Abstract
Background
The role of the domestic cat as definitive host for Echinococcus multilocularis and thus in environmental contamination with eggs has not yet been entirely resolved. This study aimed to assess the prevalence of E. multilocularis and other gastrointestinal parasites in Swiss domestic cats and to compare the diagnostic sensitivity of different methods for the detection of intestinal taeniid infection.
Methods
Faecal samples from 146 cats were included in the study. Faecal samples only were available from 55 cats; for the other 91 cats, necropsy was performed in addition to faecal sample testing. All (n = 146) faecal samples were analysed by a combined sedimentation/flotation technique (44% ZnCl2) and by the sodium acetate-acetic acid-formalin (SAF) sedimentation technique; when sufficient material was available (n = 121 samples) the Baermann-Wetzel technique was also used. Additionally, all samples were analysed by two coproantigen (copro)-quantitative PCRs (qPCR): (i) a multiplex qPCR able to detect and differentiate between E. multilocularis, Echinococcus granulosus sensu lato and Taenia spp./other cestodes (CEST-qPCR) and (ii) an E. multilocularis-specific qPCR (EM-qPCR). Finally, the intestines were examined macroscopically and microscopically for parasite stages at necropsy (n = 91) and using an intestinal scraping technique (IST) (n = 64).
Results
Of the 146 cats examined, 24 (17.1%) were infected by intestinal parasites, namely Hydatigera (syn. Taenia) taeniaeformis (8.9%), Toxocara cati (6.1%), Capillaria sp. (3.4%), hookworms (3.4%), Mesocestoides litteratus (1.4%), Giardia sp. (1.4%), Cystoisospora rivolta (1.4%), Cystoisospora felis (0.7%), Toxoplasma gondii (0.7%), Hammondia hammondi (0.7%) and Strongyloides sp. (0.7%). Necropsy and the IST revealed adult H. taeniaeformis in 12 animals, of which eight faecal samples were positive by the CEST-qPCR (sensitivity = 67%) and six samples by the sedimentation/flotation technique (sensitivity = 50%). No E. multilocularis infection was detected in the sampled cats. Using Bayesian latent class analysis, the mean posterior prevalence probability was 0.0% (95% confidence interval 0–0.83%) for E. multilocularis.
Conclusions
There was no evidence of E. multilocularis infection among the 146 cats examined, suggesting that the prevalence of this parasite is low (< 1%) in the Swiss domestic cat population. Nonetheless, some of the sampled cats were infected by parasites that have rodents as intermediate hosts, demonstrating successful predation by these cats, and some were infected with zoonotic parasites. Cats therefore should not be disregarded as potential hosts for E. multilocularis and other zoonotic parasites.
Graphical Abstract

Similar content being viewed by others
Introduction
In Switzerland, an estimated 1.8 million domestic pet cats live with a human population of approximately 8.6 million people, i.e. about 30% of all households have at least one cat [1]. Further, 100,000–300,000 stray cats roam freely in Switzerland [2]. This large cat population and the distinctive proximity of cats to humans, often including children and elderly people, make them a potential source of zoonotic pathogens, including parasites such as Toxoplasma gondii and Toxocara cati. The prevalence of these parasites in cats varies strongly depending on the geographical region of origin, frequency of veterinary care, lifestyle (stray, shelter, privately owned) and presence of intermediate and transport hosts.
Echinococcus multilocularis is a widely distributed parasite in the Northern hemisphere and is the cause of alveolar echinococcosis (AE) in humans, an emerging zoonotic disease [3, 4]. Foxes and other canids are the main definitive hosts of this parasite and responsible for environmental contamination with eggs [5,6,7]. Many rodent species serve as intermediate hosts [8, 9]. Additionally, a wide spectrum of mammal species can become accidental hosts and develop AE, such as humans, non-human primates, domestic pigs, Eurasian and Canadian beavers (Castor fiber and C. canadensis) and even domestic dogs [10,11,12,13]. Switzerland has been considered highly endemic for this parasite for decades, alongside other European countries, Russia, central Asia and western China [4, 14]. In Switzerland, the prevalence of E. multilocularis can reach up to 53% in the fox population, mainly in the northern and western parts [4] of the country, with a positive correlation between fox densities and the incidence of AE in humans [15]. Urban fox populations observed in many European countries lead to environmental contamination in the proximity of humans and their pets [16,17,18].
Comparative studies between foxes, dogs, raccoon dogs and cats suggest that cats play a much smaller role in the environmental contamination with E. multilocularis eggs than other definitive hosts. Cats showed a lower infection rate and shorter patent period, developed a lower worm burden and were estimated to have a lower biotic potential (total eggs excreted) than all other host species [6, 7]. On the other hand, cats can be a potential source of environmental contamination with E. multilocularis eggs in highly endemic areas [19]. Additionally, cat ownership, especially linked with unsupervised outdoor access of the cats, has been identified as a significant risk factor for human AE in case–control studies [20,21,22]. The majority of domestic cats in Switzerland are estimated to have unsupervised outdoor access [1]. Furthermore, even pet cats display a distinct predatory behaviour and will therefore occasionally prey on infected intermediate hosts, thus supporting the life-cycles of many parasites that affect not only felines but that can also play an important role in public health due to their zoonotic nature, such as E. multilocularis, T. cati and T. gondii.
Studies on E. multilocularis in cats generally report a very low prevalence in most western European countries. However, most studies utilized coprology as the detection method, which is well known to have a low sensitivity for taeniid eggs [23, 24]. Therefore, the aim of our study was to assess the prevalence of E. multilocularis, as well as of other zoonotic endoparasites, in domestic cats in Switzerland using different diagnostic approaches: routine coprological (copro) methods, copro-quantitative PCR (qPCR) techniques, the intestinal scraping technique (IST) followed by sedimentation and flotation of the flow-through and histopathology. Simultaneously, we compared the diagnostic performance of these various techniques for the detection of taeniid infections.
Methods
Sample collection
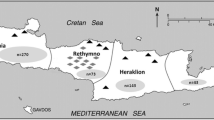
From March to September 2022, a total of 146 faecal samples were collected from privately owned cats, predominantly from the northwestern region of Switzerland (Fig. 1). Faecal samples or large intestine contents were obtained either from the routine diagnostic laboratory at the Institute of Parasitology, University of Bern (group 1, n = 55) or during necropsy (group 2, n = 91). The dead cats were disposed of at the Institute of Animal Pathology, University of Bern and kept frozen at − 20 °C until used for teaching and research purposes. Only cats older than 3 months of age were included in the study. Exact information on age, health status and outdoor access was not available for all animals.
Routine coprology
Collected faecal samples or large intestine contents collected at necropsy were stored at 4 °C for a maximum of 3 days until analysis. All samples (n = 146) were analysed by: (i) the combined sedimentation/flotation technique using a 44% zinc chloride solution (specific density [S.D.] = 1.3) as flotation medium [25] and (ii) the sodium acetate-acetic acid-formalin (SAF)—concentration technique [25]. In addition, when sufficient material was available (n = 121), the Baermann–Wetzel technique was performed [25]. Parasite stages were photographed and measured using a digital image processing system (Nikon NIS-Elements—Microscope Imaging Software; Nikon Corp., Tokyo, Japan) and identified through morphological characterization [25]. Isospora-type oocysts were further differentiated into Toxoplasma-like and Cystoisospora species by their respective size [25], and Toxoplasma-like oocysts were further differentiated by PCR. Eggs of Taenia spp., Hydatigera spp., and Echinococcus spp. were grouped as taeniid eggs and further differentiated by PCR.
Necropsy, histopathology and intestinal scraping technique
During necropsy, the intestines of 91 cats were cut open longitudinally and macroscopically examined for intestinal parasites. Detected nematodes and cestodes were stored for morphological and/or molecular characterization. For histopathological examination, sections of the duodenum and proximal jejunum were obtained [7], fixed in 4% neutral buffered formalin and embedded in paraffin. Multiple sections were prepared (thickness: 4 μm), mounted on glass slides and stained with haematoxylin and eosin (HE). Finally, the samples were analysed by light microscopy.
For 64 cats, the whole intestines were frozen at − 80 °C for at least 4 days. The IST was adapted from methods described by Hofer et al. [26]. Briefly, the intestinal mucous layer was scraped from the entire length of the small intestine using glass slides and transferred to a vessel containing 180 ml of 0.9% NaCl solution; the suspension was subsequently filtered applying a 3-step sieving technique, with filtration through a 2-mm, then a 1-mm and lastly a 250-μm mesh sieve. The remnants on each sieve were separately placed in a round plastic dish and analysed under a stereomicroscope (magnification 40×). The flow-through was allowed to sediment for 24 h, following which the supernatant was discarded, and the sediment was additionally investigated for parasite products by sucrose flotation (S.D. = 1.3).
Molecular diagnostics
DNA extraction for the faecal/intestinal contents was performed using a commercial kit (Quick-DNA Fecal/Soil Microbe Miniprep Kit, Zymo Research Corp., Orange, CA, USA) according to the manufacturer’s instructions. DNA from adult stages of helminths that were found in the intestine during necropsy was extracted using the commercial DNeasy Blood & Tissue Kit (QIAGEN GmbH, Hilden, Germany) following the manufacturer's instructions.
CEST-quantitative PCR
All faecal/intestinal content samples were analysed with a specific TaqMan-probe-based multiplex real-time PCR (qPCR) able to simultaneously detect and distinguish between DNA from E. multilocularis, Echinococcus granulosus sensu lato (E. granulosus s.l.) and other cestodes (CEST-qPCR). This CEST-qPCR targeted mitochondrial genes for NADH dehydrogenase subunit 1 (nad1) and the small subunit of ribosomal RNA (rrnS) by using primers originally described by Trachsel et al. [27] for conventional PCR. The design of the probes was based on the use of the programme Primer3 Input, version 0.4.0 (https://bioinfo.ut.ee/primer3-0.4.0). For qPCR detection, TaqMan fluorescence probes were designed to discriminate among E. multilocularis (probe EMR-TAQ labelled with Cy5), E. granulosus (probe EGR-TAQ labelled with FAM) and other taeniids (probes CEST-TAQ 1–5 labelled with HEX) in a multiplex format (Table 1). Primers and TaqMan probes were purchased from Microsynth (Balgach, Switzerland). Reactions were performed in a final volume of 10 μl containing 5 μl of commercial master mix (SensiFAST™ Probe No-ROX Kit; Meridian Bioscience, Cincinnati, OH, USA), 0.5 μl of primer mix (containing 2 μM of primers CEST1, CEST2, CEST3, CEST4, and 16 μM of primer CEST5; Table 1), 0.1 μl detection probe mix containing seven probes (Table 1) at a concentration of 10 µM each, 0.4 µl of H2O and 4 μl of sample DNA. Positive (DNA extracts from E. multilocularis, E. granulosus and Taenia saginata) and negative (H2O) controls were used in each run. DNA amplification was performed in the CFX96 qPCR detection system (Bio-Rad Laboratories AG, Cressier, Switzerland) starting with 1 cycle of 95 °C for 5 min, followed by 50 cycles of 10 s at 95 °C and 30 s at 58 °C. PCR products were measured after each cycle by the detection of light emission from the different fluorophores at the end of the 58 °C incubation step. For analysis of the results, CFX manager software version 1.6 (BioRad Laboratories AG) was used. Reactions with a cycle threshold (Ct) of < 50 cycles were defined as positive.
Echinococcus multilocularis-specific qPCR
All faecal/intestinal content samples were analysed for the presence of E. multilocularis DNA based on the amplification of a part of the mitochondrial gene rrnL with a specific TaqMan-qPCR (ES-qPCR) in a CFX96 qPCR detection system (Bio-Rad Laboratories AG), as previously described [28]. The same general qPCR reagents previously mentioned for the CEST-qPCR were used. The detection limit of this qPCR has been reported as one egg of E. multilocularis in 0.5 g of faecal sample [28].
qPCR Toxoplasma gondii
Samples positive for Toxoplasma-like oocysts were further investigated with a TaqMan-based real-time PCR as previously described by Pardo Gil et al. [29] to confirm T. gondii oocysts and distinguish them from the morphologically similar oocysts of Hammondia spp.
Conventional PCR and sequencing
DNA extracted from cestode stages collected during necropsy, IST and intestinal content samples positive for Taenia spp. in the CEST-qPCR were analysed by a conventional multiplex-PCR as described by Trachsel et al. [27]. Samples with Toxoplasma-like oocysts, negative for T. gondii, were additionally investigated with a conventional PCR targeting the 18S ribosomal RNA (rRNA) fragment employing COC-1 and COC-2 primers [30]. Positive, amplified products were purified (DNA Clean & Concentrator-5; Zymo Research, Irvine, CA, USA) and sent for Sanger sequencing (Microsynth, Balgach, Switzerland). The obtained sequences were aligned with the Geneious Prime software (version 2023.0.4) and then compared with GenBank entries using NCBI BLAST.
Statistical analysis
The observed prevalence and exact binomial 95% confidence intervals (CIs) were calculated for each parasite according to Clopper and Pearson [31], with the exception of E. multilocularis. For E. multilocularis, the confirmed EM-qPCR results were analysed in a Bayesian framework using latent class analysis as described by Branscum et al. [32]. We used peer-reviewed published literature available at the time to inform our priors and build the statistical model. We used beta prior distributions for the sensitivity (Se) and the specificity (Sp) of the EM-qPCR as follows:
These parameters were based on Knapp et al. [28] and were determined as follows: aSe = 25, bSe = 4, aSp = 26 and bSp = 2.
The infection prevalence π was modelled using a mixture distribution as follows:
where τ is the probability that the disease is present in the cat population. Again, the parameters of the prior distribution were based on published literature. Two studies assessed the prevalence of E. multilocularis in Swiss cats, one of which reported one positive sample of 263 tested (0.4%) [33] and the second reported zero prevalence [34]. Zero prevalence was also reported in three of seven European countries [35, 36]; the prior probability τ was therefore set to 0.5. In the available literature, the highest reported prevalence was 6% in Poland [48]; therefore, the prior prevalence parameters were set to aπ = 1 and bπ = 50, so that 95% of the prior probability density function fell below 6%.
The data analysis was performed with WinBUGS (version 1.4.3) [37] called from R (version 4.2.1) (www.cran.r-project.org) through the R2WinBUGS (version 2.1-21) [38] package. We run three chains for 10,000 iterations and discarded the first 500 as a burn-in and thinned to every fifth sampled value.
Sensitivity calculation of sedimentation/flotation and CEST-qPCR
The sensitivity of faecal flotation and the CEST-qPCR was estimated using post-mortem recovery of adult parasites as the gold standard. It was calculated as the ratio between the number of true positives correctly detected by the respective technique and the total number of true positives detected by necropsy.
Results
Taking into consideration all applied methods (routine coprological methods, necropsy, IST, copro-qPCRs and histopathology), 25 of the 146 cats (17.1%; 95% CI 11.4–24.2) were positive for intestinal parasites, but none of the animals showed E. multilocularis infection (Table 2).
Coprological examination
With routine faecal coprology (i.e. combined sedimentation/flotation technique, sodium acetate-acetic acid-formalin—concentration technique and Baermann), 12.3% (18/146; 95% CI 7.5–18.8) of the samples tested positive for parasite eggs, oocysts, cysts and/or larvae. Taeniids and T. cati were the most frequently detected parasites, with a prevalence of 4.1% (6/146; 95% CI 1.5–8.7) each, followed in order of decreasing prevalence by Capillaria sp. (3.4% [5/146]; 95% CI 1.1–7.8), hookworms (2.1% [3/146]; 95% CI 0.4–5.8), Giardia sp. (1.4% [2/146]; 95% CI 0.2–4.8), Cystoisospora rivolta (1.4% [2/146] 95% CI 0.2–4.8), Cystoisospora felis (0.7% [1/146]; 95% CI 0–3.7), T. gondii (0.7% [1/146]; 95% CI 0–3.7) (confirmed by qPCR) and Strongyloides sp. (0.7% [1/146] 95% CI 0–3.7) (confirmed by morphological identification of third-stage larvae in coproculture) (Table 2). Taeniid eggs were only detected in samples from necropsied cats (6.6% [6/91]; 95% CI 2.5–13.8).
Necropsy, histopathology and IST
Adult helminths were macroscopically visible during necropsy in 16/91 cats (17.6%; 95% CI 10.4–27.0) (Table 2). Histopathology revealed coccidian parasite stages in one cat, which was positive for C. rivolta oocysts by coprology. Echinococcus spp. infection was not detected by either macroscopical examination or histopathology in any of the 91 necropsied cats.
The IST and/or flotation of IST sediment in 64 cats allowed the detection of additional parasites in seven cases, i.e. a taeniid egg, Mesocestoides litteratus (immature worms), Ancylostoma tubaeforme (male worms), T. cati (immature worms) and T. gondii-like oocysts (further identified as Hammondia hammondi by PCR, see following text) (Table 2).
Copro-qPCRs
The CEST-qPCR analyses of faecal/intestinal content detected 10/146 (6.9%; 95% CI 3.3–12.2) samples positive for DNA of Taenia spp./other cestodes (Table 2). No E. multilocularis or E. granulosus s.l. DNA was detected. The E. multilocularis-specific EM-qPCR analysis was negative for all 146 faecal/intestinal content samples.
Conventional PCRs and sequencing
The conventional multiplex-PCR analysis identified 16 positive samples with primers CEST3-CEST5 (Taenia spp. and other cestodes). Fourteen of these sequences (220 bp, with trimmed primer regions) were obtained from 11 adult worms found in 10 cats (two of the worms were derived from the same cat), faeces of two cats and IST of one cat and were deposited in GenBank (Accession no. OR268952–OR268965). These sequences showed 99.1–100% identity with Hydatigera taeniaeformis sensu lato (including Hydatigera kamiyai) (GenBank ID JQ663994 and MN505198). Five different haplotypes were identified: one haplotype (1) was detected in nine of the samples (OR268953–OR268961), a second haplotype (2) was detected in two samples (OR268952 and OR268962, with one of the sequences [OR268952] being 38 bp shorter) and the other three haplotypes (3–5) (OR268963-OR268965) were observed in one sample each (Fig. 2). It is interesting to note that the two analysed worms from the same cat showed different haplotypes.
Two of the sequences (223 bp, with trimmed primer regions) obtained from a faecal sample and one adult worm had an identity of 99.6% and 100% with M. litteratus (GenBank ID MN505210 among others), respectively, and were submitted to GenBank (Accession no. OR268982–OR268983). Oocysts detected by flotation of IST sediment were identified as H. hammondi by COC1-COC2-PCR and sequencing (100% identity with GenBank AH008381, KT184369, KF854253).
Diagnostic methods comparison
In 91 cats, three diagnostic methods were implemented for the detection of cestodes, including (i) macroscopic evaluation of the intestine during necropsy; (ii) faecal sedimentation-flotation; and (iii) molecular diagnostics with copro-qPCR. Infection with adult taeniids (i.e. H. taeniaeformis) was detected in 13.2% (95% CI 7.0–21.9) of these cats during necropsy (12/91), in 6.6% (95% CI 2.5–13.8) of cats with the flotation technique (6/91) and in 9.9% of cats (95% CI 4.6–17.5) in the CEST-qPCR analysis (9/91) (Table 3). All samples positive for taeniid eggs in faecal flotation were also positive in the CEST-qPCR analysis (6/6). From the six samples positive for taeniids in necropsy and negative in coprology, two were also positive by qPCR. One of the cats negative for taeniids in necropsy and faecal flotation tested positive in the CEST-qPCR; in the flotation test of IST sediment from this cat a single taeniid egg was observed. The sensitivity of the CEST-qPCR and the flotation technique were estimated to be 66.7% and 50%, respectively, when compared to the necropsy findings (Table 3).
Prevalence of infections considering all applied methods
Overall, considering all applied methods, the most frequently found parasite was H. taeniaeformis (13/146 cats [8.9%]; 95% CI 4.8–14.7), followed by T. cati (9/146 cats [6.1%]; 95% CI 2.9–11.4) (Table 2). Capillaria sp. was found in 5/146 cats (3.4%; 95% CI 1.1–7.8), hookworms in 5/146 cats (3.4%; 95% CI 1.1–7.8), M. litteratus in 2/146 cats (1.4%; 95% CI 0.2–4.8), Giardia sp. in 2/146 cats (1.4%; 95% CI 0.2–4.8), C. rivolta in 2/146 cats (1.4%; 95% CI 0.2–4.8), C. felis in one cat (0.7%; 95% CI 0–3.7), T. gondii in one cat (0.7%; 95% CI 0–3.7), H. hammondi in one cat (0.7%; 95% CI 0–3.7) and Strongyloides sp. in one cat (0.7%; 95% CI 0–3.7). Infection with helminths (21/146 [14.4%]; 95% CI 9.1–21.1) was more prevalent than infection with protozoans (7/146 [4.8%]; 95% CI 1.5–8.7) (Table 2).
Prevalence of co-infections
Cats found to be infected with a single gastrointestinal parasite (14/146 [9.6%]; 95% CI 5.3–15.6) were more numerous than cats co-infected with two parasite species (7/146 [4.8%]; 95% CI 1.5–8.7) or three different parasite species (3/146 [2%]; 95% CI 0.4–5.9). One cat (ID PVK25) was even co-infected with five parasites (Table 2).
Prevalence of E. multilocularis
No E. multilocularis was detected in this study. The most likely prevalence was 0%, with a median and mean of the posterior density function of 0.0% and 0.12%, respectively, and a 95% upper credible limit of 0.83%. To check the influence of the prior information on the posterior prevalence, we re-ran the model, varying the prevalence beta priors and the τ parameters. Overall, the results were robust against prior changes. Using flat prior for the prevalence led to lower posterior prevalence values, whereas using flat prior and simultaneously increasing prior on τ to ≥ 0.99 led to higher posterior prevalence estimates. The posterior prevalence estimation (%) after varying model priors is shown in Additional file 1: Table S1.
Discussion
The results of the present study provide an overview of the status of gastrointestinal parasite infections in the Swiss domestic cat population, with a focus on cestode infections and a bias for the north-western regions of Switzerland. These regions historically have a high prevalence of E. multilocularis in foxes [4]. Only a few studies have focused on E. multilocularis prevalence in domestic cats in Switzerland: Zottler et al. [34] showed negative results for E. multilocularis in cats, while Deplazes et al. [33] found 1 of 263 cats (0.4%) to be positive for the parasite after necropsy.
A reliable diagnosis of E. multilocularis infection in definitive hosts can only be achieved post-mortem (i.e. sedimentation and counting technique [SCT], IST and shaking in a vessel technique [SVT] [39,40,41]), which is a major limitation for studies in pet canids and felids. Arecoline purgation was widely used in the past, but the controversial sensitivity [42, 43] and animal welfare issues linked to this technique have led to the abandonment of the method in most countries. Different coproantigen enzyme-linked immunosorbent assays (ELISAs) have been developed and tested for the detection of E. multilocularis infection in foxes, dogs and cats, and although they showed promising results, they are not widely used [33, 44]. Several copro-PCRs have been developed for the identification of E. multilocularis, which could present a good solution for monitoring E. multilocularis in both domestic and wild carnivores [24, 27, 28, 45]. Nevertheless, the diagnostic sensitivity of these techniques may be negatively affected in cats, in which this parasite has been reported to exhibit a low biotic potential [6]. Therefore, we used both a multiplex qPCR (based on Trachsel et al. [27]), which additionally allowed for detection of other cestode species, and a highly sensitive and specific qPCR for E. multilocularis [28].
Despite our considerable effort in using various diagnostic techniques, no E. multilocularis infection was detected in our sampled population. The prevalence estimation of E. multilocularis of 0% with an upper credible limit of 0.83% in cats in Switzerland, obtained by the Bayesian latent class analysis in the present study, is in accordance with previous findings reporting the prevalence of E. multilocularis in cats in Europe of between 0% and 0.38% [33, 34, 36]. However, one study from Poland reported a 6% prevalence of E. multilocularis in cats [48], and several copro-PCR studies on cat faecal samples collected in rural areas and kitchen gardens in France reported high prevalences of 1.2–9.3% [19, 35, 46, 47]. In these latter studies, deposition of multiple faecal samples by the same infected cats cannot be excluded; therefore, we did not include them in the priors for the Bayesian latent class analysis.
Taking the entire Swiss cat population into consideration (1.8–2.1 million cats [1, 2]), we could expect that up to 21,000 cats might be infected. The high densities of these pets and their common access to intermediate hosts can outweigh the low infection rates. Hegglin et al. [49] provided evidence showing that the total contribution of cats to environmental contamination with E. multilocularis eggs is < 0.2%, with other carnivores being responsible for the rest [49]. Nevertheless, in high-endemic areas, cats were shown to be a source of local environmental contamination [19]. Bastien et al. [47] showed that the density of cat faeces was significantly higher within kitchen gardens than outside them, while there was not significant difference in the distribution of fox faeces. These data indicate that cats from highly endemic areas could represent a source of contamination for human households. Therefore, even if cats rarely excrete eggs and are to be considered of lower epidemiological significance, in highly endemic areas [50], a higher deworming frequency of cats with unsupervised outdoor access and/or in contact with children, elderly and immunosuppressed persons is recommended.
In this study, the overall (17.1%; 95% CI 11.4–24.2) and coprological (12.3%; 95% CI 7.4–18.8) parasite infection rates were similar to those observed by Zottler et al. [34] in privately owned cats in Switzerland (11.7%). However, the latter study showed a markedly higher prevalence in stray cats (77.4%) and cats from shelters (21.8%), results that were confirmed by other studies [34, 51]. The latter cats are at a higher risk of parasite infection because of their dependence on hunting and scavenging for food, which exposes them to contaminated environments and intermediate and paratenic hosts; in addition, they usually do not receive anthelmintic treatments.
The results of the present study showed that mono-infections were more prevalent than co-infections with ≥ 2 intestinal parasites and that helminths were detected more frequently than protozoans, confirming the outcomes of other studies [34, 52, 53]. However, it is noteworthy that almost half of the positive cats (11/25) were co-infected by ≥ 2 intestinal parasites. Several intestinal parasites were found, including H. taeniaeformis, T. cati, Capillaria sp., A. tubaeforme, M. litteratus, Giardia sp., C. felis, C. rivolta, Strongyloides sp., T. gondii and H. hammondi, some of which are of potential zoonotic concern. The most frequently detected parasites in our study were H. taeniaeformis and T. cati.
In this study, all detected taeniid worms and eggs were identified as H. taeniaeformis, which was confirmed by molecular methods. Hydatigera taeniaeformis infection in cats is exclusively the result of predation on small mammals. Results from necropsy revealed a higher prevalence of H. taeniaeformis (13.2%, 12/91) than did copro-qPCR (9.9%, 9/91) and coprology (6.6%, 6/91) of the same samples, showing that the copro-qPCR was more sensitive than the coprological examination for taeniid infections. This result is in line with those of previous studies [24, 39, 43]. Coprology could lead to an underestimation of infection prevalence, especially when using only flotation techniques. Possible causes of this apparent lower sensitivity are the presence of immature worms, a low number of adult worms and intermittent egg shedding or shedding of a low number of eggs. This observation could be extrapolated to infections with other taeniid species, such as E. multilocularis infection in cats, with this parasite showing a lower biotic potential (total eggs excreted) in cats than in other host species [6, 7]. The overall prevalence in our study was higher than that observed by necropsy alone because in one of the samples no adult parasites were detected, only a single egg by the IST and confirmed by PCR. This result could be due to the expulsion of adults after treatment or the intestinal passage of eggs. Interestingly, five different haplotypes of H. taeniaeformis were identified, with one of the haplotypes being overrepresented (Fig. 2). Noteworthy, one of the cats harboured worms corresponding to two different haplotypes, suggesting a high genetic variability of this parasite. In addition, it is important to mention that rare cases of human infection with H. taeniaeformis have been reported [54].
Neither Dipylidium caninum nor Diphyllobothrium latum, two non-taeniid tapeworms, were detected in this study. The absence of these parasites is not surprising, considering that recent studies have indicated a low prevalence of these parasites in the Swiss cat population [34, 59]. Therefore, detecting them within a small sample size is less likely; their DNA would, however, have been detected in the CEST-qPCR [27].
Toxocara cati was detected in 9/146 cats (6.2%), which is in line with the prevalence reported in other studies from Europe, ranging from 4.7% to 35% [34, 51,52,53, 55]. The relatively low prevalence observed in this study may be associated with the fact that only privately owned cats were analysed and that kittens aged < 3 months were excluded. Toxocariasis is an important zoonosis that can cause dermatological, visceral, neural and ocular disorders [56]. Also, it would appear that T. cati plays a more important role in environmental contamination and the spread of human toxocariasis than previously thought [57].
Hookworms are zoonotic nematodes with a worldwide impact on human health, causing cutaneous larva migrans (CLM) and “eosinophilic enteritis” [58], among other pathologies. We detected hookworm infection in 5/146 (3.6%) cats by either coprology (n = 3) or the IST (n = 2; only male A. tubaeforme worms). This prevalence is among the highest in Europe; however, previous studies relied on coprology alone [34, 51, 52, 55, 59, 60]. No further identification of hookworm and Capillaria sp. eggs found in coprology was attempted and, therefore, the risk of these parasite stages being present due to intestinal passage (feed contamination, preyed rodents, or birds) cannot be ruled out.
Strongyloides species that have previously been reported in cats include S. felis, S. planiceps, S. tumefaciens and S. stercoralis. Little data on the prevalence of these parasites are available to date, but cats have been shown to harbour the potentially zoonotic S. stercoralis and to develop similar pathological lesions as humans, raising the question of whether the parasite could be of importance in a one-health context [61]. Strongyloides sp. larvae were detected in one cat in the present study, but unfortunately the attempt at molecular identification failed.
The epidemiological role of domestic cats in the spread of zoonotic Giardia species is still debated; however, cats have been shown both to have a similar rate of G. cati and G. duodenalis infection as dogs and also to harbour a greater proportion of G. duodenalis than dogs [62]. The reported prevalence for Giardia spp. in cats in Europe has a very wide range from 0.7% to 12.6%. Barutzki et al. [60] reported that cats aged < 3 months and between 3 and 6 months showed significantly higher infection rates with Giardia spp. than did older cats. In this context, our exclusion of animals aged < 3 months from the study could explain the rather low prevalence of Giardia spp. detected in our study. In addition, the freezing of cats before necropsy and sample collection may also have influenced our results since Giardia was not detected in any of the 91 analysed intestinal contents.
Felids are the only hosts that can excrete T. gondii and H. hammondi oocysts and thus are responsible for environmental contamination [63]. Toxoplasmosis can lead to major health problems in immunocompromised patients and can have fatal consequences for the foetus if seronegative mothers are infected during pregnancy [64]. Hammondia hammondi is not a zoonotic protozoan parasite, but since its oocysts are not distinguishable from those of T. gondii by microscopical examination, molecular methods are required for the differential diagnosis. Our result of 0.7% prevalence for both T. gondii and H. hammondi nicely falls within the range previously reported in Switzerland (0.4 and 0.6%, respectively) [34, 65].
Conclusion
Despite an intensive search, no intestinal infection with E. multilocularis was detected in any of the 146 cats included in this study. However, at least 10% (15/146) of the cats in our sample harboured H. taeniaeformis, M. litteratus and/or H. hammondi, suggesting that they successfully preyed on small mammals that can be natural intermediate hosts for E. multilocularis. Because of the relatively small sample size, we cannot rule out the possibility that cats may excrete E. multilocularis eggs at a low prevalence (< 0.83%) and thus contribute to household and environmental contamination. Future studies should be done on a larger sample size to address this possibility.
Availability of data and materials
All relevant raw data are given in the manuscript and Additional file 1, and sequences have been deposited in GenBank (Accession no. OR268952–OR268965 and OR268982–OR268983).
References
Verband für Heimtiernahrung. Anzahl der Haustiere in der Schweiz nach Tierart im Jahr 2022 (in Tausend) 2022. https://de.statista.com/statistik/daten/studie/373879/umfrage/anzahl-der-haustiere-in-der-schweiz-nach-tierart/. Accessed 10 Jan 2023.
Stiftung für das Tier im Recht. Kastrationspflicht für Freigänger-Katzen in der Schweiz. https://www.tierimrecht.org/de/ueber-uns/kampagnen/kastrationspflicht-fur-freiganger-katzen-der-schweiz/. Accessed 8 Jan 2023.
WHO. Report of the WHO/FAO/OIE joint consultation on emerging zoonotic diseases/in collaboration with the Health Council of the Netherlands. 2004. https://apps.who.int/iris/handle/10665/68899. Accessed 10 Jan 2023.
Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol. 2017;95:315–493.
Da Silva AM, Bastien M, Umhang G, Boué F, Bastid V, Boucher J-M, et al. Soil contamination by Echinococcus multilocularis in rural and urban vegetable gardens in relation to fox, cat and dog faecal deposits. Parasite. 2021;28:74.
Kapel C, Torgerson PR, Thompson R, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36:79–86.
Thompson R, Kapel C, Hobbs R, Deplazes P. Comparative development of Echinococcus multilocularis in its definitive hosts. J Parasitol. 2006;132:709–16.
Beerli O, Guerra D, Baltrunaite L, Deplazes P, Hegglin D. Microtus arvalis and Arvicola scherman: key players in the Echinococcus multilocularis life cycle. Front Vet Sci. 2017;4:216.
Tanner F, Hegglin D, Thoma R, Brosi G, Deplazes P. Echinococcus multilocularis in Graubünden: Verbreitung bei Füchsen und Vorkommen potentieller Zwischenwirte. Schweiz Arch Tierheilkd. 2012;148:501–10.
Ćirović D, Pavlović I, Kulišić Z, Ivetić V, Penezić A, Ćosic N. Echinococcus multilocularis in the European beaver (Castor fiber L.) from Serbia: first report. Vet Rec. 2012;171:100.
Corsini M, Geissbühler U, Howard J, Gottstein B, Spreng D, Frey CF. Clinical presentation, diagnosis, therapy and outcome of alveolar echinococcosis in dogs. Vet Rec. 2015;177:569.
Rehmann P, Gröne A, Lawrenz A, Pagan O, Gottstein B, Bacciarini LN. Echinococcus multilocularis in two lowland gorillas (Gorilla g. gorilla). J Comp Pathol. 2003;129:85–8.
Knapp J, Meyer A, Courquet S, Millon L, Raoul F, Gottstein B, et al. Echinococcus multilocularis genetic diversity in Swiss domestic pigs assessed by EmsB microsatellite analyzes. Vet Parasitol. 2021;293:109429.
Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722.
Schweiger A, Ammann RW, Candinas D, Clavien P-A, Eckert J, Gottstein B, et al. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis. 2007;13:878.
Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84.
König A, Romig T. Fox tapeworm Echinococcus multilocularis, an underestimated threat: a model for estimating risk of contact. Wildlife Biol. 2010;16:258–66.
Robardet E, Giraudoux P, Caillot C, Boue F, Cliquet F, Augot D, et al. Infection of foxes by Echinococcocus multilocularis in urban and suburban areas of Nancy, France: influence of feeding habits and environment. Parasite. 2008;15:77–85.
Knapp J, Combes B, Umhang G, Aknouche S, Millon L. Could the domestic cat play a significant role in the transmission of Echinococcus multilocularis? A study based on qPCR analysis of cat feces in a rural area in France. Parasite. 2016;23:42.
Conraths FJ, Probst C, Possenti A, Boufana B, Saulle R, La Torre G, et al. Potential risk factors associated with human alveolar echinococcosis: systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11:e0005801.
Kern P, Ammon A, Kron M, Sinn G, Sander S, Petersen LR, et al. Risk factors for alveolar echinococcosis in humans. Emerg Infect Dis. 2004;10:2088–93.
Kreidl P, Allerberger F, Judmaier G, Auer H, Aspöck H, Hall AJ. Domestic pets as risk factors for alveolar hydatid disease in Austria. Am J Epidemiol. 1998;147:978–81.
Umhang G, Forin-Wiart MA, Hormaz V, Caillot C, Boucher JM, Poulle ML, et al. Echinococcus multilocularis detection in the intestines and feces of free-ranging domestic cats (Felis s. catus) and European wildcats (Felis s. silvestris) from northeastern France. Vet Parasitol. 2015;214:75–9.
Kolapo TU, Bouchard É, Wu J, Bassil M, Revell S, Wagner B, et al. Copro-polymerase chain reaction has higher sensitivity compared to centrifugal fecal flotation in the diagnosis of taeniid cestodes, especially Echinococcus spp, in canids. Vet Parasitol. 2021;292:109400.
Deplazes P, Eckert J, Mathis A, Von Samson-Himmelstjerna G, Zahner H. Parasitology in veterinary medicine. 1st ed. Wageningen: Academic Publishers; 2016. p. 526–32, 550.
Hofer S, Gloor S, Müller U, Mathis A, Hegglin D, Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zürich, Switzerland. Parasitology. 2000;120:135–42.
Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–20.
Knapp J, Millon L, Mouzon L, Umhang G, Raoul F, Ali ZS, et al. Real time PCR to detect the environmental faecal contamination by Echinococcus multilocularis from red fox stools. Vet Parasitol. 2014;201:40–7.
Pardo Gil M, Hegglin D, Briner T, Ruetten M, Müller N, Moré G, et al. High prevalence rates of Toxoplasma gondii in cat-hunted small mammals—evidence for parasite induced behavioural manipulation in the natural environment? Int J Parasitol Parasites Wildl. 2023;20:108–16.
Ho M, Barr B, Marsch AE, Anderson ML, Rowe JD, Tarantal A, et al. Identification of bovine Neospora parasites by PCR amplification and specific small-subunit rRNA sequence probe hybridization. J Clin Microbiol. 1996;34:1203–8.
Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13.
Branscum AJ, Gardner IA, Johnson W. Bayesian modeling of animal- and herd-level prevalences. Prev Vet Med. 2004;66:145–63.
Deplazes P, Alther P, Tanner I, Thompson RCA, Eckert J. Echinococcus multilocularis coproantigen detection by enzyme-linked immunosorbent assay in fox, dog, and cat populations. J Parasitol. 1999;85:115–21.
Zottler EM, Bieri M, Basso W, Schnyder M. Intestinal parasites and lungworms in stray, shelter and privately owned cats of Switzerland. Parasitol Int. 2019;69:75–81.
Umhang G, Bastien M, Bastid V, Poulle M-L, Boué F. High variability in the number of E. multilocularis eggs in cat feces collected in the field. Parasitol Int. 2022;89:102583.
Dyachenko V, Pantchev N, Gawlowska S, Vrhovec MG, Bauer C. Echinococcus multilocularis infections in domestic dogs and cats from Germany and other European countries. Vet Parasitol. 2008;157:244–53.
Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–37.
Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. J Stat Softw. 2005;12:1–16.
Conraths FJ, Deplazes P. Echinococcus multilocularis: Epidemiology, surveillance and state-of-the-art diagnostics from a veterinary public health perspective. Vet Parasitol. 2015;213:149–61.
Deplazes P, Eckert J. Veterinary aspects of alveolar echinococcosis—a zoonosis of public health significance. Vet Parasitol. 2001;98:65–87.
Umhang G, Woronoff-Rhen N, Combes B, Boué F. Segmental sedimentation and counting technique (SSCT): an adaptable method for qualitative diagnosis of Echinococcus multilocularis in fox intestines. Exp Parasitol. 2011;128:57–60.
Hartnack S, Budke CM, Craig PS, Jiamin Q, Boufana B, Campos-Ponce M, et al. Latent-class methods to evaluate diagnostics tests for Echinococcus infections in dogs. PLoS Negl Trop Dis. 2013;7:e2068.
Ziadinov I, Mathis A, Trachsel D, Rysmukhambetova A, Abdyjaparov TA, Kuttubaev OT, et al. Canine echinococcosis in Kyrgyzstan: using prevalence data adjusted for measurement error to develop transmission dynamics models. Int J Parasitol. 2008;38:1179–90.
Deplazes P, Gottstein B, Eckert J, Jenkins DJ, Ewald D, Jimenez-Palacios S. Detection of Echinococcus coproantigens by enzyme-linked immunosorbent assay in dogs, dingoes and foxes. Parasitol Res. 1992;78:303–8.
Dinkel A, Von Nickisch-Rosenegk M, Bilger B, Merli M, Lucius R, Romig T. Detection of Echinococcus multilocularis in the definitive host: Coprodiagnosis by PCR as an alternative to necropsy. J Clin Microbiol. 1998;36:1871–6.
Poulle ML, Bastien M, Richard Y, Josse-Dupuis É, Aubert D, Villena I, et al. Detection of Echinococcus multilocularis and other foodborne parasites in fox, cat and dog faeces collected in kitchen gardens in a highly endemic area for alveolar echinococcosis. Parasite. 2017;24:29.
Bastien M, Vaniscotte A, Combes B, Umhang G, Germain E, Gouley V, et al. High density of fox and cat faeces in kitchen gardens and resulting rodent exposure to Echinococcus multilocularis and Toxoplasma gondii. Folia Parasitol (Praha). 2018;65:2018.002 https://doi.org/10.14411/fp.2018.002.
Karamon J, Sroka J, Dąbrowska J, Bilska-Zając E, Zdybel J, Kochanowski M, et al. First report of Echinococcus multilocularis in cats in Poland: a monitoring study in cats and dogs from a rural area and animal shelter in a highly endemic region. Parasit Vectors. 2019;12:313.
Hegglin D, Deplazes P. Control of Echinococcus multilocularis: strategies, feasibility and cost-benefit analyses. Int J Parasitol. 2013;43:327–37.
Deplazes P, Gottstein B, Schnyder M, Frey CF, Nett C. Bekämpfung von Würmern (Helminthen) bei Hunden und Katzen. 2015. https://www.esccap.ch/demo/wp-content/uploads/2019/01/ESCCAP_2019_Schema_Entwurmung_Katze_D_Lay01.pdf. Accessed 20 Jan 2023.
Becker AC, Rohen M, Epe C, Schnieder T. Prevalence of endoparasites in stray and fostered dogs and cats in Northern Germany. Parasitol Res. 2012;111:849–57.
Riggio F, Mannella R, Ariti G, Perrucci S. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet Parasitol. 2013;193:78–84.
Beugnet F, Bourdeau P, Chalvet-Monfray K, Cozma V, Farkas R, Guillot J, et al. Parasites of domestic owned cats in Europe: co-infestations and risk factors. Parasit Vectors. 2014;7:1–13.
Deplazes P, Eichenberger RM, Grimm F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int J Parasitol Parasites Wildl. 2019;9:342–58.
Bourgoin G, Callait-Cardinal MP, Bouhsira E, Polack B, Bourdeau P, Roussel Ariza C, et al. Prevalence of major digestive and respiratory helminths in dogs and cats in France: results of a multicenter study. Parasit Vectors. 2022;15:1–12.
Fisher M. Toxocara cati: an underestimated zoonotic agent. Trends Parasitol. 2003;19:167–70.
Lee ACY, Schantz PM, Kazacos KR, Montgomery SP, Bowman DD. Epidemiologic and zoonotic aspects of ascarid infections in dogs and cats. Trends Parasitol. 2010;26:155–61.
Epe C. Intestinal nematodes: biology and control. Vet Clin North Am Small Anim Pract. 2009;39:1091–107.
Giannelli A, Capelli G, Joachim A, Hinney B, Losson B, Kirkova Z, et al. Lungworms and gastrointestinal parasites of domestic cats: a European perspective. Int J Parasitol. 2017;47:517–28.
Barutzki D, Schaper R. Results of parasitological examinations of faecal samples from cats and dogs in Germany between 2003 and 2010. Parasitol Res. 2011;109:45–60.
Wulcan JM, Dennis MM, Ketzis JK, Bevelock TJ, Verocai GG. Strongyloides spp. in cats: a review of the literature and the first report of zoonotic Strongyloides stercoralis in colonic epithelial nodular hyperplasia in cats. Parasit Vectors. 2019;12:1–12.
Pallant L, Barutzki D, Schaper R, Thompson RCA. The epidemiology of infections with Giardia species and genotypes in well cared for dogs and cats in Germany. Parasit Vectors. 2015;8:2. https://doi.org/10.1186/S13071-014-0615-2.
Dubey JP, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Yang YR, Su C. All about toxoplasmosis in cats: the last decade. Vet Parasitol. 2020;283:109145.
Schnieder T, Bauer C, Boecking O, Conraths FJ, Daugschies A, Deplazes P, et al. Veterinärmedizinische parasitologie, 6th ed. Stuttgart: Parey; 2006.
Berger-Schoch AE, Herrmann DC, Schares G, Müller N, Bernet D, Gottstein B, et al. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet Parasitol. 2011;177:290–7.
Acknowledgements
We thank Dr. Diana Gliga and Dr. Miguel Pardo Gil for their support, and Caroline Müller, Christine Salvisberg, Rahel Ziegler and Ursula Kurath for excellent technical assistance. Our thanks are also extended to Dr. Lisa Thomann for proofreading of the manuscript, Dr. Eric Tielemans for his support in elaborating the protocol and Dr. Stephanie Krammer-Lukas and Dr. Alexandre Scherer for their regular feedback throughout the project.
Funding
This study was funded by Boehringer-Ingelheim Animal Health, France.
Author information
Authors and Affiliations
Contributions
RFJ: sample acquisition, analysis, interpretation of data and draft of the manuscript. NM1: data acquisition and analysis, revision of the manuscript. NM2: data analysis, Bayesian modelling, revision of the manuscript. GM: Data acquisition and analysis, revision of the manuscript. LA: conception of the work, revision of the manuscript. WB: data acquisition, analysis and interpretation, revision of the manuscript. CFF: conception and design of the work, data analysis and interpretation, revision of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No animal was sampled or euthanized for the purpose of this study. The cat holders consented to the anonymous use of the samples for teaching and research purposes at the involved institutes by signing the request form (www.ipa.vetsuisse.unibe.ch/unibe/portal/fak_vetmedizin/b_dept_infdipath/inst_ipa/content/e243890/e1244385/f-p002d_Untersuchungsantrag_ger.pdf).
Consent for publication
Not applicable.
Competing interests
One of the authors, LA, is an employee of Boehringer-Ingelheim Animal Health, France.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1
: Posterior prevalence estimation (%) after varying model priors in the Bayesian latent class analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Furtado Jost, R., Müller, N., Marreros, N. et al. What is the role of Swiss domestic cats in environmental contamination with Echinococcus multilocularis eggs?. Parasites Vectors 16, 353 (2023). https://doi.org/10.1186/s13071-023-05983-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05983-y