Abstract
Background
Thelaziosis is a neglected vector-borne disease caused by parasitic nematode worms of the genus Thelazia which affects various hosts. Limited attention has been given to ungulate-associated Thelazia species. Current diagnosis of thelaziosis and the identification/differentiation of species heavily relies on morphological features. Therefore, we conducted an epidemiological study in Romanian cattle, with the aim to obtain morphological and molecular data that can be used for species identification.
Methods
The eyes of 705 slaughtered cattle were sampled and subjected to morphological identification, morphometric analysis, and molecular characterization. PCR amplification and sequence analysis were performed based on the cytochromec oxidase subunit 1 (COI) gene. Statistical tests assessed the correlations between infection parameters and ecological or biogeographical factors. A novel PCR method was developed based on the consensus sequence from each species. Specific forward primers were designed for each of the three species, and a reverse primer (COIintR) was used for all reactions. A consensus thermal profile was established by gradient PCR amplification of each species separately.
Results
Of the sampled cattle, 19.3% were infected with Thelazia spp. Prevalence varied significantly with ecogeographical factors. A total of 585 Thelazia nematodes were recovered, with T. rhodesi being the most abundant, followed by T. skrjabini and T. gulosa. Morphometric and molecular analyses supported the morphological identification, yielding unique sequences for each species. From the 59 T. rhodesi specimens sequenced, 29 unique sequences were obtained, with a 99.1–99.85% nucleotide identity to the only other COI sequence present in GenBank®. All nine T. gulosa isolates were unique (99.37–100% nucleotide identity to other sequences), while T. skrjabini specimens displayed 98.47–100% nucleotide identity to the sole available sequence.
Conclusions
Bovine thelaziosis is prevalent in Romania, raising concerns for animal welfare and potential economic impacts. Infected cattle grazing alongside vulnerable wild ruminants, such as the European bison, may affect conservation efforts. Our newly developed multiplex PCR shows promise as a valuable surveillance tool, enabling the detection of occult infections in apparently healthy animals through lachrymal secretion testing.
Graphical Abstract

Similar content being viewed by others
Background
Thelaziosis is a neglected vector-borne disease caused by parasitic nematode worms of the genus Thelazia. Adult nematodes of this genus infest the conjunctival sack of their host, while the first three larval stages develop in secretophagous flies. Parasites of this genus infect a wide range of wild and domestic mammals, including humans, and more rarely wild birds. Over the past century, the disease, especially in livestock and horses, has been thoroughly studied due to its relatively wide geographical distribution, which seems to be ubiquitous outside the polar regions, and impact on animal welfare [1,2,3,4,5,6,7,8,9,10,11]. The introduction of macrocyclic lactones in the 1980s seems to be associated with an apparent decrease in the number of reported cases of thelaziosis in livestock [12]. As a result, with the exception of a plethora of studies on the emergence of Thelazia callipaeda in Europe [13,14,15,16,17,18,19,20], over the past decades only a few studies have focused on Thelazia species associated with domestic and wild ungulates [7, 21,22,23,24,25,26]. Moreover, except for the well-documented zoonotic role of the carnivore-associated species T. callipaeda [27,28,29] and Thelazia californiensis [30], few studies have reported human infections caused by the cattle-associated Thelazia gulosa in the USA [31, 32].
Three Thelazia species (i.e. T. rhodesi, T. skrjabini and T. gulosa) are found in domestic and wild bovines in Europe. These are mainly vectored by the face fly Musca autumnalis [6], but other species of genus Musca are occasionally involved in the transmission of these nematodes [1]. However, the current epidemiology of bovine thelaziosis in Europe is poorly documented, with few published reports in the last decades. Similarly, in Romania, after the first reports of Thelazia species from the early and mid-twentieth century [33,34,35], there have been no studies until a recent clinical report [25].
The differentiation of these three cattle-associated species has been routinely based on morphological criteria. However, little information is currently available on the morphology of immature stages and sub-adults. Moreover, identification based on genetic analysis has been attempted only on a few occasions and then only on isolated cases [22, 25, 36, 37].
In this context, our aim was to perform an extensive cross-sectional epidemiological study in cattle slaughtered in Romania and to document new morphological and molecular data to facilitate the specific identification of species.
Methods
Sampling
Between January 2021 and June 2022, the eyes of 705 domestic bovines (Bos taurus, n = 698; Bubalus bubalis, n = 7) that had been slaughtered at six different abattoirs from Romania were collected. The following data were collected and recorded for each animal: species, sampling date, age, sex, geographic origin and breed (if available) (Additional file 1: Raw data of abattoir samples Spreadsheet). The sampling protocol used was as previously described [26].
Morphology and morphometry
Each individual nematode specimen was identified based on morphological characteristics according to developmental stage and the available keys [1, 6, 38]. A detailed morphometric analysis was performed on all undamaged nematodes, which included 18 parameters for fourth-stage larae (L4), 19 parameters for fifth-stage larvae (L5), 21 parameters for adult males and 27 parameters for adult females (Figs. 1, 2, 3, 4). The minimum/maximum and median values were calculated for each parameter. All measurements were made under an Olympus BX61 microscope (Olympus Corp., Tokyo, Japan supported by its dedicated software (Cell F version 3.1).
Morphological features of Thelazia gulosa (a, b), Thelazia rhodesi (c, d) and Thelazia skrjabini (e, f) males. a Tail of T. gulosa with two spicules (S1, S2), as well as the preanal papillae (Prp). b Proximal part of T. gulosa male, with the buccal capsule (Bc), esophagus (Eso) and nerve ring (NR) highlighted. c Tail of T. rhodesi with measurements of the longer spicule (S1), tail width near the anal pore (TW) and length from the anal pore to the tip of the tail. d Measurements of the parameters located in the proximal end of a T. rhodesi male, anterior width (AntW, halfway between the esophageal-intestinal junction and proximal buccal capsule), width of the esophagus at the junction with the intestine (EsoJ), maximum width of the esophagus (EsoM), anterior width of the esophagus (EsoA, the location of the measurement being the halfway point between the anterior width and the proximal width of the buccal capsule), total striation length (TStL, the distance is divided by the number of striations present) and the anterior nerve ring distance (ANR, the distance between the halfway point of the nerve ring and the proximal end of the esophagus). e Tail end of a T. skrjabini male presenting two spicules (S1, S2) and preanal papillae (Prp). f Proximal end of a T. skrjabini male with the buccal capsule (Bc), esophagus (Eso) and esophageal-intestinal junction (EsoJ) highlighted
Morphological features of Thelazia gulosa (a, b), Thelazia rhodesi (c, d) and Thelazia skrjabini (e, f) females. a Tail of a T. gulosa female, with the anal pore encircled (Ao). b Proximal end of a T. gulosa female, with the genital pore (Vlv) and uterus (U) highlighted. c Tail of a T. rhodesi female, with measurements of tail width (TW), tail length (TL), striation size (Str). d Proximal end of a T. rhodesi female, with measurements including anterior width (AntW, halfway between the esophageal-intestinal junction and proximal buccal capsule), width of the esophagus at the junction with the intestine (EsoJ), maximum width of the esophagus (EsoM), anterior width of the esophagus (EsoA, the location of the measurement being the halfway point between the anterior width and the proximal width of the buccal capsule), total striation length (TStL, the distance is divided by the number of striations present) and the anterior nerve ring distance (ANR, the distance between the halfway point of the nerve ring and the proximal end of the esophagus) and the distance between the genital pore (Vlv) and the proximal width of the buccal capsule (VlvD). e Tail of a T. skrjabini with measurements of TW, TL and anal pore length (APL). f Proximal end of a T. skrjabini female with measurements of VlvD
Morphology of larvae of Thelazia gulosa (a, b), Thelazia rhodesi (c) and Thelazia skrjabini (d). a Larvae (L) outside of the ruptured uterus of a T. gulosa female. b Larvae within the same T. gulosa female. c Larvae (L) within the uterus of a gravid T. rhodesi female. d Larvae (L) and eggs with larva (LE) within and outside of a T. skrjabini gravid female
Morphology of eggs of Thelazia gulosa (a), Thelazia rhodesi (b–d) and Thelazia skrjabini (e) gravid females. a Gravid T. gulosa female with blastomeryzed eggs (EmE) and eggs containing first-stage larvae (LE). b Nonblastomeryzed eggs (UnEE) in a gravid T. rhodesi female c, d EmE and LE in T. rhodesi. e EmE and LE in T. skrjabini
Molecular characterization
DNA extraction, PCR amplification and sequence analysis
Genomic DNA was isolated from 87 specimens individually (59 T. rhodesi, 19 T. skrjabini and nine T. gulosa) using a commercial kit (ISOLATE II Genomic DNA Kit; Meridian Bioscience, London, UK). The nematodes were further analyzed by PCR amplification targeting an approximately 670-bp region of the mitochondrial cytochrome c oxidase I gene (COI) using the COIintF/COIintR primer pair, as described in [39]. All amplicons were purified (ISOLATE II PCR and Gel Kit; Meridian Bioscience) and sent to Macrogen Europe (Amsterdam, The Netherlands) for bidirectional sequencing. The chromatograms were assembled and edited using Geneious software (Biomatters Ltd., Auckland, New Zealand), and the consensus sequences obtained were compared to those of other Thelazia spp. isolates available in the GenBank® database by Basic Local Alignment Search Tool (BLAST) analysis. Calculation of genetic distances and the phylogenetic analysis were conducted using MEGA X software [40]. The analysis involved a total of 64 nucleotide sequences: 52 obtained during the present study, 11 sequences of Thelazia spp. retrieved from GenBank®, and one sequence of Dirofilaria immitis as the outgroup. The sequences were aligned using the MUSCLE algorithm, and the evolutionary history was inferred by using the maximum likelihood method and Tamura-Nei model [41]. A discrete Gamma distribution was used to model evolutionary rate differences among sites [5 categories (+ G, parameter = 0.2101)].
Multiplex PCR design and testing
Based on the consensus sequence obtained for each of the three species, specific forward primers were designed (Additional file 2: TIF file) following the criteria of Sharrocks [42]: T_rh_F (5′-CTTACTTTTAYGGCTTTGTTAATA-3′), T_Sk_F (5′-CCAGCCCGAAATGAGAATG-3′) and T_Gu_F (5′-GTTGGATCAAATGAGTATA-3′). The reverse primer COIintR (5′-ATAAGTACGAGTATCAATATC-3′) was used for all reactions. The consensus thermal profile was established by gradient PCR amplification of each species separately. Specificity and sensitivity tests were conducted in a final reaction volume of 25 μl containing 12.5 μl My Taq® Red PCR Mastermix (Meridian Bioscience), 1 μl (10 pmol) of each forward primer, 1 μl (30 pmol) reverse primer COIintR, 6.5 μl of PCR water and 2 μl of template DNA. The amplification profile was as follows: 5 min of initial denaturation at 95 °C; 35 cycles of denaturation at 95 °C for 45 s, annealing at 55 °C for 45 s and extension at 72 °C for 45 s; and a final extension at 72 °C for 5 min.
Statistical analysis
The collected data were grouped into several categories. The average age, expressed in months, of infected and uninfected cattle was used for the analyses. Seasons were defined as spring (March–May), summer (June–August), autumn (September–November) and winter (December–February) for both 2021 and 2022 according to the sampling date. Data on geographical origin were separated into categories of biogeoregions, ecoregions and altitude intervals. The following biogeoregions present in Romania were included: Pannonian (P), Steppic (S), Alpine (A) and Continental ©). The ecoregions included in the study were: Pannonian Mixed (PMF), East European forest steppe (EEFS), Carpathian montane coniferous forests (CMCF), Central European mixed forests (CEMF), Pontic steppe (PS) and Balkan mixed forests (BMF). Altitude intervals were arbitrarily chosen in order to include comparable sample sizes as follow: 0–200, 201–400, 401–600, > 600 m a.s.l.
Statistical analysis was performed using the Chi-square (χ2) test for the following datasets: distribution of Thelazia spp. and T. rhodesi by season, average age, sex, biological ecoregions, ecoregions, altitude intervals and breed. Due to a low sample size, no statistical assessments were performed separately for T. skrjabini and T. gulosa. Chi-square was also used to assess the differences between the presence of Thelazia spp. in the left and right eyes. The intensity of infection according to season, ecoregion and altitude interval was assessed by means of analysis of variance (ANOVA) testing.
The correlations between the intensity of infection and season, ecoregion, altitude and altitude interval were evaluated using the Spearman’s rho or Pearson's correlation coefficient, as appropriate. The correlation between the occurrence of Thelazia spp. and season, ecoregion and altitude interval was assessed by the point-biserial coefficient.
The calculation of prevalence and 95% confidence intervals (95% CI) and the Chi-square and ANOVA tests were performed using EpiInfo 7 software (U.S. Centers for Disease Control and Prevention [CDC], Atlanta, GA, USA), and correlations were assessed using the free on-line tool available at https://www.statskingdom.com/.
Mapping
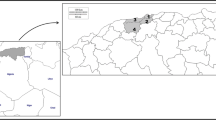
Maps were constructed using the QGis version 3.26 software (2022; https://www.qgis.org/en/site/) using the following open-access layers: BioRegions2016 (https://www.eea.europa.eu/en) and Digital map of European ecological regions (https://www.eea.europa.eu/en). Results were exported to Microsoft Excel (Microsoft Corp., Redmond, WA, USA), using the intersect option.
Results
Epidemiology
Of the 705 domestic bovines sampled, 136 (19.3%) were infected by nematodes belonging to genus Thelazia, with 95 positive animals (69.9%) harboring a unilateral infection and the remaining 41 (30.2%) having an infection in both eyes. The left eye was significantly more frequently infected (χ2 = 19.72; df = 1; P < 0.0001) than the right eye (77.9% vs. 51.5% of positive animals). The prevalence of Thelazia nematodes across the different categories is shown in Table 1. Statistically significant differences in prevalence values were observed for season (χ2 = 88.67; df = 3; P < 0.0001) and altitude intervals (χ2 = 10.21; df = 3; P = 0.016) (Table 1). Significant differences were also found between infected and uninfected animals according to the average age (P = 0.017) (Additional file 3: Table 2). Significant differences were also found between breeds linked to a Thelazia spp. infection (χ2 = 14.62; df = 2; P = 0.0007). The breeds subjected to analysis were Bălțată Românească, Holstein and Mixed breeds, based on sample sizes adequate for testing.
Of the correlations tested, the intensity according to season for both Thelazia spp. (r = 0.3; P < 0.0001) and T. rhodesi (r = 0.34; P < 0.0001) indicated a slight positive relationship. Moreover, the intensity according to ecoregions for Thelazia spp. (r = 0.007; P = 0.04) and T. rhodesi (r = 0.1; P = 0.007) also indicated the presence of a slightly positive relationship (Additional file 3: Tables 9, 14, 16, 25, 30, 32). The point-biserial correlation also indicated a slight positive and significant relationship between prevalence of Thelazia spp. and seasons (r = 0.28; P < 0.0001).
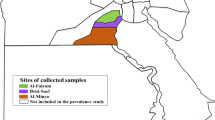
A total of 585 Thelazia spp. nematodes were recovered, with a mean intensity of infection of 4.3 (median = 3 nematodes/animal) (Table 2). Thelazia rhodesi was the most abundant of all Thelazia species, with 458 nematodes in different stages identified, followed by T. skrjabini (109 specimens) and T. gulosa (18 specimens). Also, a total of 11 co-infections were found, of which nine (81.81%) included T. skrjabini + T. rhodesi; the remaining two co-infections (18.18%) were with T. skrjabini + T. gulosa. There were no co-infections with T. rhodesi + T. gulosa. Maps on the distribution of Thelazia species are shown in Figs. 5 and 6.
Morphometry
Morphometrical analysis is shown in Additional file 4: Raw data spreadsheet and Additional file 5: Min Max Med spreadsheet. Moreover, a value-based distinction was made between the egg types present in gravid females: The median measurements for larvated eggs measured were 39.2/29.4 µm for T. rhodesi and 41.8/28.0 µm for T. skrjabini. The medium measurements for blastomeryzed eggs were 32.8/23.6 µm for T. rhodesi, 30.8/21.7 µm for T. skrjabini and 18.3/13.9 µm for T. gulosa. Non-blastomeryzed eggs were only recorded in T. rhodesi (21.5/15.6 µm) and T. skrjabini (15.5/12.9 µm) (Fig. 4).
Molecular analysis
The results of the genetic analysis were in complete agreement with the morphological identification of species. For T. rhodesi, from the 59 sequenced specimens analyzed, a total of 29 unique sequences were obtained, having a 99.1–99.85% identity to the only T. rhodesi COI sequence existing in GenBank® (Accession no. MT511659). All nine T. gulosa isolates were unique, having a 99.37–100% identity to the other two existing T. gulosa COI sequences (Accession nos. AJ544881, OL362019). From the 19 T. skrjabini specimens, 14 unique sequences were obtained, having a 98.47–100% identity to the single T. skrjabini COI sequence from GenBank® (Accession no. OL362009). The identity of the sequences in our study was between 98.7 and 100% for T. rhodesi, 97.94–100% for T. skrjabini and 98.9–99.84% for T. gulosa (Additional file 6: Distance value comparison Spreadsheet).
The sequences obtained during the current study were deposited in GenBank under the following accession numbers: OQ988094-OQ988102 for T. gulosa; OQ988118-OQ988146 for T. rhodesi; and OQ988148-OQ988161 for T. skrjabini. A bootstrap consensus tree was drawn, based on 62 nucleotide sequences, with a total of 528 positions in the final dataset (Fig. 7).
Bootstrap consensus tree inferred from 1000 replicates. The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches (values < 40% not shown). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The red squares represent sequences obtained in the current study; the black squares represent existing sequences in GenBank
The multiplex PCR yielded bands of the expected sizes for all of the three species (approx. 360 bp for T. gulosa, approx. 480 bp for T. skrjabini and approx. 580 for T. rhodesi), both in single amplification and in various combinations (Additional file 7: Multiplex PCR validation TIF file).
Discussion
The results of our study showed that bovine thelaziosis is a common, but seemingly neglected disease in Romania. In Romania, three Thelazia species (T. skrjabini, T. gulosa and T. rhodesi) have been identified, and these they can be differentiated by our novel multiplex PCR. Moreover, the current study represents the most detailed phylogenetic analysis of genus Thelazia performed up to date. The molecular analysis confirmed the identity of all three species based on morphological findings. From an evolutionary standpoint, T. rhodesi clusters together with T. callipaeda, while T. gulosa and T. skrjabini form a different clade, which also includes T. lacrymalis and T. californiensis. Thelazia skrjabini seems to be more closely related to the equine-specific species, Thelazia lacrymalis (Fig. 7; Additional file 6: Distance value comparison Spreadsheet).
Thelaziosis was a prominent disease of livestock in the Old World during the first half of the twentieth century, with the majority of reports originating in eastern European [43,44,45] and Mediterranean regions [46,47,48]. The second half of the century saw the disease’s distribution increase, ultimately spreading throughout most of the continent [1, 2, 48,49,50,51,52,53], with sporadic reports from Africa [54, 55], Asia [56], Australia [57] and the Americas [58]. In the past 40 years, coinciding with the wide use of macrocyclic lactones and the expansion of T. callipaeda in pets, wildlife and humans in Europe, thelaziosis in large herbivores has gone mostly unnoticed. The few reports that emerged [6, 12, 24,25,26, 59, 60] are a testament to its apparent decline. However, our data, obtained on an extensive dataset, are proof that thelaziosis is very much present, with almost one in the five cows tested in our study found to have harbored an infection with at least one species of Thelazia. In Romania, studies on the epidemiology of genus Thelazia in domestic cattle date back to the beginning of the 1970s [35], with a similar reported prevalence of 22.4%, as revealed also by eye examination in slaughterhouses in the north-eastern part of the country.
In our study, an effect of seasonality was evident, both for the prevalence and intensity of infection (Table 1). This is likely linked to the seasonality of the vector but also to the routine bi-annual deworming protocols performed by field veterinarians [61], which likely influenced the low prevalence levels in spring and early summer. Prevalence spikes occurred in late summer and autumn. The high prevalence in February could be attributed to the origin of the animals, most belonging to small individual extensive livestock production systems (Additional file 1: Raw data of abattoir samples Spreadsheet, Geographic origin tab). Such systems are subject to less restrictive regulations, leading to precarious hygiene, fewer preventive measures (ANSVSA president Order 208/2022 for implementing identification and registration of bovines, ovine, caprine, swine, camelids, cervinae and reindeer, https://legislatie.just.ro/Public/DetaliiDocumentAfis/263889) and higher parasitic prevalence and intensity [62,63,64].
Our statistical analysis revealed significant differences in the prevalence of Thelazia spp. between altitude intervals (Table 1; Additional file 3: Table 5). Consequently, altitude appears to act as a limiting factor, probably more so against the dipteran vectors rather than the parasite itself [65]. However, altitude seldom is the sole cause of a species inadaptability, whereas the associated environmental conditions (temperature, radiation output, precipitation and wind turbulence) appear to constitute a much more plausible explanation [66]. These factors could also explain the variability in the distribution of Thelazia spp. and its main vector, M. autumnalis, at different altitude levels, more specifically between 400 and 600 m a.s.l. The authors of several studies have concluded that wind speed is directly proportional with altitude [67, 68]. Nevertheless, a limitation of our study resides in the fact that > 50% of samples in the 400-to–600 m a.s.l. interval were collected in mid to late spring, when the prevalence was minimal (Additional file 1: Raw data of abattoir samples Spreadsheet, Date and Altitude Tab).
Specific ecological conditions (climate, habitat types or interactions with other organisms) (Fig. 6) may primarily shape the distribution of Thelazia. Both the overall prevalence of Thelazia spp. and that of T. rhodesi varied significantly according to ecoregion (Table 1). This variation could be related to different vector activity, seasonality or abundance [65] but also to the sample bias, as the BMF and PS ecoregions were sampled during the peak prevalence season (July–October), whereas the CEMF and EEFS ecoregions were mostly sampled in the spring and early summer. No statistically significant differences were found between biogeoregions (Fig. 5) for Thelazia spp. prevalence.
The overall intensity of infections varied greatly among animals, with a maximum value of 41 nematodes per animal (Table 2). The overall median intensity per animal was lower than that reported in a study from Italy [6] and in a previous study from Romania [35]. The median intensity of T. skrjabini was similar to values reported in Canada [69]. The highest intensity values were recorded in October and November and the lowest intensity was recorded in the spring, in line with previous records [35].
Co-infections with different Thelazia species seems to be rare (1.6%), as shown also by Dulceanu [35] and Moolenbeek and Surgeoner [58]. This is probably related to the evident dominance of T. rhodesi and the more sporadic occurrence of T. skrjabini and T. gulosa in Romania. These findings are similar to those reported in a study from Italy [6]; however, the prevalence and intensity of T. skrjabini in the current study was higher than that of T. gulosa. Demiaskiewicz et al. [24] also reported the presence of T. gulosa and T. skrjabini in European bison populations, with a higher intensity in T. gulosa than T. skrjabini. In North America, on the other hand, T. gulosa and T. skrjabini seem to be dominant, whereas T. rhodesi is absent [70].
Our morphometric analysis showed for the first time the size differences between the different egg types within the uterus of gravid T. rhodesi, T. gulosa and T. skrjabini females. This was previously shown in T. callipaeda [13]. Our study also provides, for the first time, the morphometric values for L4 and L5 of T. skrjabini, T. rhodesi and T. gulosa.
Conclusions
Bovine thelaziosis seems to be relatively common in Romania, and most likely also in other geographical regions with similar ecological and animal husbandry conditions. Although clinical reports of thelaziosis are rare, the presence of ocular nematodes in cattle could represent an animal welfare concern, as well as having a negative economic impact due to decreased production. Moreover, the co-existence of infected cows with wild susceptible ruminants such as European bison (as the case of Romania which has three reintroduction sites) might impact the conservation efforts of the latter. The newly developed multiplex PCR described here could be a useful tool for the surveillance of occult infections in affected hosts by testing lachrymal secretions in apparently healthy animals.
Availability of data and materials
All data generated or analysed during this study are included in this published article as well as its additional data. Sequences generated in this study are available in GenBank (OQ988094-OQ988102; OQ988118-OQ988146; OQ988148-OQ988161).
Abbreviations
- BMF:
-
Balkan mixed forests
- CEMF:
-
Central European mixed forests
- CMCF:
-
Carpathian montane coniferous forests
- EEFS:
-
East European forest steppe
- PMF:
-
Pannonian mixed forests
- PS:
-
Pontic steppe
References
Skrjabin KI, Soboley AA, Ivanshkin VM. Spirurata of animals and man and the diseases caused by them part 4: Thelazioidea. In: Skrjabin KI, editor. Essentials of nematodology, vol. XVI. Moscow: Academy of Sciences of the USSR; 1967. p. 24–32.
Arbuckle JB, Khalil LF. A survey of Thelazia worms in the eyelids of British cattle. Vet Rec. 1978;102:207–10. https://doi.org/10.1136/vr.102.10.207.
Overend DJ. Thelazia gulosa in cattle. Aust Vet J. 1983;60:126–7.
Kennedy MJ, Murray J, Treichel DTBA. First report of immature Thelazia skrjabini (Nematoda: Thelazioidea) from the eye of a white-tailed deer, Odocoileus virginianus. J Wildl Dis. 1993;29:159–60.
Kennedy MJ, MacKinnon JD. Site segregation of Thelazia skrjabini and Thelazia gulosa (Nematoda: Thelazioidea) in the eyes of cattle. J Parasitol. 1994;80:501–4.
Giangaspero A, Tieri E, Otranto D, Battistini ML. Occurrence of Thelazia lacrymalis (Nematoda, Spirurida, Thelaziidae) in native horses in Abruzzo region (central eastern Italy). Parasite. 2000;7:51–3. https://doi.org/10.1051/parasite/2000071051.
Munang’andu HM, Chembensofu M, Slamudaala VM, Munyeme M, Matandiko W. Thelazia rhodesii in the African Buffalo, Syncerus caffer, Zambia. Korean J Parasitol. 2011;49:91–4.
Djungu DFL, Retnani EB, Ridwan Y. Thelazia rhodesii infection in cattle in Kupang District. Trop Biomed. 2014;31:844–52.
Anvari D, Sharifi N, Hashemi SH. First report of isolation and identification of Thelazia lacrymalis nematodes in horses from Iranshahr city. In: Proceedings of the Third National Congress of Equine Health and Diseases, 29 April–1 May 2025, Shiraz. p. 42.
Hassan EB, Moshaverinia A, Sheedfar F, McCowan C, Bazargani TT, Hosseinzadeh A, et al. A report of the unusual lesions caused by Thelazia gulosa in cattle. Vet Parasitol Reg Stud Rep. 2017;7:62–5.
Das J, Kuldeep SK, Singh R. Surgical removal of eyeworm in indigenous cow. J Entomol Zool Stud. 2021;9:1487–9.
Tweedle DM, Fox MT, Gibbons LM, Tennant K. Change in prevalence of Thelazia species in bovine eyes in England. Vet Rec. 2005;157:555–6.
Otranto D, Lia RP, Buono V, Traversa D, Giangaspero A. Biology of Thelazia callipaeda (Spirurida, Thelaziidae) eyeworms in naturally infected definitive hosts. Parasitology. 2004;129:627–33.
Otranto D, Cantacessi C, Dantas-Torres F, Brianti E, Pfeffer M, Genchi C, et al. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: helminths and arthropods. Vet Parasitol. 2015;213:24–37.
Tudor P, Bădicu A, Mateescu R, Tudor N, Mateescu C, Ionaşcu I. First report of canine ocular thelaziosis in the Muntenia Region, Romania. Parasitol Res. 2016;115:1741–4.
Mihalca AD, Ionică AM, D’Amico G, Daskalaki AA, Deak G, Matei IA, et al. Thelazia callipaeda in wild carnivores from Romania: new host and geographical records. Parasit Vectors. 2016;9:350.
Gama A, Pires I, Canado M, Coutinho T, Lopes AP, Latrofa MS, et al. First report of Thelazia callipaeda infection in wild European rabbits (Oryctolagus cuniculus) in Portugal. Parasit Vectors. 2016;9:236.
Ionică AM, Deak G, Matei IA, D’Amico G, Cotuţiu VD, Gherman CM, et al. Thelazia callipaeda, an Endemic Parasite of Red Foxes (Vulpes vulpes) in Western Romania. J Wildl Dis. 2016;54:829–33.
Dumitrache MO, Gyorke A, Mircean M, Benea M, Mircean V. Ocular thelaziosis due to Thelazia callipaeda (Spirurida: Thelaziidae) in Romania: first report in domestic cat and new geographical records of canine cases. Parasitol Res. 2018;117:4037–42.
Cotuțiu V-D, Mihalca AD, Hołówka KA, Ionică AM, Cazan CD, Gherman CM. European hares, Lepus europaeus, represent a reservoir host for Thelazia callipaeda in Romania. Pathogens. 2022;11:1225. https://doi.org/10.3390/pathogens11111225.
Dubay SA, Williams ES, Mills K, Berger-Fields AM. Bacteria and nematodes in the conjunctiva of mule deer from Wyoming and Utah. J Wildl Dis. 2000;36:783–7.
Otranto D, Tarsitano E, Traversa D, Giangaspero A, De Luca F, Puccini V. Differentiation among three species of bovine Thelazia (Nematoda: Thelaziidae) by polymerase chain reaction-restriction fragment length polymorphism of the first internal transcribed spacer ITS-1 (rDNA). Int J Parasitol. 2001;31:1693–8. https://doi.org/10.1016/s0020-7519(01)00279-x.
Khasatiya CT, Dabhi DM, Mahala DR, Chaudhari PP. Ocular worm in non-descript buffalo: a case report. Indian J Field Vet. 2008;4:67–8.
Demiaszkiewicz AW, Moskwa B, Gralak A, Laskowski Z, Myczka AW, Kołodziej-Sobocińska M, et al. The Nematodes Thelazia gulosa Railiet and Henry, 1910 and Thelazia skrjabini Erschov, 1928 as a cause of blindness in European Bison (Bison bonasus) in Poland. Acta Parasitol. 2020;65:963–8. https://doi.org/10.1007/s11686-020-00243-w.
Deak G, Ionica AG, Oros NV, Gherman CM, Mihalca AD. Thelazia rhodesi in a dairy farm in Romania and successful treatment using eprinomectin. Parasitol Int. 2021;80:102–83. https://doi.org/10.1016/j.parint.2020.102183.
Cotuțiu VD, Ionică AM, Lefkaditis M, Cazan CD, Hașaș AD, Mihalca AD. Thelazia lacrymalis in horses from Romania: epidemiology, morphology and phylogenetic analysis. Parasit Vectors. 2022;15:425. https://doi.org/10.1186/s13071-022-05532-z.
Wei X, Liu B, Li Y, Wang K, Gao L, Yang Y. A human corneal ulcer caused by Thelazia callipaeda in Southwest China: case report. Parasitol Res. 2020;119:3531–4.
Dolff S, Kehrmann J, Eisermann P, Dalbah S, Tappe D, Rating P. Case report: Thelazia callipaeda eye infection: the first human case in Germany. Am J Trop Med Hyg. 2020;102:350–1.
do Vale D, Lopes AP, Fontes MC, Silvestre M, Cardoso L, Coelho AC. Systematic review on infection and disease caused by Thelazia callipaeda in Europe: 2001–2020. Parasite. 2020;11:98.
Singh K, Khindria A. First case of human ocular Thelaziasis from India caused by Thelazia californiensis: a case report. IOSR-JDMS. 2018;17:24–7.
Bradbury RS, Breen KV, Bonura EM, Hoyt JW, Bishop HS. Case report: conjunctival infestation with Thelazia gulosa: a novel agent of human thelaziasis in the United States. Am J Trop Med Hyg. 2018;98:1171–4. https://doi.org/10.4269/ajtmh.17-0870.
Bradbury RS, Gustafson DT, Sapp SGH, Fox M, de Almeida M, Boyce M, et al. A second case of human conjunctival infestation with Thelazia gulosa and a review of T. gulosa in North America. Clin Infect Dis. 2018;70:518–20. https://doi.org/10.1093/cid/ciz469.
Udriski G, Ciurea I. Ophtalmie vermineuse chez le boeuf. Arab Vet. 1921;2:43–4.
Nicolaescu S. Telaziosa in Cic-tia Verinara C. raiom Corabia. Lucrare de Diploma (nr 2148/1) Bucuresti:Faculty of Veterinary Medicine; 1962.
Dulceanu N. Cercetari cu privire la localizarea speciilor si intensivitatea infestatiei cu thelazii la taurine. Lucrari Stiintifice. II. Zootechnie-Medicina Veterinara. 1971 (Helm. Abstr. 43, Abstr. no. 2939*1974).
Otranto D, Tarsitano E, Traversa D, De Luca F, Giangaspero A. Molecular epidemiological survey on the vectors of Thelazia gulosa, Thelazia rhodesi and Thelazia skrjabini (Spirurida: Thelaziidae). Parasitology. 2003;127:365–73.
Filip-Hutsch K, Laskowski Z, Myczka AW, Czopowicz M, Moskwa B, Demiaszkiewicz AW. The occurrence and molecular identification of Thelazia spp. in European bison (Bison bonasus) in the Bieszczady Mountains. Sci Rep. 2022;12:22508. https://doi.org/10.1038/s41598-022-27191-x.
Naem S. Morphological differentiation among three Thelazia species (Nematoda: Thelaziidae) by scanning electron microscopy. Parasitol Res. 2007;101:145–51.
Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. https://doi.org/10.1093/molbev/msy096.
Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;3:512–26. https://doi.org/10.1093/oxfordjournals.molbev.a040023.
Sharrocks AD. The design of primers for PCR. In: Griffin HG, Griffin AM, editors. PCR technology, current innovations. CRC Press: London; 1994. p. 5–11.
Vasiliev M. Invasion thelasiques ophtalmiques chez les bovins a abattoir de Zagreb en 1930. Rev Genet Med Vet. 1933;42:28–9.
Iamandi GG, Teclu M. L'ophtalmie vermineuse des ruminants en Roumanie. Annales de Parasitologie Huaine et Comparée. 1937;330–2.
Klesov MD. The biology of the nematode Thelazia rhodesii Desmarest, 1827. Doklady Akad Nauk SSSR. 1949;66:309–11.
Jordano D. Primeros casos de conjuntivitis verminosa en terneros debideos a la “Thelazia rhodesi” (Desmarest, 1827). Zootecnia. 1945;28–31.
Di DL. alcuni casi di infezione del sacco congiuntivale dei bovini da Thelazia. Clin Vet (Milano). 1949;72:108–18.
Gajewski D. Thelazia infection on slaughter cattle in Warsaw. Med Vet. 1963;19:259–60.
Eckert J, Stober M, Schmidt H. Occurence of eye worms (Thelazia) in cattle in northwest Germany. In: Rep. III. Int. Meet. Dis. Cattle. Copenhagen; 1964. p. 506–15.
Güralp N, Oğuz T. Türkiye’de mandalarda (Bubalus bubalis) thelaziose. Ankara Univ Vet Fak Derg. 1970;17:109–13.
Corba J. Prispevok k profylaktickej dehelmintizacii telaziozneho hovadzieho dobytka. Folia Vet. 1970;14:105–8.
Kolstrup N. Fund af ojeorm Thelazia skrjabini i dansk kvaeg. Nord Vet Med. 1974;26:459–62.
Perrier F. La thélaziose bovine en France, revue bibliographique et enquête épidémiologique. PhD thesis; 1993.
Ikeme MM. Keratoconjunctivitis in cattle in the plateau area of Northern Nigeria. A study of Thelazia rhodesi as a possible aetiological agent. Bull Epizoot Dis Afr. 1967;15:363–367.
Chartier C, Eboma KE. La thelaziose oculaire des bovins en Ituri (Haut-Zaire): epidemiologie et clinique. Rev Med Vet. 1988;139:1053–8.
Okoshi S, Kitano N. Studies of Thelaziasis of cattle. I. Thelazia skrjabini Erschow, 1928 found in Japan. Nihon Juigaku Zasshi. 1966;28:11–5.
Smael MG. Observations on the occurrence of Thelazia or eyeworm infection of cattle in Northern New South Wales. Aust Vet J. 1968;44:516–21.
Moolenbeek WJ, Surgeoner GA. Southern Ontario survey of eyeworms, Thelazia gulosa and Thelazia lacrymalis in cattle and larvae of Thelazia spp. in the face fly, Musca autumnalis. Can Vet J. 1980;21:50–2.
Medl N. Thelazia lacrymalis (Gurlt, 1831) beim Pferd - epidemiologische und histopathologische Untersuchungen un kritische retrospektive Betrachtund der klinischen Bedeutung. In: Inaugural-Dissertation zur Erlangung der tiermedizinischen Doktorwürde der Tierärztlichen Fakultät der Ludwig-Maximilians Munich; 2007. p. 39–44.
Bras ALL. Estude epedilogico geospacial de Thelazia spp em bovinos da regiao de alentejo. PhD thesis. Lisbon: Universidade Técnica de Lisboa, Faculdade de Medicina Veterinária; 2012.
Dărăbuș G, Constantin N, Constantinoiu C, Cosoarbă I, Cozma V, Didă I, et al. Strongilidoze digestive. In: Constantin N, Constantinoiu C, Cosoarbă I, Cozma V, Dărăbuș G, Didă I, et al., editors. Tratat de Medicină Veterinară. București: Risoprint; 2014. p. 648–92.
Waterhouse A. Animal welfare and sustainability of production under extensive conditions—A European perspective. Appl Anim Behav Sci. 1996;49:29–40.
Turner SP, Dwyer CM. Welfare assessment in extensive animal production systems: challenges and opportunities. Anim Welfare. 2007;16:189–92.
Temple D, Manteca X. Animal welfare in extensive production systems is still an area of concern. Front Sustain Food Syst. 2020;4:545902.
Valiela I. An experimental study of the mortality factors of larval Musca autumnalis DeGeer. Ecol Monogr. 1969;39:199–225.
Hodkinson ID. Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev. 2005;80:489–513.
Sommerfeld M, Crawford C, Monahan A, Bastigkeit I. LiDAR-based characterization of mid-altitude wind conditions for airborne wind energy systems. Wind Energy. 2019;22:1101–20.
Tar K. Some statistical characteristics of monthly average wind speed at various heights. Renew Sust Energ Rev. 2008;12:1712–24.
Kennedy MJ, Moraiko DT. The eyeworm, Thelazia skrjabini in cattle in Canada. Can Vet J. 1987;28:254–5.
Otranto D, Traversa D. Thelazia eyeworm: an original endo- and ecto-parasitic nematode. Trends Parasitol. 2005;21:1–4. https://doi.org/10.1016/j.pt.2004.10.008.
Acknowledgements
The authors would like to thank all the former students and Ph.D. candidates involved in the sampling procedure: Borza Emil, Opriș Diana, Balmoș Oana, Toma-Naic Andra and dr. Hortvath Cintia. We would also like to acknowledge the availability and professionalism of the personnel in the slaughterhouses where we collected our samples, as well as the management of the following enterprises: Sancom AS SRL and S.C. Barbarul SRL. The work of AM Ionică was conducted within the framework of Grant No. TE49/2022, by UEFISCDI Romania. The publication was supported by funds from the National Research Development Projects to finance excellence (PFE)-14 (ID 546) granted by the Romanian Ministry of Research, Innovation and Digitalization.
Funding
This research received funding from the Internal Grant “Soluții” 24843/05.11.2021.
Author information
Authors and Affiliations
Contributions
VDC, AMI and ADM conceived the structure of the study. VDC and AMI established the methodology of sampling and molecular analysis. CMG, MM and ADM established the framework in which the samples could be collected from the slaughterhouses. VDC, SDB, TD, CDC, MM and CMG collected samples from the abattoirs. VDC and TD examined the samples and developed the database for the morphological identification of all recovered nematodes. CDC processed the molecular samples and prepared them for sequencing. AMI designed the primers and the protocol for the multiplex PCR method. CDC and AMI tested and validated the results of the multiplex method. AMI processed and analyzed the statistical findings from the database. CAC provided the visualization components, image editing and graphical abstract. VDC wrote the draft and the manuscript. AMI and ADM reviewed and assisted in the editing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Excel spreadsheet (.xlsx).
Raw data of abattoir samples.
Additional file 2:
TAG Image file format (.tif). Alignment of consensus sequences of the three Thelazia species and the position of the designed forward primers.
Additional file 3:
Excel spreadsheet (.xlsx). Statistical tests performed on the database.
Additional file 4:
Excel spreadsheet (.xlsx). Raw data of all measurements performed on recovered Thelazia specimens.
Additional file 5:
Excel spreadsheet (.xlsx). Minimum, maximum and median values for all the parameters measured in the raw data file.
Additional file 6:
Excel spreadsheet (.xlsx). Distance value comparison between original and available sequences.
Additional file 7:
TAG Image file format (.tif). Multiplex PCR validation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cotuțiu, VD., Ionică, A.M., Dan, T. et al. Diversity of Thelazia spp. in domestic cattle from Romania: epidemiology and molecular diagnosis by a novel multiplex PCR. Parasites Vectors 16, 400 (2023). https://doi.org/10.1186/s13071-023-06012-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06012-8