Abstract
Background
Anaplasma phagocytophilum is characterized by a worldwide distribution and distinguished from other Anaplasmataceae by the broadest range of mammalian hosts and high genetic diversity. The role carnivores play in the life cycle of A. phagocytophilum in Europe is uncertain. Currently, only the red fox is considered a suitable reservoir host. In this study, we focused on native and invasive medium-sized carnivore species that live in sympatry and represent the most abundant species of wild carnivores in Poland.
Methods
A total of 275 individual spleen samples from six carnivore species (Vulpes vulpes, Meles meles, Procyon lotor, Nyctereutes procyonoides and Martes spp.) were screened combining nested PCR and sequencing for A. phagocytophilum targeting a partial groEL gene with subsequent phylogenetic analysis inferred by the maximum likelihood method.
Results
The DNA of A. phagocytophilum was detected in 16 of 275 individuals (5.8%). Eight unique genetic variants of A. phagocytophilum were obtained. All detected haplotypes clustered in the clade representing European ecotype I. Three variants belonged to the subclade with European human cases together with strains from dogs, foxes, cats, and wild boars.
Conclusions
While carnivores might have a restricted role in the dissemination of A. phagocytophilum due to their relatively low to moderate infection rates, they hold significance as hosts for ticks. Consequently, they could contribute to the transmission of tick-borne infections to humans indirectly, primarily through tick infection. This underscores the potential risk of urbanization for the A. phagocytophilum life cycle, further emphasizing the need for comprehensive understanding of its ecological dynamics.
Graphical Abstract

Similar content being viewed by others
Background
Anaplasma phagocytophilum is a gram-negative alpha-proteobacterium infecting neutrophils. It is characterized by a broad distribution [1, 2] and distinguished from other Anaplasmataceae bacteria by the widest range of mammalian hosts and high genetic diversity [3]. Based on studies focused on ecology and genetic diversity, the species of A. phagocytophilum consists of at least four major ecotypes, of which only ecotype I has been proven to infect humans in Europe so far [4, 5]. The main hosts of ecotype I are ungulates [6, 7], dogs, cats, horses [8,9,10], and various wild mammals in urban or suburban areas, such as red foxes (Vulpes vulpes) [11,12,13], hedgehogs (Erinaceus sp.) [14,15,16,17], and wild boars (Sus scrofa) [18,19,20]. European ecotype I of A. phagocytophilum is mainly transmitted by the tick Ixodes ricinus, characterized by low host specificity [21, 22]. To some extent, the nest-dwelling I. hexagonus, which is known to parasitize hedgehogs, red foxes, and European badgers, is involved in the circulation of ecotype I of A. phagocytophilum [23, 24]. Occasionally, species from other tick genera tested positive for the presence of A. phagocytophilum DNA; however, their significance is currently unknown [25,26,27,28].
In Europe, A. phagocytophilum has been detected by molecular methods in wild carnivores from six families: Canidae, Ursidae, Mustelidae (Caniformia), Felidae, Procyonidae, and Viverridae (Feliformia) [3, 29,30,31]. Although the role of wild carnivores as reservoir hosts for this pathogen in Europe is uncertain, some species such as raccoon dogs and red foxes are capable of transmitting A. phagocytophilum in nature. In this study, we focused on native and invasive medium-sized carnivore species living in sympatry and representing the most abundant species of wild carnivores in Poland. Thus, the objectives of this study were to understand the genetic diversity of A. phagocytophilum in wild invasive and native carnivores with overlapping ranges and to investigate the possibility of cross-species transmission of genetic variants (including zoonotic ones) of A. phagocytophilum between these species.
Materials and methods
Study area and sampling
The carcasses of red fox, raccoon dog, raccoon, badger, and marten were collected in the forestry of Ruszów (51° 24′ 00.1″ N 15° 10′ 12.2″ E) in the Lower Silesia County in Poland during the predator control, which was part of the program for the reintroduction of capercaillie (Tetrao urogallus) in the Lower Silesian Forest (project LIFE11 NAT /PL/428) in the years 2017–2019. All carcasses were frozen and transported to the Department of Parasitology, University of Wrocław. A total of 275 individual spleen samples from six carnivore species, red fox (V. vulpes) (n = 48), raccoon dog (Nyctereutes procyonoides) (n = 50), raccoon (Procyon lotor) (n = 42), badger (Meles meles) (n = 51), beech marten (Martes foina) (n = 57), and European pine marten (Martes martes) (n = 27) were collected during necropsy. All samples were kept at − 20 °C until further DNA isolation procedures.
DNA extraction, PCR protocols and sequencing
DNA was extracted from 10 mg of spleen using the commercial GeneMatrix Bio-Trace DNA Purification Kit (EURx, Poland) according to the manufacturer’s instructions. PCRs for detection of A. phagocytophilum were performed using 2 × PCRBIO Taq Mix Red (PCR Biosystems, UK). To determine the groEL ecotype of A. phagocytophilum, 1297 bp fragments of the groESL operon or (in the case of a missing amplicon) 407 bp of the groEL gene were amplified by nested PCR as previously described [18]. To distinguish two marten species (M. martes and M. foina) the rapid PCR–RFLP method described by Vercillo et al. [32] was used.
Amplicons were separated by electrophoresis in a 1.5% agarose gel stained with Midori Green Advance (Nippon Genetics Europe, Germany) gel stain and visualized under UV light. All PCR products of the expected size were excised from the agarose gels, purified, and sequenced in both directions using the amplification primers. Sequencing was performed by Macrogen Capillary Sequencing Services (Macrogen Europe, the Netherlands). The sequences obtained were processed using the Geneious 11.1.4 software [33] and compared with those available in the GenBank™ dataset by Basic Local Alignment Tool (BLAST).
Phylogenetic analysis
The phylogeny of A. phagocytophilum was constructed using eight unique groEL haplotypes detected in this study along with 65 sequences from GenBank, representing four ecotypes described by Jahfari et al. [4] and a sequence from Anaplasma platys used as outgroup. Due to unequal sequence lengths, the alignment was calculated in two steps using the MAFFT algorithm ‘Auto’ strategy for sequences > 1000 nt and the –add function for implementing sequences < 1000 nt in the alignment with final length of 1402 nt. The phylogenetic tree was inferred by the maximum likelihood method by IQTREE 1.6.5 [34]. The best-fit evolution model was selected based on the Bayesian information criterion (BIC) computed by implemented ModelFinder [35]. Branch supports were assessed by the ultrafast bootstrap (UFBoot) approximation [36] and by the SH-like approximate likelihood ratio test (SH-aLRT) [37]. Trees were visualized and edited in FigTree v1.4.1 and Inkscape 0.91.
Results
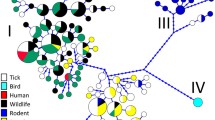
The DNA of A. phagocytophilum was detected in 16 of 275 individuals (5.8%). The number of positive animals per species ranged from one (2%) in raccoon dog to five (8.8%) in beech marten (Table 1). Three long (> 1000 nt) and 13 short (300–400 nt) sequences of the groEL gene representing 8 unique genetic variants were obtained. The major genetic variant V1 was detected in seven samples derived from four martens and a single European badger, red fox, and raccoon, respectively. Two other variants, V2 and V3, were detected in two animals each. Variant V2 was found in red fox and racoon dog, and variant V3 was detected in samples from red foxes only. The remaining variants V4–V8 were detected in one sample each from three martens, one badger, and one raccoon (Table 1). The representative sequences were submitted to the GenBank under the accession number OR167090-OR167101. In phylogenetic analyses (Fig. 1), all detected haplotypes clustered in the largest clade representing European ecotype I [4], which is closely related to isolates from the USA and forms cluster I [5]. Three variants (V1, V3, and V8) belonged to the subclade with European human cases and strains from dogs, foxes, cats, and wild boars. The remaining five variants were distributed among strains isolated from I. ricinus, European hares, carnivores, and sequences obtained from ungulates.
A Schematic representation of the maximum likelihood phylogenetic tree based on the groEL gene sequences of Anaplasma phagocytophilum representing all ecotypes. The highlighted clade representing Ecotype I is displayed in detail; bootstrap values (SH-aLRT/UFB) above the 70/70 threshold are displayed; sequence of Anaplasma platys used as an outgroup is not shown. B Detailed view of the clade representing the Ecotype I/Cluster I; sequences acquired from the GenBank database are marked by their accession number, host, and country of origin. Sequences from this study are highlighted in red and marked by the number of a respective variant. The scale bar indicates the number of nucleotide substitutions per site. C Map of Poland with a detailed locality of Ruszów Forestry sampling area
Discussion
The persistence and transmission of tick-borne pathogens in ecosystems relies upon abundance of susceptible reservoir hosts and their infestation by permissive tick species. Studies on European strains of A. phagocytophilum have shown that a wide range of animal species are involved in the circulation of this pathogen in different ecological niches [38]. Among all Anaplasma spp., A. phagocytophilum represents an assemblage with enormous genetic diversity. Clarifying which host species harbor specific strains of Anaplasma is important for understanding pathogen dynamics and for developing measures to reduce disease burden [39]. The role of carnivores in the ecoepidemiology of A. phagocytophilum is not well understood. While several wild carnivores have been implicated as possible reservoirs for A. phagocytophilum in the US, only the red fox has been considered a suitable host in Europe [12, 29, 30, 40,41,42]. Carnivores such as badgers and martens are often overlooked in studies. This information gap also affects invasive species such as raccoons and raccoon dogs, which were intentionally introduced to Europe and later spread through the continent [43]. In our study, we have shown that both native (foxes, badgers, martens) and invasive (raccoons) carnivores living in sympatry in a forest biotope are involved in the circulation of A. phagocytophilum with zoonotic potential, finding the genetic variant V1 in all examined species except raccoon dogs (Table 1, Fig. 1).
The red fox is the most widespread free-living predator in the world [44], and its role as a host for A. phagocytophilum is well documented [45, 46]. In Poland, A. phagocytophilum has been detected in foxes with prevalence ranging from 2.7% in the central part of the country [11] to 34.5% in the northeastern regions [31]. In our study, 6.2% of animals tested positive for this pathogen, which is consistent with the general trend observed for Anaplasma infections in the European fox population and supports foxes as a reservoir of A. phagocytophilum.
Only a few previous studies have focused on the role of mustelids in the circulation of A. phagocytophilum. In this study, 3.9% of badgers and 9.5% of martens were positive for A. phagocytophilum DNA. Analyses focused on badgers and martens from eastern and northern Poland detected the DNA of A. phagocytophilum in 18.7% and 41.7% of animals, respectively [31]. For comparison, the number of positive badgers from Spain and The Netherlands did not exceed 2% [39, 47]. Data on A. phagocytophilum in European marten populations are sparse. To our knowledge, this pathogen has been detected so far in a beech marten from Romania [28] and a pine marten from Hungary [45] in which ecotype I was recognized [4]. In addition, in mustelids from The Netherlands tested by quantitative polymerase chain reaction (qPCR) for several Tick Borne Pathogens (TBPs), A. phagocytophilum was detected in beech martens (1.5%), European badgers (1.8%), European polecats (Mustela putorius) (4.9%), and pine martens (22%) [39]. The observed discrepancies in overall prevalence are likely due to the specific environmental conditions under which each study was conducted, affecting tick occurrence and density. The type of tissue and molecular method used to detect pathogens may also explain the differences in results [17]. The results of our study indicate that martens are significantly more susceptible to Anaplasma infection, with a consistent increase in prevalence observed in these predators in all cited studies. The differences in distribution patterns between the two species (the pine marten has a patchy, fragmented ecogeographic distribution restricted to a narrow ecological niche, whereas the beech marten has a continuous distribution across a wide range of natural, semi-natural, and even urban habitats) may have implications for the ecoepidemiology of A. phagocytophilum, particularly in the context of rapid landscape change and intense urbanization processes. Nevertheless, the current lack of comprehensive studies makes it difficult to fully elucidate the relationships. Determining whether martens have exclusive host/reservoir competence for Anaplasma, Anaplasmataceae, or other tick-borne pathogens is a complex task that requires further investigation. Invasive carnivore species, such as raccoons and raccoon dogs, are potential reservoirs for numerous TBPs [43, 48], and we also found A. phagocytophilum in 2% of raccoon dogs and 4.7% of raccoon dogs. The prevalence of A. phagocytophilum previously observed in raccoon dogs from Poland (35.3%) [31] was higher than in Germany (23%) [47]. Kjær and colleagues reported a high clustering of A. phagocytophilum-positive ticks on individual raccoon dogs in Denmark [49]. Raccoons from Austria, the Czech Republic, Germany, and Poland [50, 51] were tested for the presence of A. phagocytophilum DNA, but the pathogen was found in only one raccoon from the latest study [43]. Our results show that raccoons are adapted to carry European variants of A. phagocytophilum. Due to their synanthropic nature and frequent use of tree holes and burrows of other animal species, raccoons can be infested with both questing and endophilic ticks, potentially bridging the enzootic cycles of A. phagocytophilum. Regarding the epidemiological impact of raccoons and raccoon dogs, these invasive species should be monitored for their possible involvement in the spread of A. phagocytophilum in different geographic regions [51].
In recent years, awareness of role of wildlife in TBPs and the possible impact on livestock, humans, and their pets has increased [52]. Knowledge of potential reservoir hosts and their ticks is necessary to develop effective surveillance and management measures for disease outbreaks and parasite cycles in wildlife [53]. Nidicolous ticks such as Ixodes hexagonus, which are commonly found on foxes and have been detected on mustelids [39, 54], deserve future attention as they may play a role as vectors for zoonotic variants of A. phagocytophilum. In addition, high population densities of predator populations are possible in European landscapes with heterogeneous habitat structure, leading to shared territories among red foxes, raccoons, and raccoon dogs [55,56,57], favoring the transmission of vectors and pathogens. The increasing distribution and numbers of foxes in urban and suburban areas make this species a bridging species between natural ecosystems and anthropogenic landscapes [39].
Conclusions
While carnivores might have a restricted role in the dissemination of A. phagocytophilum due to their relatively low to moderate infection rates, they hold significance as hosts for ticks. Consequently, they could contribute to the transmission of tick-borne infections to humans indirectly, primarily through tick infection. This underscores the potential risk of urbanization for the A. phagocytophilum life cycle, further emphasizing the need for comprehensive understanding and management of its ecological dynamics.
Data availability
Data will be made available on request.
References
Ben Said M, Belkahia H, Messadi L. Anaplasma spp. in North Africa: a review on molecular epidemiology, associated risk factors and genetic characteristics. Ticks Tick Borne Dis. 2018;9:543–55.
Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol. 2010;167:108–22.
Rar V, Tkachev S, Tikunova N. Genetic diversity of Anaplasma bacteria: twenty years later. Infect Genet Evol. 2021;91:104833.
Jahfari S, Coipan EC, Fonville M, Van Leeuwen AD, Hengeveld P, Heylen D, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014;7:365.
Jaarsma RI, Sprong H, Takumi K, Kazimirova M, Silaghi C, Mysterud A, et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit Vectors. 2019;12:328.
Stigum VM, Jaarsma RI, Sprong H, Rolandsen CM, Mysterud A. Infection prevalence and ecotypes of Anaplasma phagocytophilum in moose Alces alces, red deer Cervus elaphus, roe deer Capreolus capreolus and Ixodes ricinus ticks from Norway. Parasit Vectors. 2019;12:1–8.
Hamšíková Z, Silaghi C, Takumi K, Rudolf I, Gunár K, Sprong H, et al. Presence of roe deer affects the occurrence of Anaplasma phagocytophilum ecotypes in questing Ixodes ricinus in different habitat types of central Europe. Int J Environ Res Public Health. 2019;16:4725.
Praskova I, Bezdekova B, Zeman P, Jahn P. Seroprevalence of Anaplasma phagocytophilum in horses in the Czech Republic. Ticks Tick Borne Dis. 2011;2:111–5.
Franzén P, Aspan A, Egenvall A, Gunnarsson A, Karlstam E, Pringle J. Molecular evidence for persistence of Anaplasma phagocytophilum in the absence of clinical abnormalities in horses after recovery from acute experimental infection. J Vet Intern Med. 2009;23:636–42.
Kapiainen S. The prevalence of Anaplasma phagocytophilum in Finnish dogs. 2016. https://core.ac.uk/works/37117259.
Karbowiak G, Vichova B, Majlathova V, Hapunik J, Petko B. Anaplasma phagocytophilum infection of red foxes (Vulpes vulpes). Ann Agric Environ Med. 2009;16:299–300.
Tolnai Z, Sréter-Lancz Z, Sréter T. Spatial distribution of Anaplasma phagocytophilum and Hepatozoon canis in red foxes (Vulpes vulpes) in Hungary. Ticks Tick Borne Dis. 2015;6:645–8.
Hodžić A, Alić A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher GG. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors. 2015;8:88.
Földvári G, Jahfari S, Rigó K, Jablonszky M, Szekeres S, Majoros G, et al. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in urban hedgehogs. Emerg Infect Dis. 2014;20:496–7.
Ruszkowski JJ, Hetman M, Turlewicz-Podbielska H, Pomorska-Mól M. Hedgehogs as a potential source of zoonotic pathogens—a review and an update of knowledge. Animals. 2021;11:1754.
Silaghi C, Skuballa J, Thiel C, Pfister K, Petney T, Pfäffle M, et al. The European hedgehog (Erinaceus europaeus)—a suitable reservoir for variants of Anaplasma phagocytophilum? Ticks Tick Borne Dis. 2012;3:49–54.
Lesiczka PM, Hrazdilová K, Majerová K, Fonville M, Sprong H, Hönig V, et al. The role of peridomestic animals in the eco-epidemiology of Anaplasma phagocytophilum. Microb Ecol. 2021;82:602–12.
Hrazdilová K, Lesiczka PM, Bardoň J, Vyroubalová Š, Šimek B, Zurek L, et al. Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick Borne Dis. 2021;12:101558.
de la Fuente J, Gortazar C. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg Infect Dis. 2012;18:2094–5.
Silaghi C, Pfister K, Overzier E. Molecular investigation for bacterial and protozoan tick-borne pathogens in wild boars (Sus scrofa) from southern Germany. Vector-Borne Zoonot Dis. 2014;14:371–3.
Humair PF, Douet V, Cadenas FM, Schouls LM, Van De PI, Gern L. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J Med Entomol. 2007;44:869–80.
Hvidsten D, Frafjord K, Gray JS, Henningsson AJ, Jenkins A, Kristiansen BE, et al. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 2020;11:101388.
Jahfari S, Ruyts SC, Frazer-Mendelewska E, Jaarsma R, Verheyen K, Sprong H. Melting pot of tick-borne zoonoses: the European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasit Vectors. 2017;10:1–9.
Krawczyk AI, Van Leeuwen AD, Jacobs-Reitsma W, Wijnands LM, Bouw E, Jahfari S, et al. Presence of zoonotic agents in engorged ticks and hedgehog faeces from Erinaceus europaeus in (sub) urban areas. Parasit Vectors. 2015;8:210.
Baldridge GD, Scoles GA, Burkhardt NY, Schloeder B, Kurtti TJ, Munderloh UG. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae). J Med Entomol. 2009;46:625–32.
Bonnet SI, Binetruy F, Hernández-Jarguín AM, Duron O. The tick microbiome: why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front Cell Infect Microbiol. 2017;7:236.
Szekeres S, Claudia Coipan E, Rigó K, Majoros G, Jahfari S, Sprong H, et al. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in natural rodent and tick communities in Southern Hungary. Ticks Tick Borne Dis. 2015;6:111–6.
Wirtgen M, Nahayo A, Linden A, Garigliany M, Desmecht D. Tickborne diseases: detection of Anaplasma phagocytophilum in Dermacentor reticulatus ticks. Vet Rec. 2011;168:195.
André MR. Diversity of Anaplasma and Ehrlichia/Neoehrlichia agents in terrestrial wild carnivores worldwide: implications for human and domestic animal health and wildlife conservation. Front Vet Sci. 2018;5:293.
Matei IA, Ivan T, Ionică AM, D’amico G, Deak G, Nadas GC, et al. Anaplasma phagocytophilum in multiple tissue samples of wild carnivores in Romania. J Wildl Dis. 2021;57:949–53.
Szewczyk T, Werszko J, Myczka AW, Laskowski Z, Karbowiak G. Molecular detection of Anaplasma phagocytophilum in wild carnivores in north-eastern Poland. Parasit Vectors. 2019;12:1–5.
Vercillo F, Lucentini L, Mucci N, Ragni B, Randi E, Panara F. A simple and rapid PCR-RFLP method to distinguishing Martes martes and Martes foina. Conserv Genet. 2004;5:869–71.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9.
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74.
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–9.
Minh BQ, Nguyen MAT, von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol. 2013;30:1188–95.
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–21.
Dugat T, Lagrée AC, Maillard R, Boulouis HJ, Haddad N. Opening the black box of Anaplasma phagocytophilum diversity: current situation and future perspectives. Front Cell Infect Microbiol. 2015;5:1–18.
Hofmeester TR, Krawczyk AI, Van Leeuwen AD, Fonville M, Montizaan MGE, Van Den Berge K, et al. Role of mustelids in the life-cycle of ixodid ticks and transmission cycles of four tick-borne pathogens. Parasit Vectors. 2018;11:1–3.
Härtwig V, von Loewenich FD, Schulze C, Straubinger RK, Daugschies A, Dyachenko V. Detection of Anaplasma phagocytophilum in red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) from Brandenburg, Germany. Ticks Tick Borne Dis. 2014;5:277–80.
Sgroi G, Iatta R, Veneziano V, Bezerra-Santos MA, Lesiczka P, Hrazdilová K, et al. Molecular survey on tick-borne pathogens and Leishmania infantum in red foxes (Vulpes vulpes) from southern Italy. Ticks Tick Borne Dis. 2021;12:101669.
Lesiczka PM, Rudenko N, Golovchenko M, Juránková J, Daněk O, Modrý D, et al. Red fox (Vulpes vulpes) play an important role in the propagation of tick-borne pathogens. Ticks Tick Borne Dis. 2023;14:102076.
Myśliwy I, Perec-Matysiak A, Hildebrand J. Invasive raccoon (Procyon lotor) and raccoon dog (Nyctereutes procyonoides) as potential reservoirs of tick-borne pathogens: data review from native and introduced areas. Parasit Vectors. 2022;15:1–11.
Lindsø LK, Dupont P, Rød-Eriksen L, Andersskog IPØ, Ulvund KR, Flagstad Ø, et al. Estimating red fox density using non-invasive genetic sampling and spatial capture–recapture modelling. Oecologia. 2022;198:139–51.
Medkour H, Laidoudi Y, Marié JL, Fenollar F, Davoust B, Mediannikov O. Molecular investigation of vector-borne pathogens in red foxes (Vulpes vulpes) from southern France. J Wildl Dis. 2020;56:837–50.
Ebani VV, Verin R, Fratini F, Poli A, Cerri D. Molecular survey of Anaplasma phagocytophilum and Ehrlichia canis in red foxes (Vulpes vulpes) from central Italy. J Wildl Dis. 2011;47:699–703.
García-Pérez AL, Oporto B, Espí A, del Cerro A, Barral M, Povedano I, et al. Anaplasmataceae in wild ungulates and carnivores in northern Spain. Ticks Tick Borne Dis. 2016;7:264–9.
Hildebrand J, Perec-Matysiak A, Popiołek M, Merta D, Myśliwy I, Buńkowska-Gawlik K. A molecular survey of spotted fever group rickettsiae in introduced raccoons (Procyon lotor). Parasit Vectors. 2022;15(1):162. https://doi.org/10.1186/s13071-022-05280-0.
Kjær LJ, Jensen LM, Chriél M, Bødker R, Petersen HH. The raccoon dog (Nyctereutes procyonoides) as a reservoir of zoonotic diseases in Denmark. Int J Parasitol Parasites Wildl. 2021;16:175–82.
Duscher T, Hodžić A, Glawischnig W, Duscher GG. The raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor)—their role and impact of maintaining and transmitting zoonotic diseases in Austria. Central Europe Parasitol Res. 2017;116:1411–6.
Hildebrand J, Buńkowska-Gawlik K, Adamczyk M, Gajda E, Merta D, Popiołek M, et al. The occurrence of Anaplasmataceae in European populations of invasive carnivores. Ticks Tick Borne Dis. 2018;9:934–7.
Otranto D, Cantacessi C, Pfeffer M, Dantas-Torres F, Brianti E, Deplazes P, et al. The role of wild canids and felids in spreading parasites to dogs and cats in Europe Part I: protozoa and tick-borne agents. Vet Parasitol. 2015;213:12–23.
Sutor A, Schwarz S, Conraths FJ. The biological potential of the raccoon dog (Nyctereutes procyonoides, Gray 1834) as an invasive species in Europe-new risks for disease spread? Acta Theriol (Warsz). 2014;59:49–59.
Hofmeester TR, Jansen PA, Wijnen HJ, Coipan EC, Fonville M, Prins HHTT, et al. Cascading effects of predator activity on tick-borne disease risk. Proc R Soc B Biol Sci. 1859;2017:20170453.
Márton M, Markolt F, Szabó L, Heltai M. Niche segregation between two medium-sized carnivores in a hilly area of Hungary. Ann Zool Fenn. 2014;51:423–32.
Kauhala K, Holmala K. Landscape features, home-range size and density of northern badgers (Meles meles). Ann Zool Fenn. 2011;48:221–32.
Holmala K, Kauhala K. Habitat use of medium-sized carnivores in southeast Finland- key habitats for rabies spread? Ann Zool Fenn. 2009;46:233–46.
Acknowledgements
The carnivores’ carcasses were collected during the predator control operation conducted as a part of the program to re-introduce the capercaillie (Tetrao urogallus) in the Lower Silesian Forest financed by the European Commission, the National Fund for Environmental Protection and Water Management, and the Polish State Forests (Grant LIFE11 NAT/PL/428). We are grateful to Janusz Kobielski and Marcin Popiołek, PhD, DSc, for their help in collecting the material. We thank Weronika Hildebrand, DVM, for help with laboratory work.
Funding
KH was supported by the project National Institute of Virology and Bacteriology (Programme EXCELES, ID Project No. LX22NPO5103)—Funded by the European Union—Next Generation EU.
Author information
Authors and Affiliations
Contributions
PL, KH: methodology; PL, IM: formal analysis; KH, JH, APM: funding acquisition: PL, KH: original draft writing; all authors were responsible for editing and review; all authors approved the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The approval of the Ethics Committee was not required because the material for the research was obtained from the predator control operation.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lesiczka, P.M., Myśliwy, I., Buńkowska-Gawlik, K. et al. Circulation of Anaplasma phagocytophilum among invasive and native carnivore species living in sympatry in Poland. Parasites Vectors 16, 368 (2023). https://doi.org/10.1186/s13071-023-05996-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05996-7