Abstract
Background
Recent data from Ghana indicates that after seven rounds of annual mass drug administration (MDA) there is still sustained transmission albeit at low levels in certain areas where Anopheles melas, An. gambiae s.s., Mansonia and Culex species are the main biting mosquitoes. Anopheles gambiae s.l. and An. funestus are the known vectors in Ghana and a recent report indicated that An. melas could transmit at low level microfilaraemia. However, because An. melas is not found everywhere there was the need to determine whether any of the other culicine species could also be playing a role in the transmission of LF.
Methods
Indoor mosquitoes collected once a month for three months using pyrethrum spray catches in six communities within the Kommenda-Edina-Eguafo-Abirem (KEEA) District, Central Region of Ghana were morphologically identified, dissected and examined for the presence of W. bancrofti. Additionally, stored mosquito samples collected during previous years in 8 communities from the Gomoa District also in the Central Region were similarly processed. The identities of all W. bancrofti parasites found were confirmed using an established PCR method.
Results
A total of 825 indoor resting mosquitoes comprising of 501 Anopheles species, 239 Mansonia species, 84 Culex species and 1 Aedes species were dissected and examined for the presence of W. bancrofti. Mansonia africana had infection and infectivity rates of 2.5%. and 2.1% respectively. Anopheles gambiae s.l. had an infection rate of 0.4% and a similar infectivity rate. None of the Culex sp. and Aedes sp were found with infection. From the stored mosquitoes the infection and infectivity rates for M. africana were 7.6% (N = 144) and 2.8% respectively whilst the corresponding rates for M. uniformis were 2.9% (N = 244) and 0.8%.
Conclusions
This is the first report of Mansonia species as vectors of lymphatic filariasis (LF) in Ghana and in West Africa since that of 1958 in Guinea. The revelation of a hitherto unrecognised vector which is possibly more efficient in transmission than the recognised ones has a profound implication for elimination of lymphatic filariasis programmes in the sub-region.
Similar content being viewed by others
Background
Wuchereria bancrofti, the causative agent of lymphatic filariasis (LF) is transmitted by mosquito species belonging to Anopheles, Aedes, Culex and Mansonia depending on the geographic area. In East Africa, Anopheles and Culex species are known vectors but in West Africa only Anopheles species are the reported vectors of the disease. In Ghana An. gambiae s.l and An. funestus are the main vectors [1, 2] with An. pharoensis playing a minor role [3]. Several previous studies have determined that Culex species are refractory to W. bancrofti in West Africa [4, 5] and Ghana [6]. Although Mansonia species are identified among biting mosquito populations in LF endemic areas of Ghana their role in LF transmission are as yet unknown and only one report of vectorial status in West Africa has been published [7].
The Global Programme to Eliminate Lymphatic Filariasis (GPELF) strategy is mass drug administration to entire endemic communities. This strategy is based on the assumption that when circulating microfilariae (mf) levels are reduced below a certain threshold that decreases availability of mf to vectors, the transmission of W. bancrofti will be interrupted, especially if the vectors are anophelines [8]. This reasoning is based on the observation that anophelines exhibit the process of ‘facilitation’ whereby the number of ingested mfs developing to infective stages (L3) increases with increasing number of ingested mfs. The opposite, which is the process of ‘limitation’ is observed in culicines [9, 10]. Thus the successful elimination of LF by MDA is considered to be achievable in areas with Anopheles-W. bancrofti relationships compared to Culex or Aedes or Mansonia – W. bancrofti combinations [11]. This indicates that an understanding of the local vector-parasite combination is critical for the elimination of LF.
Since Anopheles species are the known vectors in Ghana it was assumed that after 5 – 6 rounds of once a year of MDA with a combination of ivermectin and albendazole it should be possible to interrupt transmission of LF [8, 12]. Recent evaluations of the impact of 6 rounds of MDA in Ghana have revealed residual transmission is still ongoing in some communities (Dr Nana Biritwum unpublished data). Collections of mosquitoes in these LF endemic areas have shown large numbers of Culex and Mansonia species and while it has been established that Culex does not transmit the disease in these areas [6], the status of Mansonia is not known. We therefore conducted an initial investigation regarding the involvement of Mansonia species in LF transmission in communities in one of these endemic areas i.e. Komenda-Edina-Eguafo-Abirem (KEEA) District. When infective mosquitoes were found by this initial study we then revisited stored Mansonia mosquitoes which had been collected during previous entomology studies at another endemic district i.e. Gomoa District and found all the developmental stages of W. bancrofti in Mansonia species. We report in this article the results obtained and discuss the implications for LF elimination in Ghana.
Methods
Description of study sites
KEEA District
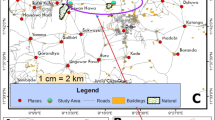
The district is located in the Central Region of Ghana between longitude 1°20’ West and 1°40’ West, and latitude 5°05’ North and 5°15’ North. There had been 5 MDAs in this area by 2008 and a parasitological survey carried out in the study communities found seven people positive for W. bancrofti out of a total of 799 screened (0.88%). The number of microfilariae per 100 μl of blood ranged from 3 to 279 using the counting chamber method. The mosquito species found in this area are An. gambiae s.l., An. funestus, An. pharoensis, Aedes species, Culex species and Mansonia species.
Gomoa District
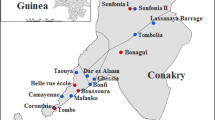
The Gomoa district is also located in the Central Region and found between longitudes 0°22’ West and 0°54’ West, and latitudes 5°14’ North and 5°35’ North. There has been 7 MDA’s in this area and the last microfilariaemia prevalence was 0.4%. Anopheles gambiae s.l, An. funestus, An. pharoensis, Culex species, Aedes species and Mansonia species are the known biting mosquitoes in the district.
Field sampling of mosquitoes
KEEA collections
Indoor resting adult mosquitoes were collected from randomly selected compounds in six villages Sanka, Epoano, Atabadze, Anyinase, Ponkrom, Bandor in the KEEA District. The collections were done on two consecutive days in each month between 0500 H and 0800 H by a team of eight collectors during August, October and December 2009 using PSC method. All knocked-down mosquitoes were picked and placed on moist filter paper lining appropriately labelled Petri dishes and transported to the laboratory for processing.
Gomoa collections
The stored mosquitoes used were collected between, 2000 – 2003 and were part of those obtained from July to December each year during entomological studies aimed at monitoring and evaluating MDA activities from 2000 – 2008. These were mosquitoes caught indoors using the HLC method simultaneously in all eight communities from 1800 H to 0600 H each day for four consecutive days for each of the six months. The mosquitoes collected at the time were kept in paper cups, transported to the laboratory, killed and sorted out according to genus to separate Anopheles species from the rest, including Mansonia spp, which were stored over silica gel until they were used for this study.
Processing of mosquitoes
Each mosquito was identified using the morphological keys of Gillies and De Meillon [13] and Gillies and Coetzee [14] and sorted out into the different species of Aedes Anopheles Culex and Mansonia.
All mosquitoes collected from KEEA were processed for W. bancrofti infections, while only the stored Mansonia spp from Gomoa District were processed for this study, because the Anopheles and Culex species obtained there had been used by earlier studies [Boakye DA, unpublished data].
Mosquito dissections
The head, thorax and abdomen of each mosquito were separated and each part placed in a drop of 1% saline solution on a slide. In the case of An. gambiae s.l. the legs were removed and placed in 1.5 ml Eppendorf tubes for molecular identification of the species complex. Each of the body parts were dissected under a dissecting microscope for the presence of W. bancrofti. The stored Mansonia spp were re-hydrated initially for 5 min in 1% saline solution in 96-well microtitre plates before dissecting as described above.
Molecular identification of An. gambiae species complex
Molecular identification of W. bancrofti
The dried carcass of dissected mosquitoes together with any W. bancrofti larvae found on each slide was scraped into a 1.5 ml Eppendorf tube and then homogenized in phosphate buffered saline (PBS). The genomic DNA was extracted using the DNeasy Tissue Kit (QIAGEN Inc., USA) following the manufacturer’s protocol. Polymerase chain reaction to confirm that parasites observed were W. bancrofti was then carried out using the method of Ramzy et al. (1997) [18].
Ethical considerations
Ethical approval for both studies was obtained from the Institutional Review Board of the Noguchi Memorial Institute for Medical Research and verbal/written consents were obtained from each local volunteers who participated in the indoor human landing catches (HLC) during July to December which correspond to the period of highest mosquito breeding in Gomoa. Prior consents to conduct pyrethrum spray catches (PSC) in rooms were also obtained from the occupants.
Results
A total of 825 mosquitoes comprising of 501 (61%) Anopheles spp, 239 (29%) Mansonia spp, 84 Culex spp (10%) and 1 Aedes spp from KEEA were studied. Seventy (70) An. gambiae s.l. were further identified as An. gambiae s.s (n = 58) and An. melas (n = 3) while nine did not amplify. Thus the ratio of An. gambiae: An. melas was approximately 20:1. Twenty-two of the An. gambiae s.s were further identified as M (n = 15) and S (n = 7) forms. All the 239 Mansonia spp were identified as M. africana.
All the 825 mosquitoes from the KEEA were dissected and five M. africana and two An. gambiae s.s were found to be infective. One M. africana was infected with one L2 stage of the parasite (Table 1). Thus the estimated infection and infectivity rates for M. africana were 2.5% and 2.1% respectively with An. gambiae s.l having an infectivity rate of 0.4%. Table 2 shows the details of the infections in individual Mansonia species from Sanka where approximately 67% of the total Mansonia species from KEEA were obtained. In this community, the five infective Mansonia mosquitoes found harboured a total of 26 L3s, and three had as many as 6, 7 and 11 L3s respectively.
Three hundred and eighty-eight stored Mansonia sp. from the Gomoa District collected between 2000–2003 were examined. Of these 144 were identified as M. africana and 244 were M. uniformis. Eleven M. africana were found infected with all stages of the parasite with four of them with infective stages, while seven M. uniformis were found infected with two of them with the infective stage. The distribution of the W. bancrofti stages among the two Mansonia species are also given in Table 3. The infection and infectivity rates for M. africana were 7.6% and 2.8% respectively while those for M. uniformis were 2.9% and 0.8 respectively.
The identity of W. bancrofti was confirmed by PCR.
Discussion
In studies on the vectors of lymphatic filariasis in Africa, species of biting mosquitoes collected include the genera Aedes, Culex Anopheles and Mansonia. Species of these genera are known to transmit lymphatic filariasis in one place or another worldwide. However, in Africa only Culex and Anopheles are reported as vectors. Culex species are important vectors in urban areas of East Africa [19, 20], but not in West Africa. A recent study carried out on Culex species in Ghana revealed that they are refractory [6]. In West Africa, including Ghana, Anopheles species have been reported as the only vectors of W. bancrofti[1–3], although there has been an earlier report of L3 of W. bancrofti in M. uniformis from Boké, Guinea [7]. Even though Mansonia species are known vectors of filariasis in some endemic countries in Asia they had not been considered as vectors in Africa. A recent study however, revealed that infective M. uniformis was possible in laboratory experiments but this was not the case in the wild [21]. Therefore, this is the first report of infection of all stages of W. bancrofti in Mansonia species in Ghana and the only one in West Africa since 1958.
The global strategy for the elimination of LF is based on the vector –parasite relationships of facilitation and limitation. Anopheles vectors of W. bancrofti have been reported to exhibit facilitation and hence elimination is feasible through MDA alone where they are the vectors. Thus West Africa is among the areas where LF elimination through MDA is expected to progress without any hitch. However, in some sites in Ghana and Burkina Faso, the prevalence of infection is not what is expected after 5 to 8 years of annual MDA. For example in the KEEA district a study conducted after 6 annual MDA showed a prevalence of 2.1% (23/1107) in some neighbouring communities to the study sites (D. Boakye unpublished report). There have been various explanations for this apparent residual infection and non-compliance by infected people to take the drugs have been postulated as was found in Haiti [22]. Others have hypothesized that on-going transmission could account for the prevalence observed and that not all Anopheles species may exhibit facilitation [23]. A recent study by Amuzu et al. at the Gomoa District sites showed that An. melas may be exhibiting limitation and could be responsible for transmission at low level parasitaemia [24]. It has also been suggested that even if vectors pick up parasites after MDA, the number of parasites ingested could be too low to sustain effective transmission. In this study, we found an individual infective Mansonia species that harboured up to 11 L3 larvae. This indicates that an infective bite could transmit many parasites that could set up an active infection. Hence, transmission could be sustained even if at a low level. Relatively, therefore, it does appear that Mansonia species could be more important compared to An. gambiae species especially at low level microfilaraemia.
Previous studies in some parts of West Africa and in Accra Ghana as summarized by Laurence, shows that Mansonia africana was more widely distributed than Mansonia uniformis and that their distribution does not completely coincide [25]. We found this to be the case since both study sites have similar ecology and housing structure but Mansonia uniformis was localized in only one study area. Even though sampling at both sites was carried out in the rainy season, the study was limited by the fact that it was not carried out at the same time. The sampling methods used at the study sites were also different and these could have been a contributing factor for the differences in the distribution of the two species.
There is currently an ethical debate on the use of the human landing collection (HLC) for sampling vector species. However, HLC provides accurate estimates of transmission indices due to anthropophilic vectors, which are difficult to obtain from other collection methods. For example, mosquitoes obtained from other methods such as the indoor residual spraying collections require analysis of bloodmeals to indicate the level of anthropophily (human biting index). In the current study, the mosquitoes processed from the Gomoa district were part of sampling to accurately determine the impact of MDA on transmission of W. bancrofti hence the use of HLC.
Conclusions
The observation from this study that M. uniformis and M. africana are vectors in the study sites show that the transmission system could be more complex than expected in some areas in West Africa. Mansonia species are seemingly important vectors, which have not been factored in the elimination strategy in the sub-region and therefore could have critical implications. Their distribution, biology and vectorial importance should be studied as well as the need to study local vectorial systems so as to complement MDA with vector control where necessary for the goals of GPELF to be achieved.
References
Dunyo SK, Appawu M, Nkrumah FK, Baffoe-Wilmot A, Pedersen EM, Simonsen PE: Lymphatic filariasis on the coast of Ghana. Trans R Soc Trop Med Hyg. 1996, 90: 634-638. 10.1016/S0035-9203(96)90414-9.
Dzodzomenyo MD, Dunyo SK, Ahorlu CK, Coker WZ, Appawu MA, Pedersen EM, Simonsen PM: Bancroftian filariasis in an irrigation project community in southern Ghana. Trop Med Int Hlth. 1999, 4: 13-18. 10.1046/j.1365-3156.1999.00354.x.
Appawu MA, Dadzie SK, Baffoe-Wilmot A, Wilson MD: Lymphatic filariasis in Ghana: entomological investigation of transmission dynamics and intensity in communities served by irrigation systems in the Upper East Region of Ghana. Trop Med Int Health. 2001, 6: 511-516. 10.1046/j.1365-3156.2001.00737.x.
Subra R, Mouchet J: Culex pipens fatigans Wiedemann in West Africa and its possible role in the transmission of Bancroft’s filariasis. WHO mimeograph document. 1967, WHO/FIL/67.73
Zielke E, Kuhlow F: On the inheritance of susceptibility for infection with Wuchereria bancrofti in Culex pipiens fatigans. Tropenmed Parasitol. 1977, 28: 68-78.
Aboagye-Antwi F: Studies on the roles of Culex and Anopheles species in the transmission of Wuchereria bancrofti (Spirurida: Filariidae) in the Gomoa District of Ghana. MPhil thesis University of Ghana, Zoology Department. 2003
Toumanoff C: Filariose humaine et sa transmission dans la Basse-Guinée (Estuaire du Rio Nunez). Bull Soc Pathol Exo. 1958, 51: 908-912.
Weber RH: Can anopheline-transmitted filariasis be eradicated?. J Trop Med Hyg. 1991, 94: 241-244.
Bain O: Transmission des filarioses. Limitation des passages de microfilaires ingérées vers l’hémocèle des vecteurs; interprétation. Ann Parasit Hum Com. 1971, 46: 613-631.
Brengues J, Bain O: Passage des microfilaires de l’estomac vers l’hemocele du vecteur, dans les couples Wuchereria bancrofti − Anopheles gambiae A, Wuchereria bancrofti – Aedes aegypti et Setaria labiatopapillosa − Aedes aegypti. Cah ORSTOM Serie Entomologie Medicale et Parasitologie. 1972, 10: 235-249.
Duerr H-P, Dietz K, Eichner M: Determinants of the eradicability of filarial infections: a conceptual approach. Trends Parasitol. 2005, 21 (2): 88-96. 10.1016/j.pt.2004.11.011.
Ottesen EA, Duke BO, Karam M, Behbehani K: Strategies and tools for the control/elimination of lymphatic filariasis. Bull WHO. 1997, 75: 441-450.
Gillies MT, De Meillon B: The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). South African Institute for Medical Research. 1968
Gillies MT, Coetzee M: A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region). South African Institute for Medical Research. 1987
Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V: A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987, 37: 37-41.
Scott JA, Brogdon WG, Collins FH: Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993, 49 (4): 520-529.
Fanello C, Santolamazza F, della Torre A: Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002, 16: 461-464. 10.1046/j.1365-2915.2002.00393.x.
Ramzy RM, Farid HA, Kamal IH, Ibrahim GH, Morsy ZS, Faris R, Weil GJ, Williams SA, Gad AM: A polymerase chain reaction assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Trans Roy Soc Trop Med & Hyg. 1997, 91: 156-160. 10.1016/S0035-9203(97)90205-4.
Magayuka SA, White GB: Hybrid compatibilities and susceptibility of Culex pipiens fatigans Wied. to Wuchereria bancrofti (Cobbold) in East Africa. Bull WHO. 1972, 46: 801.805-
Mwandawiro CS, Fujimaki Y, Mitsui Y, Katsivo M: Vectors of Bancroftian filariasis in Kwale District, Kenya. E Afr Med J. 1997, 74 (5)
Onapa AW, Pedersen EM, Reimert CM, Simonsen PE: A role for Mansonia uniformis mosquitoes in the transmission of lymphatic filariasis in Uganda?. Acta Trop. 2007, 101 (2): 159-168. 10.1016/j.actatropica.2007.01.003.
Boyd A, Won KY, McClintock SK, Donovan CV, Laney SJ, Williams SA, Pilotte N, Streit TG, de Rochars MVE Beau, Lammie PJ: A community-based study of factors associated with continuing transmission of lymphatic filariasis in Leogane, Haiti. PLoS Negl Trop Dis. 2010, 4 (3): e640-10.1371/journal.pntd.0000640.
Boakye DA, Wilson MD, Appawu MA, Gyapong J: Vector competence for Wuchereria bancrofti of the Anopheles populations in the Bongo district of Ghana. Ann Trop Med Parasitol. 2004, 98 (5): 501-508. 10.1179/000349804225003514.
Amuzu H, Wilson MD, Boakye DA: Studies of Anopheles gambiae s.l (Diptera: Culicidae) exhibiting different vectorial capacities in lymphatic filariasis transmission in the Gomoa district, Ghana. Parasite Vectors. 2010, 3: 85-10.1186/1756-3305-3-85.
Laurence BR: The biology of two species of mosquito, Mansonia africana (Theobald) and Mansonia uniformis (Theobald), belonging to the subgenus Mansonioides (Diptera, Culicidae). Bull Entomolo Res. 1960, 51: 491-517. 10.1017/S0007485300055127.
Acknowledgements
This study was partly funded by a WHO/TDR grant award to DAB (WHO/TDR grant No. A000693 jointly supported by Liverpool Centre for Neglected Tropical Diseases and GlaxoSmithKline), the Gates Foundation grant to the LF Support Center, Atlanta and the African Regional Postgraduate Programme for Insect Scientists (ARPPIS) fellowship award to JU by the International Institutes Cooperation Programme (SII), Netherlands. We appreciate very much the technical support rendered by Samson Otoo, Sakyi Kojo Yirenkyi, Joseph Osei Nyarko and Kojo Frempong of the Parasitology Department, NMIMR. The support of the chiefs, volunteers and the entire communities in the Gomoa and KEEA district are duly acknowledged. Professor Alexander Nyarko, Director of NMIMR is acknowledged for granting permission to publish.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All the authors have contributed significantly to this study. DAB, MDW JG and DA-G contributed intellectually to the conceptualization and design of the study. Also DAB, MDW and HB prepared the manuscript. HB, JU, MC, carried out the laboratory and field studies. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ughasi, J., Bekard, H.E., Coulibaly, M. et al. Mansonia africana and Mansonia uniformis are Vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasites Vectors 5, 89 (2012). https://doi.org/10.1186/1756-3305-5-89
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-5-89