MD, Donnelly MJ (2007) Ecological zones rather than molecular forms predict genetic differentiation in the malaria vector Anopheles gambiae s.s. in Ghana. Genetics 175:751–761
Zacharias I, Dimitriou E, Koussouris T (2005) Integrated water management scenarios for wetland protection: application in Trichonis Lake. Environ Modell Softw 20:177–185
Zedler JB, Nelson AP (2001) Handbook for restoring tidal wetlands. CRC Press, New York, pp 271–334
Zou L, Miller SN, Schmidtmann ET (2006) Mosquito larval habitat mapping using remote sensing and GIS: implications of coalbed methane development and West Nile virus. J Med Entomol 43:1034–1041
Zou L, Miller SN, Schmidtmann ET (2007) A GIS tool to estimate West Nile virus risk based on a degree-day model. Environ Monit Assess 129:413–420
Acknowledgement
We wish to acknowledge the assistance of Mr. Aubrey Chandica who took on the challenge of constructing Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dale, P.E.R., Knight, J.M. Wetlands and mosquitoes: a review. Wetlands Ecol Manage 16, 255–276 (2008). https://doi.org/10.1007/s11273-008-9098-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-008-9098-2