Abstract
Background
Equine thelaziosis is a neglected vector-borne parasitic disease in modern veterinary medicine, lacking recent reports. It is transmitted by Musca autumnalis, and potentially other Muscidae species, by ingesting the lachrymal secretions of its equine host. The distribution of both Thelazia lacrymalis and its intermediate hosts remains largely unknown throughout Europe, with most studies dating back 20 years. The aim of this study was to assess the presence, prevalence and distribution of T. lacrymalis in horses from Romania.
Methods
The eyes of 273 horses, slaughtered at two abattoirs from the Northwestern and Western regions of Romania, were examined for the presence of T. lacrymalis between March and November 2021. Upon detection, the nematodes were collected and morphologically identified using the keys from literature. Following identification, one specimen from each animal was selected for molecular analysis while the rest underwent detailed morphometric measurements. Mapping and distribution, according to ecoregions, was done using the QGis 3.20 software, while sequences obtained were compared to those available in GenBank through BLAST analysis using the MEGA X software.
Results
Of the 273 animals sampled, 12 (4.39%) were positive for Thelazia spp. infection. Eighty-seven nematodes were recovered, all morphologically identified as T. lacrymalis. The intensity of infestation varied between one and 33 nematodes/animal while five animals presented a bilateral infestation and seven a unilateral one. The highest prevalence was encountered in Pannonian ecoregion (12.12%) while the lowest was in the Alpine ecoregion (0%). Seventy-five intact specimens underwent detailed morphometric analysis, of the 18–20 parameters, resulting in notable differences in striation lengths compared to the data available in other reports. BLAST analysis identified a 96.46–98.60% similarity to the only other COI gene sequence available for T. lacrymalis.
Conclusions
The current study represents the first report of T. lacrymalis in horses in Romania. The low prevalence rates are probably linked to the wide use of macrocyclic lactones.
Graphic Abstract

Similar content being viewed by others
Background
Thelaziosis is a parasitic disease caused by nematodes of genus Thelazia in the conjunctival sack of their hosts. The intermediate hosts for various Thelazia species are non-biting secretophagous flies, which ingest the first stage larvae during their meal on lachrymal secretions [1]. For Thelazia spp. infecting large ruminants and horses, Musca autumnalis seems to be the main vector. Musca domestica as well as other Muscidae species have also been suggested as vectors [2, 3]. The disease has garnered much attention within the past 2 decades, following the emergence of Thelazia callipaeda in carnivores and other hosts, including humans, throughout much of Europe [4]. Additionally, occasional reports of cases in large ruminants still emerge from time to time in Europe [5, 6]. In Romania, Thelazia spp. were so far found in domestic and wild carnivores [7,8,9,10] and cattle [6, 11].
In horses, the disease is poorly studied, and its epidemiology remains largely unknown. The only species reported in horses is Thelazia lacrymalis, first described in Germany in the nineteenth century [12]. The species is cosmopolitan, ranging from Asia to the Americas and Europe [3, 13,14,15,16,17,18,19]. However, reports of thelaziosis in horses in Europe are scarce, with the last published case in 2007 in Germany [20]. With the exception of the former USSR, where equine thelaziosis has been reported in the region of Bashkortostan (at the very eastern limit of geographical Europe) [3], equine thelaziosis has not been documented in Eastern Europe. One report of T. lacrymalis was published from Switzerland in horses imported from Poland and Hungary, but the infection site of horses is not identified with certainty [2].
The aim of our study was to investigate the occurrence of Thelazia spp. in horses from Romania. Additionally, we provide detailed morphometric data to improve the species description.
Materials and methods
Samples consisted of both eyes belonging to 273 horses slaughtered at two different abattoirs from Northwestern and Western Romania between March and December 2021 (Table 1). For each horse, the following data were collected: sampling date, age, sex and origin (locality, geographic coordinates, altitude and ecoregion).
In the slaughterhouse, the eyes of each animal were removed along with adjacent tissues, namely eyelids and lachrymal glands, without perforating the conjunctival sack and individually placed in a sealed zip bag. Any visible Thelazia worms were collected in a 1.5-ml plastic tube with saline and placed in the same zip bag as the eyes they belonged to. All samples were then transported to the Department of Parasitology and Parasitic Diseases of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca for detailed examination. Upon arrival, the samples were transferred to a refrigerator prior to examination, which was done in maximum 48 h.
Each eye and adjacent structures were carefully examined by opening the lateral canthus followed by the eversion of the eye globe. The third eyelid was inverted and partially detached allowing the lachrymal ducts to be dissected. Subsequently, each eye was flushed with physiological saline along with their corresponding bag into a Petri dish. The content of the Petri dish was examined under a zoom stereomicroscope. All collected nematodes were placed in vials with physiological saline and kept in a refrigerator until morphological identification.
Each nematode was morphologically identified to species and developmental stage based on morphological keys described in literature [3, 16, 21]. The undestroyed specimens were preserved in 4% formalin solution and processed by detailed morphometric analysis including 19 parameters in adult males and larvae and 20 in adult females, as shown in Table 3. Morphological identification and measurements were carried out using the Olympus microscope (Olympus BX61) and dedicated software. The number of nematodes was independently recorded for each animal, by stage and sex.
One nematode from each horse was randomly selected and stored in 70% ethanol for further molecular characterization. DNA was extracted individually from 12 nematodes using ISOLATE II Genomic DNA Kit (Bioline Meridian Bioscience, Luckenwalde, Germany), according to the manufacturer’s instructions, and stored at − 20 °C until further use. A PCR amplification targeting the mitochondrial cytochrome oxidase I (COI) gene region (670 bp) was performed in 25 μl reaction volume, containing 12.5 μl My Taq® Red PCR Mastermix (Bioline Meridian Bioscience, Luckenwalde, Germany), 6.5 μl of ultrapure water, 1 μl (10 pmol) of each of the two previously described primers [22], COIintF 5′-TGATTGGTGGTTTTGGTAA-3′ and COIintR 5′-ATAAGTACGAGTATCAATATC-3′, and 4 μl aliquot of isolated DNA. One negative control (PCR water) was included. The PCR was performed using a C1000™ Thermal Cycler (Bio-Rad, London, UK), with the following conditions: initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 45 s and annealing at 47 °C for 45 min with extension at 72 °C for 1 min. A final extension at 72 °C for 5 min was performed. Amplification products were visualized by electrophoresis on 1.5% agarose gel stained with ECO Safe 20,000 × Nucleic Acid Staining Solution (Pacific Image Electronics, New Taipei, Taiwan), and their molecular weight was assessed by comparison to a molecular marker (HyperLadder™ 100 bp, Bioline Meridian Bioscience, Luckenwalde, Germany). The quality of the samples was visually assessed via gel electrophoresis before samples underwent purification. All PCR products were purified using the ISOLATE II PCR and Gel Kit (Bioline Meridian Bioscience, Luckenwalde, Germany) and sent for sequencing in both directions (Macrogen Europe, Amsterdam, The Netherlands). The attained chromatograms were assembled, and consensus sequences were edited and translated to corresponding proteins using Geneious 4.8.5 software (Biomatters Ltd., Auckland, New Zealand). The consensus sequences were compared to those available in the GenBank® database by means of Basic Local Alignment Search Tool (BLAST).
The statistical analysis was performed using EpiInfo™ 7 software (CDC, USA). The frequency prevalence and 95% confidence interval (CI) of infestation were calculated both overall and according to various categories (Table 1). The differences among categories were assessed by means of Chi-square testing. Mapping and distribution were carried out using the QGis software (version 3.20).
The evolutionary analyses were conducted using MEGA X software [23]. The analysis involved 18 nucleotide sequences: 12 attained within the present study, five sequences of Thelazia spp. retrieved from GenBank and one Dirofilaria immitis sequence as outgroup. The sequences were aligned using the MUSCLE algorithm, and the evolutionary history was inferred by using the maximum likelihood method and Tamura-Nei model [24]. A discrete gamma distribution was used to model evolutionary rate differences among sites.
Results
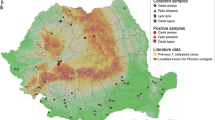
Of the 273 animals sampled, 12 (4.39%) were positive for Thelazia spp. infestation (Table 1). A total of 87 nematodes were collected (Table 2). Of the infected animals, five presented a bilateral infestation (41.66%) while the rest had a unilateral one (58.33%). The intensity varied between one and 33 nematodes per animal (mean intensity 7.25 nematodes/infected horse and median of 4) with an intensity between one and 20 nematodes/infested eye (mean intensity of 2.66 and 4.58 nematodes per eye, respectively). The adult female-to-male ratio was 3.4 to 1. All individuals were morphologically identified as adults and larvae (L5) of T. lacrymalis. There were no statistically significant differences between the prevalence in any of the considered categories (sex, age group, altitude, ecoregion). Of the four ecoregions from which samples were collected, T. lacrymalis was found in three (Fig. 1).
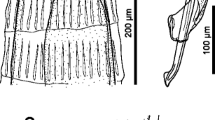
Of the 85 T. lacrymalis specimens, 75 were selected for a detailed morphometric analysis (Fig. 2) (16 adult males, 36 adult females, 22 female L5). The results are shown in Table 3.
Morphological features of T. lacrymalis. A1: Cervical region of a T. lacrymalis male; A2: tail of a T. lacrymalis male; A3: total length of a T. lacrymalis male; B1: cervical region of a T. lacrymalis female, with the nerve ring (Nr) positioned in the lower third of esophagus and the vulvar opening (Vlv) positioned distally from esophago-intestinal junction; B2: tail of a T. lacrymalis female; C1: contents of ruptured uterus of a gravid T. lacrymalis female with measurements; C2: cervical striations in a T. lacrymalis female with measurements; C3: gravid T. lacrymalis female with a highlighted egg. Abbreviations: BC, buccal capsule; Eso, esophagus; Sp, spicule; Prp, pre-anal papillae; Ao, anal orifice; Pap, post-anal papillae; Lrv, larvae; Eme, eggs containing larvae; Ume: blastomerized eggs; Str, striations
All 12 specimens selected for molecular analysis were successfully sequenced, and 11 unique sequences of the COI gene were obtained. The BLAST analysis revealed a 96.46–98.60% nucleotide similarity to the only T. lacrymalis COI isolate from GenBank (AJ271619). The similarity to other species of Thelazia (e.g. Thelazia gulosa AJ544881, Thelazia rhodesi MT511659, T. callipaeda AM042556) ranged between 85.05 and 88.91%. Our sequences were deposited in GenBank under the accession numbers listed in Table 4. Phylogenetic relationships are presented in Fig. 3.
Phylogenetic tree. The bootstrap consensus tree inferred from 1000 replicates. The percentage of trees in which the associated taxa clustered together is shown next to the branches (only values > 50% are shown). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 18 nucleotide sequences. There were a total of 571 positions in the final dataset
Discussion
The lack of data over the past decades concerning the distribution of Thelazia in large herbivores could be attributed to the appearance of more affordable and potent anthelmintics [25] as well as the overall lack or mildness of clinical signs. Although equine thelaziosis is rarely diagnosed, it may represent an animal welfare concern due to the chronical and sometimes irreversible development of the disease or concurrent diseases [13, 26] combined with its potentially rapid spread in larger herds.
In terms of geographical distribution, equine thelaziosis has been occasionally reported in Europe in Russian Federation, England, France, Germany, Sweden and Italy [3, 14,15,16,17, 20, 27]. The current study acts as the first report of equine thelaziosis in the last 15 years in Europe, and the first from Romania and Eastern Europe, with the exceptions of old reports from the former USSR. Although the prevalence is relatively low, we consider that the lack of reports is related to the limited interest of researchers in this disease and the probably limited or absent awareness of veterinary clinicians. The low prevalence could also be attributed to applications of general oral deworming protocols used in horses, which include the use of either ivermectin or fenbendazole [28]. There were no noticeable differences in overall prevalence values during different seasons, the overall value remaining at around 5% throughout the year. Adults have been encountered during every season. L5 females were found only from July to the end of October.
During the present study, no statistically relevant results could be quantified because of the low sample number and wide distribution of both geospatial location and developmental stages. It can be however presumed that altitude is a determining factor in the occurrence of thelaziosis. In this study there were no infested animals originating from the alpine ecoregion (0 of 45); therefore, a plausible explanation could be the decreased vector abundance and shorter seasonal activity at higher altitudes [29,30,31].
Although clinical signs have been associated with the presence of adults, in most species, it has been theorized that the death of larvae within the lachrymal glands could be responsible for the appearance of coalescing granulomas following the administration of oxybendazole in horses [32]. This theory also underlines the importance of larvae in the pathological processes inherent to conjunctivitis, dacryocystitis and ultimately keratoconjunctivitis sicca [33].
The morphological description was done to improve the data availability for the identification of this poorly known species. Our results provide additional data on 18–20 parameters (Table 3), extending the previously documented variations, in adult parasites (both mature and immature stages) as well as comparing our findings to those available in other studies.
Despite the low number of COI gene sequences for nematodes of genus Thelazia available for the phylogenetic analysis, we showed that T. lacrymalis represents a separate clade within the genus.
Conclusion
Equine thelaziosis is present in Romania at low prevalence values, related probably to the widespread use of macrocyclic lactones. We consider equine thelaziosis a neglected disease in Europe, which requires more attention from veterinary practitioners mainly from an animal welfare point of view due to the potentially severe clinical impact.
Availability of data and materials
All data generated or analyzed during this study are included in this published article as well as its additional data. Sequences generated in this study are available in GenBank (ON024362-ON024373).
References
Otranto D, Traversa D. Thelazia eyeworm: an original endo- and ecto-parasitic nematode. Trends Parasitol. 2005;21:1–4.
Löhrer J, Hörning B. Thelazia lacrymalis beim Pferd. Schweizer Archiv für Tierheilkunde SAT : die Fachzeitschrift für Tierärztinnen und Tierärzte. 1967;109:644–53. https://doi.org/10.5169/seals-593476.
Skrjabin KI, Sobolev AA, Ivashkin VM. Spirurata of animals and man and the diseases caused by them. Part 4 thelazioidea. In: Skrjabin KI, editor. Essentials of nematodology. Moscow: Academy of Sciences of the USSR; 1971. p. 1–54.
Otranto D, Mendoza-Roldan JA, Dantas-Torres F. Thelazia callipaeda. Trends Parasitol. 2021;37:263–4. https://doi.org/10.1016/j.pt.2020.04.013.
Karbowiak G, Demiaszkiewicz AW, Pyziel AM, Wita I, Moskwa B, Werszko J, et al. The parasitic fauna of the European bison (Bison bonasus) (Linnaeus, 1758) and their impact on the conservation. Part 1. The summarising list of parasites noted. Acta Parasitol. 2014;3:363–71. https://doi.org/10.2478/s11686-014-0252-0.
Deak G, Ionică AM, Oros NV, Gherman CM, Mihalca AD. Thelazia rhodesi in a dairy farm in Romania and succesful treatment using eprinomectin. Parasitol Int. 2021;80:102–83. https://doi.org/10.1016/j.parint.2020.102183.
Ionică AM, Deak G, Matei IA, D’Amico G, Cotuţiu VD, Mihalca AD, et al. Thelazia callipaeda, an endemic parasite of red foxes (Vulpes vulpes) in Western Romania. J Wildl Dis. 2018;54:829–33. https://doi.org/10.7589/2017-10-251.
Ionică AM, Deak G, D’Amico G, Gherman CM, Mihalca AD. Thelazia callipaeda in mustelids from Romania with the European badger, Meles meles, as a new host for this parasite. Parasit Vectors. 2019;12:370.
Dumitrache MO, Gyorke A, Mircean M, Benea M, Mircean V. Ocular thelaziosis due to Thelazia callipaeda (Spirurida: Thelaziidae) in Romania: first report in domestic cat and new geographical records of canine cases. Parasitol Res. 2018;117:4037–42.
Dumitrache MO, Ionică AM, Voinițchi E, Chavdar N. First report of canine ocular thelaziosis in the Republic of Moldova. Parasit Vectors. 2019;12:505.
Dulceanu, N. Cercetari cu privire la localizarea speciilor si intensivitatea infestatiei cu thelazii la taurine. Lucrari stiintifice, Inst. Agronomic. 1971.
Gurlt EF. Lehrbuch der pathologischen Anatomie der Haustiere, pt 1. Berlin: Reimer; 1831.
Barker IK. Case report. Thelazia lacrymalis from the eyes of an Ontario horse. Can Vet J. 1970;9:186–9.
Arbuckle JB, Khalil LF. A survey of thelazia worms in the eyelids of British cattle. Vet Rec. 1978;102:207–10. https://doi.org/10.1136/vr.102.10.207.
Höglund J, Ljungström BL, Nilsson O, Lundquist H, Osterman E, Uggla A. Occurrence of Gasterophilus intestinalis and some parasitic nematodes of horses in Sweden. Acta Vet Scand. 1997;38:157–65. https://doi.org/10.1186/BF03548495.
Beelitz P, Dongus H, Schöl H, Gerhards H, Gothe R. Thelazia lacrymalis (Nematoda, Spirurida, Thelaziidae): report in a horse in Germany and contribution to the morphology of adult worms. Parasitol Res. 1997;83:627–31. https://doi.org/10.1007/s004360050309.
Giangaspero A, Tieri E, Otranto D, Battistini ML. Occurrence of Thelazia lacrymalis (Nematoda, Spirurida, Thelaziidae) in native horses in Abruzzo region (central eastern Italy). Parasite. 2000;7:51–3. https://doi.org/10.1051/parasite/2000071051.
Alsaad KM, Abbas BA, Yaseen J. Keratoconjunctivitis in drought horses in Basrah. Basrah-Iraq Bas J Vet Res. 2010;1:155–63.
Anvari D, Sharifi N, Hashemi SH. First report of isolation and identification of Thelazia lacrymalis nematodes in horse from Iranshahr city. In: Proceedings of the 3rd national congress of equine health and diseases. 29th April–1st May, 2015, Shiraz, Iran; 2015. p. 42.
Medl N. Thelazia lacrymalis (Gurlt, 1831) beim Pferd - epidemiologische und histopathologische Untersuchungen un kritische retrospektive Betrachtund der klinischen Bedeutung. Inaugural-Dissertation zur Erlangung der tiermedizinischen Doktorwürde der Tierärztlichen Fakultät der Ludwig-Maximilians. Munich, Germany; 2007. pp. 39–44.
Naem S. Ultrastructural observations on the surface of Thelazia lacrymalis (Nematoda: Spirurida. Thelaziidae Acta Vet Hung. 2005;53:205–12.
Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. https://doi.org/10.1017/s0031182000007149.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. https://doi.org/10.1093/molbev/msy096.
Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;3:512–26. https://doi.org/10.1093/oxfordjournals.molbev.a040023.
Tweedle DM, Fox MT, Gibbons LM, Tennant KV. Change in the prevalence of Thelazia species in bovine eyes in England. Vet Rec. 2005;18:555–6. https://doi.org/10.1136/vr.157.18.555.
Wollanke B, Gerhards H, Pfeghaar S. Chronisch rezidivierende Konjunktivitis infolge Thelazia lacrymalis- induzierter, chronisch abszedierender Dacryoadenetis bei einem Warmbluthengst. Pferdeheilkunde. 2004;20:131–4.
Collobert C, Bernard N, Lamidey C. Prevalence of Onchocerca species and Thelazia lacrymalis in horses examined post mortem in Normandy. Vet Rec. 1995;136:463–5. https://doi.org/10.1136/vr.136.18.463.
Dărăbuș G, et al. Strongilidoze digestive. In: Constantin N, Constantinoiu C, Cosoarbă I, Cozma V, Dărăbuș G, Didă I, et al., editors. Tratat de Medicină Veterinară. București: Risoprint; 2014. p. 648–92.
Valiela I. An experimental study of the mortality factors of larval Musca autumnalis De Geer. Ecol Monogr. 1969;39:199–225.
Hodkinson ID. Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev. 2005;80:489–513. https://doi.org/10.1017/S1464793105006767.
Trout Fryxell RT, Moon RD, Boxler DJ, Watson DW. Face fly (Diptera: Muscidae)-biology, pest status, current management prospects, and research needs. J Integr Pest Manag. 2021;12:1–18. https://doi.org/10.1093/jipm/pmaa020.
Greenberg Shari M, Plummer CE, Brooks DE, Porter M, Farina LL, Winter MD. Unilateral orbital lacrimal gland abcess in a horse. Vet Ophthalol. 2011;14:55–60. https://doi.org/10.1111/j.1463-5224.2010.00842.x.
Brooks DE, Matthews AG. Equine ophthalmology. In: Gelatt KN, editor. Veterinary ophthalmology. 4th ed. Oxford: Blackwell Publishing; 2007. p. 1165–274.
Acknowledgements
The authors thank the following students and DVMs involved in the collection and examination of the samples: Teodora Dan, Emil Borza, Diana Opriș and Alexandru Ivanciuc.
Funding
This study was performed under the framework of project Grant Number 57 PCCDI/2018, grant agency ‘The Executive Unit for Financing Higher Education, Research, Development and Innovation’ (UEFISCDI), Romania. The work of CDC was supported by a grant from the Romanian Ministry of Education and Research, CNCS—UEFISCDI, project number PN-III-P1-1.1-PD-2019-0598, within PNCDI III.
Author information
Authors and Affiliations
Contributions
VDC and ADM conceived the studies’ structure. VDC and ADH collected and examined samples from the abattoirs. VDC developed the sampling protocol and created the figures. ADH provided access to the abattoirs. AMI chose and applied the PCR protocol used in the study while CDC processed the samples. ML reviewed the study and assisted in editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cotuțiu, VD., Ionică, A.M., Lefkaditis, M. et al. Thelazia lacrymalis in horses from Romania: epidemiology, morphology and phylogenetic analysis. Parasites Vectors 15, 425 (2022). https://doi.org/10.1186/s13071-022-05532-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05532-z