Abstract
Background
Mosquitoes (Diptera: Culicidae) can have a significant negative impact on human health. The urbanization of natural environments and their conversion for agricultural use, as well as human population growth, may affect mosquito populations and increase the risk of emerging or re-emerging mosquito-borne diseases. We report on the variety and number of adult mosquitoes found in four environments with varying degrees of human impact (rural, urban, rice fields, and forest) located in a savannah zone of West Africa.
Methods
Mosquitoes were collected from two regions (Hauts-Bassins and Sud-Ouest) of Burkina Faso during five periods between August 2019 and June 2021. Sampling sites were grouped according to environment. Mosquitoes were collected using BG-Sentinel traps and double net traps, and Prokopack Aspirators. Statistical analyses were performed using R software version 4.1.2. Logistic regression, using generalised mixed linear models, was used to test the effect of environment on mosquito abundance and diversity. Alpha diversity analysis was also performed, using the vegan package.
Results
A total of 10,625 adult mosquitoes were collected, belonging to 33 species and five genera: Culex, Aedes, Anopheles, Mansonia, and Ficalbia. The most dominant species were Culex quinquefasciatus, Anopheles gambiae sensu lato and Aedes aegypti. Alpha diversity was similar in the two regions. Habitat had a significant effect on mosquito species richness, the Shannon index and the Simpson index. The rural environment had the highest species richness (n = 28) followed by the forest environment (n = 24). The highest number of mosquitoes (4977/10,625) was collected in the urban environment.
Conclusions
The species composition of the mosquito populations depended on the type of environment, with fewer species in environments with a high human impact such as urban areas and rice fields. Due to the diversity and abundance of the mosquito vectors, the human populations of all of the environments examined are considered to be at potential risk of mosquito-borne diseases.
Graphical Abstract

Similar content being viewed by others
Background
The urbanisation of the natural environment and its modification due to agricultural practices and population growth expose human communities to new ecological constraints as a consequence of (i) the extinction of certain animal and plant species; (ii) variations in climatic conditions (global warming, drought, extreme climatic events); and (iii) the risk of the emergence of viral and/or parasitic diseases through disruption of the natural cycles involving hosts and pathogens. In Burkina Faso, for example, cases of cutaneous leishmaniasis were recorded in the capital, Ouagadougou, between 2000 and 2005, probably due to the urbanisation of a peripheral district of the city which favoured contact between the rodent reservoir, the sandfly vector and the urban human population [1, 2]. Bonds et al. [3] reported that biodiversity loss is a major factor in the spread of vector-borne diseases (e.g. dengue, malaria, leishmaniasis), which in turn have negative impacts on the economy and human health. Changes in land use can alter the diversity, distribution, abundance and feeding patterns of mosquito populations due to alterations in the landscapes they occupy [4]. Increasing international travel and globalization also favour the geographical spread of mosquito species, which may thus modify the Culicidae community in the areas that they invade. For example, the mosquitoes Aedes albopictus, Aedes japonicus and Aedes koreicus, which are endemic to Asia, have colonised several European countries [5, 6]. Both Ae. aegypti and Ae. albopictus have been recently found on the island of Cyprus [7]. The recent invasion of East Africa by Anopheles stephensi, a malaria vector of urban areas in India, is extremely worrying as it could jeopardise current malaria control efforts [8].
Changes in mosquito populations can influence the transmission dynamics of emerging and re-emerging infectious diseases that are transmitted by them [4, 9]. For example, anthropogenic modifications can have a positive effect on mosquito vector populations by creating favourable breeding conditions for them [10, 11]. In recent years, studies of medically important insects such as mosquitoes have been focused on gaining a more accurate understanding of the ecology of these vectors and the interactions between them and/or the pathogens that they transmit. The aim of many of these studies was to develop effective strategies to interrupt the transmission of vector-borne diseases. However, studies describing mosquito populations in the field are scarce. Understanding the abundance and spatial distribution of mosquitoes in different landscapes subject to multiple climatic and anthropogenic disturbances is essential for assessing the risk of vector-borne disease transmission. Our study was undertaken to update information on the diversity and abundance of mosquito populations in different environments (urban, rural, rice fields and forest) characteristic of a West African savannah region.
Methods
Study sites
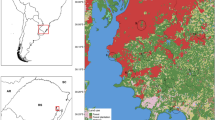
The mosquitoes were collected in the Hauts-Bassins and Sud-Ouest regions of Burkina Faso. These regions are affected by urbanisation, the increased use of land for agriculture and the development of artisanal gold mining (Fig. 1). They have an average annual rainfall of 1200 mm. The climate is tropical with two seasons: a rainy season from June to September and a dry season from October to May. The vegetation of the Hauts-Bassins region is mainly composed of tree savannah (lower total plant density) and that of the Sud-Ouest region wooded savannah (higher total plant density). Agriculture is the main economic activity in both regions, followed by artisanal gold mining in the Sud-Ouest region.
In the Hauts-Bassins region, the sampling sites were located along two road transects. Sampling started in the town of Bobo-Dioulasso. On the first transect, samples were collected in three rural areas (Banakeledaga, Sourkoudougou, Badara) and in the Vallée du Kou 3 (VK3), the rice-growing area. The ecosystem in these locations is wooded savannah on low-lying land with a very flat topography. The main crops are cereals (mainly rice in VK3) and banana and papaya. Housing mainly comprises traditional or semi-modern houses. Sampling on the second transect was carried out in two forest areas, Nasso and Dinderesso, where the dwellings are semi-modern, and agriculture is essentially cereal based.
In the Sud-Ouest region, sampling was carried out on a single road transect located between two urban areas: Diébougou and Gaoua. In addition to the two urban areas, sampling was carried out in four rural sites characterized by wooded savannah: Bapla, Tiankoura, Banlo and Bouroum-Bouroum.
Mosquito sampling and identification
Mosquito sampling was conducted during five periods (August and September 2019, June and July 2020, October 2020, May 2021, and June 2021) to cover the different climatic seasons in the region to achieve the best representativeness and optimise the sampling in terms of abundance and species richness. Sampling was carried out on 2 consecutive days. In each locality, a house was chosen for the sampling of mosquitoes outside. Three types of devices were used: a double net trap [12], a BG-Sentinel trap (Biogents, Germany) baited with BG-Lure (Biogents) and CO2 (BG trap) [13], and a Prokopack Aspirator [14]. Two double net traps were set up at two houses at least 100 m apart, one with a human volunteer and the other one with an animal (cattle) as the bait to attract mosquitoes. The human volunteer and the animal were protected by the first net, which prevented the mosquitoes from biting them. These traps were used between 6 p.m. and 7 a.m. Five BG traps were set up in five houses at 8 a.m. and operated continuously over the 2 sampling days. A Prokopack Aspirator was used to collect mosquitoes in 30 resting places (agricultural huts, abandoned houses, livestock pens and abandoned tires) around these dwellings from 6 a.m. to 11 a.m. on the 2 sampling days. The specimens were identified morphologically using identification keys [15,16,17], grouped according to date, site and environment and stored at − 80 °C for subsequent analyses.
Statistical analysis
Statistical analyses were performed with R version 4.1.2. A Kruskal–Wallis test was used to compare mean abundance between environment types. Logistic regression by generalized mixed linear models was used to test the effect of environment on mosquito abundance. Species richness and Shannon and Simpson diversity indices for the two regions and four collection environments were calculated using the vegan package [18]. Logistic regression by generalized linear models was used to test the effect of environment on each diversity index, and the emmeans package was used to compare the indices between environments.
Results
Mosquito abundance and species composition
A total of 10,625 mosquitoes were collected over the five sampling periods at 13 sites distributed across the four environments. The total number of mosquitoes was significantly different between environments (Kruskal–Wallis, χ2 = 22.29, df = 3, P < 0.001). As expected, a greater number of female mosquitoes were collected (7221/10,625, corresponding to 67.96%). Thirty-three species were identified, which belonged to the following genera: Culex, Aedes, Anopheles, Mansonia, and Ficalbia. Based on the total number of mosquitoes collected per sampling method, the Prokopack Aspirator was the most effective, followed by the BG trap, the double net plus animal trap and the double net plus human trap (Table 1). The highest diversity of mosquitoes (29 out of 33 species) was collected by the double net plus animal trap followed by the double net plus human trap (26 out of 33 species), the Prokopack Aspirator (19 out of 33 species) and the BG trap (17 out of 33 species) (Table 1). More species of Anopheles were collected in the double net traps, but the Prokopack Aspirator and the BG trap were more specific for the sampling of Ae. aegypti and Culex quinquefasciatus (Table 1). The distribution of culicids varied with environment and sampling period. Culicids were more abundant in urban areas (46.84%) and rural areas (29.28%) (Table 2). Among the mosquito genera, Culex predominated (53.92% of culicids) followed by Anopheles (23.7%) and Aedes (21.14%) (Table 2). Nine species of Anopheles were collected, predominantly from rural and rice field environments, at 39.24% and 46.22%, respectively (Table 2). Species of Aedes were most abundant in urban areas (34.04%). They represented 10.92% of the collected mosquitoes in rural areas and 11.41% in the forest environment. A total of 12 Aedes species were collected during this study, with Ae. aegypti predominating and accounting for 89.72% of the collected Aedes mosquitoes followed by Aedes vittatus at 5.65%. Nine species of Culex were identified, with Cx. quinquefasciatus being the predominant one at 78.22%. These species were predominant in the urban area at 59.41% and in the rural area at 46.90%. Other culicids, such as Aedes furcifer, Aedes fowleri, Aedes jamoti, Anopheles pretoriensis, Culex nebulosus and Culex uniformis, were observed in low numbers (Table 2).
Alpha diversity
No significant differences in species richness between the Sud-Ouest and Hauts-Bassins regions were shown by alpha diversity (P > 0.05) or Shannon (P > 0.05) and Simpson (P > 0.05) indices. Environment had a significant effect on mosquito species richness [likelihood ratio test (LRT), χ2 = 14.79, df = 3, P < 0.001), and Shannon (LRT, χ2 = 26.57, df = 3, P < 0.001) and Simpson (LRT, χ2 = 23.59, df = 3, P < 0.001)] indices. Significant differences in species richness (emmeans, Z = 3.41; SE = 0.44; P < 0.05), and diversity [Shannon (emmeans, Z = 3.92; SE = 0.26; P < 0.05) and Simpson (emmeans, Z = 3.24; SE = 0.33; P < 0.05) indices] were observed between the forest and urban environments (Fig. 2). Species richness differed slightly between the urban and rice field environments (emmeans Z = 1.95; SE = 0.49; P = 0.05). In contrast, the Shannon (emmeans, Z = 3.34; SE = 0.34; P < 0.05) and Simpson (emmeans, Z = 3.44; SE = 0.18; P < 0.05) indices showed the highest diversity of culicids in the rice field environment compared to the urban environment, where it was lowest (Fig. 2). The urban environment was less diverse than the rural environment, as shown by species richness (emmeans Z = 3.02; SE = 0.3; P < 0.05), and the Shannon (emmeans, Z = 4.35; SE = 0.19; P < 0.05) and Simpson (emmeans, Z = 4.14; SE = 0.1; P < 0.05) indices (Fig. 2).
Discussion
Human activities can modify the natural environment and provide new ecological niches that may drive a mosquito species towards adaptation or extinction. Here, we investigated the relationship between mosquito diversity and habitat modification by humans across a range of sites, comprising rural, urban, rice field and forest areas, in savannah areas of western Burkina Faso.
More mosquitoes were collected with the Prokopack Aspirator than the BG trap and the double net traps (human or animal bait). The efficacy of the Prokopack Aspirator can be explained by the fact that it is an active method that requires a technician with entomological training to search for and collect mosquitoes from potential resting sites [14]. In contrast, the BG and double net traps have been developed to capture host-seeking mosquitoes, i.e. by using a lure and CO2 as the attractants with the BG trap and a human or a bovine host for the double net trap. These collection methods are considered passive, as they only collect specimens attracted to the traps, and depend on the attractant used; the trap yields vary greatly in terms of mosquito abundance and diversity, which also depend on the context, such as the climate, environment, and type of habitat [19]. This probably explains the low density of mosquitoes collected with these types of traps during our study. It should also be noted that the types of traps we used preferentially collect female mosquitoes that usually feed on mammals [20]; for the collection of ornithophilic species, other types of traps should be used, such as bird-baited traps [21] or the recently developed nest mosquito trap [22]. In the present study, the double net plus animal trap was more effective than the double net plus human trap. This may have been due to the fact that cattle emit more CO2, which is a common host-seeking cue for mosquitoes [23], than humans. The effectiveness of these traps could also be explained by the abundance of zoophilic mosquitoes and the presence of many animals in the different environments sampled.
We collected a total of 10,625 mosquitoes representing five genera and 33 species from the four types of environments. All of the species are common members of the culicid fauna of Burkina Faso. The mosquito abundance varied greatly depending on the type of environment, with the highest abundances found in the urban and the rural sites (Table 2). The mosquito population in the urban environment had the lowest species diversity, consisting of a core community comprising the three most frequent species: Cx. quinquefasciatus, Ae. aegypti and An. gambiae. The predominance and abundance of these species in urban environments can be explained by their co-adaptation to areas in which humans live with respect to the availability of sites suitable for their larvae, trophic preferences and resting places. Culex quinquefasciatus is known to use polluted breeding sites. Population growth and urbanization lead to an increase in these potential breeding sites, and consequently the abundance of this species in cities and towns throughout the tropics [24]. In Bobo Dioulasso, Burkina Faso’s second largest city in the southwestern savannah region, Cx. quinquefasciatus was the main mosquito species identified as early as 1970 biting humans [25]. Other studies carried out in the same town confirmed its predominance and its aggressiveness towards humans [26]. Anopheles gambiae sensu lato (s.l.) mosquitoes like unpolluted stagnant water without submerged vegetation for oviposition and larval development [27, 28]. Highly urbanised city centres are not very favourable for Anopheles malaria vectors, whereas certain areas along rivers or in low-lying areas are. Anopheles arabiensis, a member of the An. gambiae s.l. complex and a major vector of malaria, which was originally distributed in the dry Sahelian regions, is now present in many West African towns [29]. In Bobo-Dioulasso, this species has become the dominant malaria vector [30, 31], whereas it was formerly present only at a low abundance [32]. In West Africa, An. arabiensis is now found in towns in the more humid areas of the forest belt of the Gulf of Guinea, such as in Nigeria [33] and Côte d'Ivoire [34]. The adaptation of this species to pollution [35] and climate change (rising temperatures, drought) is thought to promote its proliferation in West African cities. Female Ae. aegypti mostly lay their eggs in domestic and peridomestic water containers. The availability of these containers is partly due to socioeconomic activities [36, 37]. Females of this species preferentially bite humans and rest inside dwellings, and thus find all the necessary conditions to proliferate in human habitats in urban and rural areas of West Africa [38]. It is important to note the absence from our samples of the related species Ae. albopictus, the notorious Asian tiger mosquito, and particularly its absence from the urban sites near Côte d'Ivoire, Ghana and Mali, where this invasive species has recently been detected [39]. In addition, Robert et al. [32] reported certain species, such as Ae. fowleri, Ae. luteocephalus, Ae. hirsutus, in urban environments in Bobo-Dioulasso, which were not found in our study. Their absence could be due to increasing urbanization over the four last decades, which may be unfavourable for these species, which develop in natural breeding sites.
Previous studies carried out in the same western part of Burkina Faso reported the predominance of these three species in urban areas, with Cx. quinquefasciatus always being the most abundant culicid species, followed by An. gambiae s.l. and Ae. aegypti [31, 32]. A core community comprising these three species is generally found in most towns in West Africa and also in East Africa [40]. These three species can expose urban human populations to several parasitic and arboviral diseases.
Mosquitoes were more abundant in the rice fields than in the forest environment (Table 2). The rice field environment provides large areas of aquatic habitat for mosquito breeding. The dynamics of culicids in the perimeter of the Kou valley where rice is grown are essentially influenced by two factors: the season and phase of rice cultivation (watering, heading, ripening stages). Anopheles gambiae larvae, for example, are mainly present during the watering phase of the rice paddies, then disappear during the rice growth phase due to shading by the rice and eutrophication of the water [41]. In the latter study, An. gambiae and, to a lesser extent, An. funestus were the main malaria vectors present in this rice-growing area [41], whereas in our study, only An. gambiae s.l. was collected. In general, An. funestus is not very abundant in savannah rice fields in West Africa [42]. However, in the present study the mosquito population was more diverse in the forest and rural environments (Fig. 2), and there was no statistically significant difference between the populations in these two environments. The high diversity of mosquito species compared with previous studies [30, 32] in these two types of environments can be explained by the maintenance of natural areas, despite anthropogenic pressure, including in the rural environment, and the productivity of natural breeding sites (tree hollows, sheathing leaves, rocks holes, etc.) which are suitable for many sylvatic mosquito species, particularly during the rainy season when rainfall is frequent and humidity high.
We found several mosquitoes species that are vectors of parasites (Plasmodium sp.) and/or arboviruses. Species of the genera Culex, Aedes, Anopheles and Mansonia are known to transmit pathogens [43, 44]. Culex quinquefasciatus and Ae. aegypti, which are both vectors of arboviruses in tropical regions, were abundant in our four environments. Culex quinquefasciatus is a vector for West Nile virus (WNV) and Usutu virus (USUV) [45,46,47]. A recent study on the seroprevalence of WNV and USUV in Ouagadougou and Bobo-Dioulasso, the two largest cities of Burkina Faso, showed the circulation of these arboviruses in donated blood [48]. The abundance of Cx. quinquefasciatus in different types of environment could constitute a potential risk favouring the emergence of WNV and USUV. Beside the potential for pathogen transmission, females of Cx. quinquefasciatus are very aggressive towards humans and constitute a night-time nuisance due to their biting, particularly in tropical urban environments [49]. Aedes aegypti is a known vector of several arboviruses of major public health importance, including dengue virus (DENV), yellow fever virus (YFV), chikungunya virus, and Zika virus (ZKV) [50,51,52,53]. YFV, DENV and ZKV circulate in Burkina Faso [37, 54, 55]. Despite the existence of a vaccine for YFV, an epidemic occurred in southeast Burkina Faso in 1983. The outbreak, which was rural, occurred in villages and herders’ camps located close to and in gallery forests, and Ae. furcifer, a sylvatic mosquito, was the main species involved in the transmission of the disease [56]. In 1983, 1984 and 1986, seven strains of YFV were isolated in wild mosquitoes in the region of Bobo-Dioulasso in remarkably similar circumstances [57]. The mosquitoes were from sylvatic areas, never from the towns, and were found at the end of the rainy season (in October and November). Only Ae. luteocephalus was found, a predominant potential vector of YFV in the region. These findings confirm that YFV regularly circulates in the southern savanna zone of West Africa, which therefore forms part of the endemic emergence zone. Cases of YFV still occur in the country, despite the routine Expanded Program of Immunization [58]. The risk of a new YFV outbreak is increasing due to the internal displacement of people as a consequence of terrorist conflicts, which may lead to a reduction in vaccine coverage and increase the risk of YFV emergence in several localities in Burkina Faso. Urban epidemics of YFV occurred in Abidjan, the capital of Côte d'Ivoire, during the armed unrest in the country in 2000 [59]. The circulation of DENV-2 in the sylvatic mosquito Ae. luteocephalus collected in the wooded savannah area of western Burkina Faso near Bobo-Dioulasso, was reported in 1980 [60], and DENV-2 was isolated from patients in 1982 in Ouagadougou city [61]. In 1986, two strains of DENV-2 were isolated from wild mosquitoes in the Bobo-Dioulasso region [57], one from Ae. luteocephalus in the sylvatic zone, the other from Ae. aegypti in the city centre. These findings indicated that these DENV-2 variants had distinct life cycles, one urban and the other sylvatic, and that the two may coexist in the same region. Other sylvatic Aedes species, such as Ae. furcifer, Ae. vittatus, Ae. africanus and Ae. unilineatus, have been associated with arbovirus transmission in the forest zone of Senegal [62, 63], confirming that the naturally humid areas of southern West Africa are a setting for enzootic circulation of dengue viruses. Since 2013, there have been several dengue outbreaks in the main cities of the country, Ouagadougou and Bobo-Dioulasso [54, 64]. The threat of YFV and DENV, as well as other viruses transmitted by Aedes which are present in the region, such as ZKV [55], could be increased by colonization by Ae. albopictus, a potential vector of these arboviruses, which are expanding rapidly in Africa and are already present in some of the neighbouring countries of Burkina Faso [39].
In Burkina Faso, the An. gambiae complex is composed of three species, An. gambiae, An. arabiensis and An. coluzzii, which are involved in the transmission of malaria parasites to humans in the country [65]. Previous studies reported that An. arabiensis was the main malaria vector in urban areas [31] and that An. gambiae and An. coluzzii were still predominant vectors in rural and peri-urban areas [66] in the western part of Burkina Faso. Other malaria vectors, such as An. funestus and An. nili, were observed in small numbers compared with An. gambiae s.l. These vectors may be more abundant in certain contexts in favourable ecological zones and constitute locally important vectors of species of Plasmodium that infect humans [67, 68]. Anopheles stephensi, an invasive urban Asian malaria vector, was not present in our samples. The recent establishment and expansion of An. stephensi in Africa suggest that it may become a serious threat to malaria control in urban areas of the continent [8].
Conclusions
We identified 33 species of mosquitoes from four landscapes (urban, rural, rice fields and forest) in southwest Burkina Faso. Culex quinquefasciatus, An. gambiae s.l. and Ae. aegypti were the most abundant species in each environment. The species composition of the mosquito populations depended on the type of environment, with lower species diversity in highly human-modified environments such as urban areas and rice fields. The diversity and abundance of these mosquito vectors indicate that human populations in all of these environments may become more exposed to mosquito-borne diseases, in particular arboviruses, which are re-emerging or emerging in different regions of the world, including West Africa. Our main objective in the near future will be to screen the viromes (arboviruses and mosquito-specific viruses) associated with each species collected in this study to provide more information on mosquito vector-related risks in Burkina Faso.
Availability of data and materials
The datasets used during this article are fully available without restriction. Further inquiries can be directed to the corresponding authors.
References
Traoré KS, Sawadogo NO, Traoré A, Ouedraogo JB, Traoré KL, Guiguemdé TR. Preliminary study of cutaneous leishmaniasis in the town of Ouagadougou from 1996 to 1998. Bull Soc Pathol Exot. 1990;2001:52–5.
Bamba S, Gouba A, Drabo MK, Nezien D, Bougoum M, Guiguemdé TR. Epidemiological profile of cutaneous leishmaniasis: retrospective analysis of 7444 cases reported from 1999 to 2005 at Ouagadougou, Burkina Faso. Pan Afr Med J. 2013. https://doi.org/10.1160/pamj.2013.14.108.1140.
Bonds MH, Dobson AP, Keenan DC. Disease ecology, biodiversity, and the latitudinal gradient in income. Hochberg ME, editor. PLoS Biol. 2012;10:e1001456.
Meyer Steiger DB, Ritchie SA, Laurance SGW. Mosquito communities and disease risk influenced by land use change and seasonality in the Australian tropics. Parasit Vectors. 2016;9:387.
Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector-Borne Zoonotic Dis. 2012;12:435–47.
Jansen S, Cadar D, Lühken R, Pfitzner WP, Jöst H, Oerther S, et al. Vector competence of the invasive msquito species Aedes koreicus for arboviruses and interference with a novel insect-specific virus. Viruses. 2021;13:2507.
Christou M, Lippert S, Weigand A, Angelidou I, Athanasiou KC, Demetriou J, et al. First record of the invasive Asian tiger mosquito Aedes albopictus in Cyprus based on information collected by citizen scientists. J Eur Mosq Control Assoc. 2023;1–8.
Samarasekera U. A missed opportunity? Anopheles stephensi in Africa. The Lancet. 2022;400:1914–5.
Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, et al. Global consequences of land use. Science. 2005;309:570–4.
Amerasinghe FP, Ariyasena TG. Larval survey of surface water-breeding mosquitoes during irrigation development in the Mahaweli project. Sri Lanka J Med Entomol. 1990;27:789–802.
Burkett-Cadena ND, Vittor AY. Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl Ecol. 2018;26:101–10.
Tangena J-AA, Thammavong P, Hiscox A, Lindsay SW, Brey PT. The human-baited double net trap: an alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS ONE. 2015;10:e0138735.
Kröckel U, Rose A, Eiras ÁE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006;22:229–38.
Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46:1256–9.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara. Publ Afr Inst Med Res. 1987;55:1–143.
Gillies MT, De Meillon B. Anophelinae of Africa south of the Sahara. 1968;
Huang Y-M. The subgenus Stegomyia of Aedes in the Afrotropical Region with keys to the species (Diptera: Culicidae). Zootaxa. 2004;700:1.
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, Ohara RB, et al. Package ‘vegan.’ Commun Ecol Pack Version. 2013;2:1–295.
Jean Jose Nepomichene TN, Elissa N, Cardinale E, Boyer S. Species diversity, abundance, and host preferences of mosquitoes (Diptera: Culicidae) in two different ecotypes of Madagascar with recent RVFV transmission. J Med Entomol. 2015;52:962–9.
Kline DL. Semiochemicals, traps/targets and mass trapping technology for mosquito management. J Am Mosq Control Assoc. 2007;23:241–51.
Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J Med Entomol. 2006;43:83–92.
Caillouët KA, Riggan AE, Rider M, Bulluck LP. Nest mosquito trap quantifies contact rates between nesting birds and mosquitoes. J Vector Ecol. 2012;37:210–5.
McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–71.
Noori N, Lockaby BG, Kalin L. Larval development of Culex quinquefasciatus in water with low to moderate. J Vector Ecol. 2015;40:208–20.
Subra R. Biology and control of Culex pipiens quinquefasciatus Say, 1823 (Diptera, Culicidae) with special reference to Africa. Int J Trop Insect Sci. 1981;1:319–38.
Skovmand O, Ouedraogo TDA, Sanogo E, Samuelsen H, Toé LP, Baldet T. Impact of slow-release Bacillus sphaericus granules on mosquito populations followed in a tropical urban environment. J Med Entomol. 2009;46:67–76.
Carnevale P, Robert V. Les anophèles: biologie, transmission du Plasmodium et lutte antivectorielle. IRD éditions; 2017.
Mouchet J. Biodiversité du paludisme dans le monde. Arcueil: John Libbey Eurotext; 2004.
Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–97.
Dabiré KR, Diabaté A, Paré-Toé L, Rouamba J, Ouari A, Fontenille D, et al. Year to year and seasonal variations in vector bionomics and malaria transmission in a humid savannah village in west Burkina Faso. J Vector Ecol J Soc Vector Ecol. 2008;33:70–5.
Dabiré RK, Namountougou M, Sawadogo SP, Yaro LB, Toé HK, Ouari A, et al. Population dynamics of Anopheles gambiae s.l. in Bobo-Dioulasso city: bionomics, infection rate and susceptibility to insecticides. Parasit Vectors. 2012;5:127.
Robert V, Gazin P, Boudin C, Molez JF, Ouedraogo V, Carnevale P. The transmission of malaria in a wooded savannah area and a rice-growing area around Bobo Dioulasso (Burkina Faso). Ann Soc Belg Med Trop. 1985;65:201–14.
Awolola TS, Okwa O, Hunt RH, Ogunrinade AF, Coetzee M. Dynamics of the malaria-vector populations in coastal Lagos, south–western Nigeria. Ann Trop Med Parasitol. 2002;96:75–82.
Fournet F, Adja AM, Adou KA, Dahoui MMC, Coulibaly B, Assouho KF, et al. First detection of the malaria vector Anopheles arabiensis in Côte d’Ivoire: urbanization in question. Malar J. 2022;21:275.
Jones CM, Toé HK, Sanou A, Namountougou M, Hughes A, Diabaté A, et al. Additional selection for insecticide resistance in urban malaria vectors: DDT resistance in Anopheles arabiensis from Bobo-Dioulasso, Burkina Faso. PLoS ONE. 2012;7:e45995.
Namountougou M, Soma DD, Kaboré DA, Ndo S, Kientega M, Sawadogo JEM, et al. Characterisation of the breeding sites and insecticide resistance of Aedes aegypti population in the city of Bobo-Dioulaso, Burkina Faso. Afr Entomol. 2020;28:142.
Ouattara LPE, Sangaré I, Namountougou M, Hien A, Ouari A, Soma DD, et al. Surveys of arboviruses vectors in four cities stretching along a railway transect of Burkina Faso: risk transmission and insecticide susceptibility status of potential vectors. Front Vet Sci. 2019. https://doi.org/10.3389/fvets.2019.00140/full.
Egid BR, Coulibaly M, Dadzie SK, Kamgang B, McCall PJ, Sedda L, et al. Review of the ecology and behaviour of Aedes aegypti and Aedes albopictus in western Africa and implications for vector control. Curr Res Parasitol Vector-Borne Dis. 2022;2:100074.
Longbottom J, Walekhwa AW, Mwingira V, Kijanga O, Mramba F, Lord JS. Aedes albopictus invasion across Africa: the time is now for cross-country collaboration and control. Lancet Glob Health. 2023. https://doi.org/10.1016/S2214-109X(23)00046-3.
Karisa J, Muriu S, Omuoyo D, Karia B, Ngari M, Nyamwaya D, et al. Urban ecology of arboviral mosquito vectors along the Kenyan coast. J Med Entomol. 2020. https://doi.org/10.1093/jme/tjaa136.
Baldet T, Diabaté A, Guiguemdé TR. Malaria transmission in 1999 in the rice field area of the Kou Valley (Bama), (Burkina Faso). Sante Montrouge Fr. 2003;13:55–60.
Carnevale P, Guillet P, Robert V, Fontenille D, Doannio J, Coosemans M, et al. Diversity of malaria in rice growing areas of the Afrotropical region. Parassitologia. 1999;41:273–6.
Tandina F, Doumbo O, Yaro AS, Traoré SF, Parola P, Robert V. Mosquitoes (Diptera: Culicidae) and mosquito-borne diseases in Mali, West Africa. Parasit Vectors. 2018;11:467.
Ughasi J, Bekard HE, Coulibaly M, Adabie-Gomez D, Gyapong J, Appawu M, et al. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasit Vectors. 2012;5:89.
Cook CL, Huang Y-JS, Lyons AC, Alto BW, Unlu I, Higgs S, et al. North American Culex pipiens and Culex quinquefasciatus are competent vectors for Usutu virus. PLoS Negl Trop Dis. 2018;12:e0006732.
Motayo BO, Onoja BA, Faneye AO, Adeniji JA. Seasonal abundance and molecular identification of West Nile virus vectors, Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in Abeokuta, south-west Nigeria. Afr Health Sci. 2016;16:135.
Orba Y, Hang’ombe BM, Mweene AS, Wada Y, Anindita PD, Phongphaew W, et al. First isolation of West Nile virus in Zambia from mosquitoes. Transbound Emerg Dis. 2018;65:933–8.
Tinto B, Kaboré DPA, Kagoné TS, Constant O, Barthelemy J, Kiba-Koumaré A, et al. Screening of circulation of usutu and West Nile viruses: a One Health approach in humans, domestic animals and mosquitoes in Burkina Faso, West Africa. Microorganisms. 2022;10:2016.
Samuelsen H, Toé LP, Baldet T, Skovmand O. Prevention of mosquito nuisance among urban populations in Burkina Faso. Soc Sci Med. 2004;59:2361–71.
Kamgang B, Vazeille M, Yougang AP, Tedjou AN, Wilson-Bahun TA, Mousson L, et al. Potential of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to transmit yellow fever virus in urban areas in Central Africa. Emerg Microbes Infect. 2019;8:1636–41.
Monteiro FJC, Mourão FRP, Ribeiro ESD, da Rêgo MOS, da Frances PAC, Souto RNP, et al. Prevalence of dengue, Zika and chikungunya viruses in Aedes (Stegomyia) aegypti (Diptera: Culicidae) in a medium-sized city, Amazon, Brazil. Rev Inst Med Trop Sao Paulo. 2020;62:e10.
Nag DK, Payne AF, Dieme C, Ciota AT, Kramer LD. Zika virus infects Aedes aegypti ovaries. Virology. 2021;561:58–64.
Uwishema O, Eneh SC, Chiburoma AG, Fadl Elhassan WA, Abdur-Rahman Adekunle A, Rogose MS, et al. Yellow fever outbreak in Kenya: a review. Ann Med Surg. 2022. https://doi.org/10.1016/j.amsu.2022.104537.
Ouattara CA, Traore S, Sangare I, Traore TI, Meda ZC, Savadogo LGB. Spatiotemporal analysis of dengue fever in Burkina Faso from 2016 to 2019. BMC Public Health. 2022;22:462.
Tinto B, Kaboré DPA, Kania D, Kagoné TS, Kiba-Koumaré A, Pinceloup L, et al. Serological evidence of Zika virus circulation in Burkina Faso. Pathogens. 2022;11:741.
Baudon D, Robert V, Roux J, Lhuillier M, Saluzzo JF, Sarthou JL, et al. The 1983 yellow fever epidemic in Burkina Faso. Bull World Health Organ. 1986;64:873–82.
Robert V, Lhuillier M, Meunier D, Sarthou JL, Monteny N, Digoutte JP, et al. Yellow fever virus, dengue 2 and other arboviruses isolated from mosquitos, in Burkina Faso, from 1983 to 1986. Entomological and epidemiological considerations. Bull Soc Pathol Exot 1990. 1993;86:90–100.
Yaro S, Zango A, Rouamba J, Diabaté A, Dabiré R, Kambiré C, et al. Situation épidémiologique de la fièvre jaune au Burkina Faso de 2003 à 2008. Bull Société Pathol Exot. 2010;103:44–7.
Fitzner J, Coulibaly D, Ekrakouadio D, Claudeyavo J, Loukou Y, Odehourikoudou P, et al. Safety of the yellow fever vaccine during the September 2001 mass vaccination campaign in Abidjan, Ivory Coast. Vaccine. 2004;23:156–62.
Hervy J-P, Legros F, Roche J-C, Monterny N, Diaco B. Circulation du virus dengue 2 dans plusieurs milieux boisés des savanes soudaniennes de la région de Bobo-Diou-lasso (Burkina Faso). Cah-ORSTOM Entomol Méd Parasitol. 1984;22:135–43.
Gonzalez JP, Du Saussay C, Gautun JC, McCormick JB. Dengue in Burkina Faso (ex-Upper Volta): seasonal epidemics in the urban area of Ouagadougou. Bull Soc Pathol Exot Filiales. 1985;78:7–14.
Diallo D, Fall G, Diagne CT, Gaye A, Ba Y, Dia I, et al. Concurrent amplification of Zika, chikungunya, and yellow fever virus in a sylvatic focus of arboviruses in southeastern Senegal, 2015. BMC Microbiol. 2020;20:181.
Diouf B, Gaye A, Diagne CT, Diallo M, Diallo D. Zika virus in southeastern Senegal: survival of the vectors and the virus during the dry season. BMC Infect Dis. 2020;20:371.
Tarnagda Z, Cissé A, Bicaba BW, Diagbouga S, Sagna T, Ilboudo AK, et al. Dengue fever in Burkina Faso, 2016. Emerg Infect Dis. 2018;24:170–2.
Soma DD, Zogo B, de Hien DFS, Hien DA Kaboré, Kientega AS, et al. Insecticide resistance status of malaria vectors Anopheles gambiae (s.l.) of southwest Burkina Faso and residual efficacy of indoor residual spraying with microencapsulated pirimiphos-methyl insecticide. Parasit Vectors. 2021. https://doi.org/10.1186/s13071-020-04563-8.
Epopa PS, Collins CM, North A, Millogo AA, Benedict MQ, Tripet F, et al. Seasonal malaria vector and transmission dynamics in western Burkina Faso. Malar J. 2019;18:113.
Dabiré KR, Baldet T, Diabaté A, Dia I, Costantini C, Cohuet A, et al. Anopheles funestus (Diptera: Culicidae) in a humid savannah area of western Burkina Faso: bionomics, insecticide resistance status, and role in malaria transmission. J Med Entomol. 2007;44:990–7.
Soma DD, Poda SB, Hien AS, Namountougou M, Sangaré I, Sawadogo JME, et al. Malaria vectors diversity, insecticide resistance and transmission during the rainy season in peri-urban villages of south-western Burkina Faso. Malar J. 2021;20:63.
Acknowledgements
We thank the technical staff of IRSS-DRO, especially Ouari Ali, Bationo Richard, Traore S. Aboubacar and Meda G. Benson for their assistance. We would like to express our sincere appreciation for the support and kind cooperation of the inhabitants of all the sampling sites and all the volunteers during the fieldwork.
Funding
This work was funded by Montpellier University of Excellence through the French National Research Agency under the Investissements d’Avenir programme (reference ANR-16-IDEX-0006).
Author information
Authors and Affiliations
Contributions
RKD and DPAK conceived and designed the study. DDS, MK and DPAK collected the data. DPAK and MK analysed the data. DPAK drafted the manuscript. DDS, PG, SPS, GAO, PVDP, TB, SG and RKD reviewed the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol of this study was reviewed and approved by the Institutional Ethics Committee of the Institut de Recherche en Sciences de la Santé (IEC-IRSS) and registered under no. A08/2019/CEIRES. The volunteers who slept in the double net trap gave their written informed consent for participation in the study. The owner of the cattle gave written informed consent for the use of their animals in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kaboré, D.P.A., Soma, D.D., Gil, P. et al. Mosquito (Diptera: Culicidae) populations in contrasting areas of the western regions of Burkina Faso: species diversity, abundance and their implications for pathogen transmission. Parasites Vectors 16, 438 (2023). https://doi.org/10.1186/s13071-023-06050-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06050-2