Abstract
Background
Several gastrointestinal parasites that infect cats pose potential health threats for humans and animals. The present study is the first to report gastrointestinal (GIT) parasites in feces of stray cats from Gharbia governorate, Egypt. Findings were combined with those published in the earlier surveys from various Egyptian governorates, and various meta-analyses were conducted to underline the parasitic zoonoses from cats in Egypt.
Results
Out of 143 samples tested in Gharbia, 75 (52.4%) were found infected with 13 different parasites. Co-infections were observed in 49.3% of positives. Several parasites were detected, e.g., Toxocara cati (30.0%), Toxascaris leonina (22.4%), hookworms (8.4%), taeniids (4.2%), Strongyloides spp. (2.1%), Physaloptera spp. (2.1%), Alaria spp. (1.4%) and Dipylidium caninum (0.7%). Opisthorchis-like eggs were found in a single sample being the first report from cats in Africa. Oocysts of 4 coccidian parasites were identified, and a few Toxoplasma gondii-like oocysts were detected in 2 samples (1.4%). Results of the meta-analysis illustrated that occurrence of T. gondii oocysts in feces of cats from Egypt may have been overestimated in earlier studies; 1432 cats have been tested and displayed a 5 times higher pooled prevalence (11.9%) than the published global pooled prevalence for T. gondii oocysts in cats. This overestimation might have occurred because some small-sized oocysts that belong to other coccidian parasites were mis-identified as T. gondii. Toxocara cati had a high pooled prevalence (22.5%) in cats from Egypt, which is even greater than the published pooled prevalence in cats globally; however, several reports from Egypt have neglected the role of T. cati in human toxocarosis. Dipylidium caninum displayed also a high prevalence (26.7%).
Conclusion
Several zoonotic parasite species have been found in stray cats from Egypt, raising concerns about the risks to the Egyptian human population as well as environmental contamination. Prompt surveillance supervised by the government and accompanied by data dissemination will be helpful for developing effective control strategies.
Similar content being viewed by others
Background
The estimated global cat population is 700 million comprising 480 million living as strays and 220 million as pets (https://www.carocat.eu). Cats can be infected with various parasites including those inhabiting the gastrointestinal tract (GIT), which represent a growing concern from both veterinary and public health perspectives because of their impact on cat health as well as their potential to infect humans [1, 2]. In several regions worldwide, cats live mostly as strays receiving no or little veterinary care, and stray cats often have a high prevalence of GIT parasites suggesting high health risks for humans living in these regions [3].
While many studies have been conducted to investigate the GIT parasites infecting cats in Europe [2, 4,5,6], a little is known about feline GIT parasites and their zoonotic impacts in Africa [7]. However, a recent study hypothesized that cats were likely domesticated in an African country (Egypt) 4000 years ago [8, 9]. A few studies have been conducted in Egypt mostly on a small population of cats from limited regions in the country, e.g., Cairo and Giza; however, reports from some Egyptian governorates e.g., Gharbia governorate, are lack.
Cats share dogs some GIT parasites that can cause serious disease in humans, e.g., hookworms, Giardia and Cryptosporidium. Evaluating the whole situation of these parasites in dogs and cats is crucial to demonstrate how dogs or cats contribute to the epidemiology of these zoonotic parasites in a region. For example, zoonosis caused by members of the genus Toxocara (T. cati from cats and Toxocara canis from dogs) is common worldwide. The relative contribution of both species in human toxocarosis is largely unclear due to lack of valid serological assays for differentiation as well as seldom recovery of the causative larvae [10]. Recent studies analysing the global situation of Toxocara species have shown that the prevalence in cats is significantly higher than that in dogs, suggesting a substantial role of cats in human toxocarosis [11, 12]. In Egypt, toxocarosis is quite common among humans [13]; many clinical cases have been documented in the recent years and the majority of these cases have been attributed to T. canis as the causative agent [14]. This highlights the need for establishing a comprehensive study, based on the published data from various governorates, analysing the situation of Toxocara spp. in cats and dogs from Egypt, which will aid in understanding the epidemiology of human toxocarosis in this country.
The objective of the present study was to determine, for the first time, the prevalence of various GIT parasites infecting stray cats in Gharbia governorate, Egypt. In addition, efforts were made to highlight the potential relevance of GIT parasites infecting cats as a source of parasitic zoonoses in Egypt within the frame of the One Health concept.
Materials and methods
Samples collection and laboratory processing
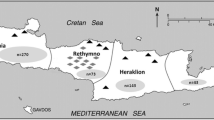
A total number of 143 fecal samples of stray cats were collected over a year (January—December 2021) from different rural areas in Gharbia governorate located in the Nile Delta, Egypt (Fig. 1). The total area of Gharbia is approximately 2000 km2. Around 5 million people are residing this governorate, with a very high population density ratio. The Nile Delta has a semi-desert climate with hot dry summers (temperature 31—34 °C in average) as well as warm (9 -19 °C) slightly rainfall (100–200 mm) winters [15, 16].

Source of original map before modifications: https://d-maps.com/carte.php?num_car=25356&lang=en
Map of Egypt illustrating the study area.
Samples were collected within 15 batches (each composed of 5–15 samples) from different areas. All cat fecal samples available to us from the sampled areas were collected. Many samples were collected in a fresh state after watching the cats depositing and burying their feces in soil around resident houses or animal farms. Other samples were collected from different sandy spots and displayed different degrees of dehydration due to their earlier deposition. Samples were stored at 4 °C immediately after collection and treated under strict hygienic conditions. In the next day, samples were grossly inspected and examined under the stereoscopic microscope (M6C-9, USSR) to identify any tapeworm segments or adult nematodes present, then were tested by means of routine sedimentation followed by the modified Wisconsin sucrose (specific gravity > 1.27) flotation technique [17, 18]. The recovered parasitic stages were identified morphologically based on pervious keys [19,20,21]. Coccidian oocysts recovered during examination were invitro-sporulated at room temperature using 2.5% potassium dichromate. No further procedures for delimitation of some species (e.g., hookworms) were conducted. Micrographs were captured using a binocular microscope (Carl Zeiss, Oberkochen, Germany) equipped with 12 MP camera (AmScope®, USA). Results were statistically analysed using a chi-square test. The 95% confidence intervals of a proportion including continuity correction and odds ratios were calculated using www.vassarstats.net. Differences with p < 0.05 were considered significant.
Data collection and analysis
A systematic electronic search was conducted to collect all published articles on GIT parasites infecting cats in Egypt. The international databases (Google Scholar, PubMed, Scopus, and Science Direct) were searched by two of the authors (EE and BE) using the following keywords: cat, feline, Egypt, stray, gastrointestinal parasites, faeces, Toxocara cati, hookworms, taeniids, Dipylidium caninum, Strongyloides, Toxoplasma gondii, Sarcocystis, Isospora, Giardia and Cryptosporidium. The Boolean operators “AND” and “OR” were used to connect the entry terms. Websites of the local databases: Egyptian knowledge bank (http://www.ekb.eg) and the Egyptian university libraries consortium (http://srv4.eulc.sdu.eg/eulc_v5/libraries/start.aspx) were consulted to collect articles published in local journals. Libraries of Faculty of Veterinary Medicine, Mansoura University, as well as Parasitology Department, Faculty of Medicine, Ain Shams University, Egypt were screened to collect articles published in local journals that had no electronic copies. The collected articles were screened for eligibility by EE and BE and any discrepancies were discussed with the first author (IA). Articles were defined eligible when the study 1) was conducted on cats from Egypt, 2) available in full text, 3) published as a research article, 4) found positive samples for any GIT parasite in cats, and 5) had a defined number of tested and positive samples. Articles that did not meet these criteria were excluded. For example, articles on non-GIT parasites in cats and articles available only as conference or proceeding abstracts as well as review articles.
Two of the authors (EE and BE) independently extracted important findings of the eligible studies, e.g., study sub-region, sample size, number of positives, diagnostic methods, parasites detected and mode of life (stray/household). Data were tabulated in Microsoft Excel spreadsheets, then used for various meta-analyses conducted in the present study using the software OpenMeta[Analyst] [22]. All analyses were computed based on a 95% confidence interval, and the pooled estimates that represented prevalences of the included parasites were determined employing the random effects model coupled to the DerSimonian-Laird method. The heterogeneity among the included studies was calculated using the I2 statistic and the heterogeneity were considered high when I2 values exceeded 50%. Subgroup analyses were conducted to investigate variations in prevalence of the included parasites according to the Egyptian regions, mode of life of the tested cats (stray or household) and to the detection method used (fecal examination or intestinal necropsy). Publication bias was not assessed in the present study because it is not considered relevant for prevalence studies [23].
Results
Prevalence of GIT parasites infecting stray cats in Gharbia
Seventy-five (52.4%) out of 143 faecal samples collected from stray cats in Gharbia, had eggs/oocysts of at least one parasitic species. Single (26.7%; n = 38) and mixed (25.9%; n = 37) infections were detected almost in equal rates. Samples from 28 (19.6%) and 8 (5.6%) cats had dual and triple infections respectively, whereas a single sample (0.7%) had quadruple infection (Table 1).
Eggs/oocysts of 13 GIT parasites were detected in the tested samples comprising 9 helminth species (5 nematodes, 2 tape worms and 2 flukes) as well as 4 protozoan parasite species (Table 2). Toxocara cati was the most frequently detected parasite (n = 43; 30.0%) followed by Toxascaris leonina (n = 32; 22.4%). Toxocara cati-T. leonina combination represented the most frequently detected form of dual infections (see Additional file 1). Eggs of other helminths were detected at lower rates: hookworms (8.4%), taeniids (4.2%), Strongyloides spp. (2.1%), Physaloptera spp. (2.1%), Dipylidium caninum (0.7%) and Alaria spp. (1.4%) (Fig. 2). Opisthorchis-like eggs were interestingly detected in a single sample. Eggs measured 20–30 X 10–20 µm, yellow brownish-coloured and contained mature miracidia. Eggs were operculated and had prominent opercular shoulders and abopercular knob (Fig. 2F). Other helminth eggs (e.g., Hymenolepis diminuta, Anoplocephalid spp. and poultry ascarids) were occasionally observed, but were considered as spurious parasites.
The coccidium Cystoisospora (9.1%) was the most frequently detected protozoa. Two Cystoisospora spp. were identified; Cystoisospora felis (4.2%) and Cystoisospora rivolta (4.9%). One sample displayed mixed infection with the two species. Infections were mostly less intense; however, a C. felis-infected sample had high oocyst count (18,400 oocyst per gram). Oocysts of both species were found unsporulated, but occasionally early sporulated oocysts containing 2 sporoblasts were observed in old deposited fecal samples. Oocysts of C. felis (n = 40) were ovoid, large (30–50 X 25–35 μm) with a length–width ratio of 1.3–1.4 (Fig. 3A). Oocyst walls were 1.3 μm thick and smooth. No micropyle, polar granule or oocyst residual body were detected. Sporocysts measured 20–25 X 15–20 μm. Cystoisospora rivolta oocysts (n = 20) were ovoid to somewhat ellipsoidal, medium-sized (18–25 X 15–25 μm) with a length–width ratio of 1.1–1.3, and had thin (0.5 μm) smooth walls. No micropyle, polar granule or oocyst residual body were observed. Sporocysts were broadly ellipsoidal and measured 13–15 X 10–12 μm (Fig. 3B).
Micrographs of protozoan oocysts isolated from stray cats in Gharbia governorate, Egypt. A Cystoisospora felis unsporulated (single arrow) and sporulated (double arrows) oocysts, B Cystoisospora rivolta unsporulated (B1) and sporulated (B2) oocysts, C T. gondii-like oocyst, D Sarcocystis spp. sporocyst (D1) and oocyst (D2), (E1-2) unsporulated different Eimeria spp. oocysts, (E3) sporulated Eimeria spp. oocyst
A few T. gondii-like oocysts (3–5) were detected in 2 samples. Oocysts were small-sized (11–13 μm), spherical to subspherical, filled with the sporont and had double-layered colourless oocyst walls (Fig. 3C). No polar granules were observed. Unfortunately, no T. gondii-like sporulated oocysts were recovered from these 2 samples after invitro sporulation, most likely due to the limited oocyst number. Sarcocystis spp. (7.0%) was also noticed, frequently as individual oval sporocysts (9–11 µm) with 4 sporozoites (Fig. 3D1), but occasionally as fragile thin-walled sporulated oocysts of different sizes (14.5- 17 µm) (Fig. 3D2). Unsporulated oocysts of different sizes (12–35 μm) were also observed in 10 additional samples. Oocysts were spherical, subspherical, oval or ovoid, and had micropyles, which in some oocysts was shallow and barely seen. After sporulation, tetrasporocystic dizoic oocysts were recovered, confirming their identity as Eimeria spp. This type of oocysts was considered as spurious parasites (Figs. 3E1-3).
Overall prevalence of the most common GIT parasites infecting cats in Egypt
Studies describing various GIT protozoa infecting cats in Egypt are scarce with fragmentary results. Findings of these studies on Toxoplasma gondii and Cystoisospora were included in the meta-analysis conducted considering the very important zoonoses of the former. Toxoplasma gondii-like oocysts have been observed in feces of 196 out of 1432 cats tested in 12 datasets, giving rise to a pooled prevalence of 11.9% (95% CI: 8.2 – 15.7%) (Fig. 4). In addition, T. gondii antibodies have been detected in sera of 601 out of 1186 cats resulting in a much higher pooled prevalence (49.7%, 25.9 – 73.5%). On the other hand, 21 datasets have described GIT helminths infecting 1866 cats in Egypt (Tables 3, 4). Of these datasets, 11 determined the overall prevalence in 1147 cats, and 683 were found infected with a high pooled prevalence (62.5%, 45.5 – 79.5%). Several helminths were detected after examining either adult worms recovered during intestinal necropsy or eggs detected during faecal examination. Of these helminths, 6 (T. cati, T. leonina, hookworms, D. caninum, taeniids and Heterophyes heterophyes) have been frequently observed. Eighteen datasets have observed T. cati in 377 out of 1745 cats from various Egyptian governorates, giving rise to a high pooled prevalence (22.5%, 16.1 – 28.9%). The prevalence significantly differed (p-value = 0.0346) according to the detection method used; 1277 cats diagnosed via intestinal necropsy had a double prevalence (32.7%) in comparison to 468 cats diagnosed via fecal examination (16.4%). Wild cats (Felis sylvestris) have been tested in a single dataset and had also a high T. cati prevalence 58.7% (44.5 – 72.9%) (Table 3, Fig. 5). The other Ascarid “T. leonina” displayed a lower prevalence (9.5%) in 1128 cats tested in 8 datasets (Table 3). Based on testing of 690 cats in 6 datasets, hookworms had the lowest pooled prevalence (3.2%, 1.2 – 5.1%) among the studied helminths infecting cats in Egypt (Table 3). On the contrary, D. caninum displayed the highest pooled prevalence (26.7%, 18.4 – 34.9%); this cestode has been detected in 266 out of 1254 cats tested in 13 datasets (Fig. 6). Like T. cati, the prevalence was significantly (p-value = 0.0012) higher in 450 cats (44.7%) tested via intestinal necropsy than in 804 cats tested via fecal examination (7.6%) (Table 4). Taeniid eggs and/or adult worms have been observed in 168 out of 1162 cats with a pooled prevalence of 14.4% (8.9 – 20.0%) (Table 4). A similar pooled prevalence (13.1%, 5.8 – 20.3%) was estimated for the trematode parasite “H. heterophyes” that has been detected in 69 out of 617 cats tested in 7 datasets (Table 4).
Discussion
The vast majority of cats in Egypt live as strays roaming everywhere and contributing to the occurrence as well as endemicity of several parasites, including those pose potential health risks for humans. The present study is the first to report GIT parasites in stray cats from Gharbia governorate, Egypt, and a high overall prevalence (52.4%) was determined, which is consistent with what have been reported from cats in various Egyptian governorates (see Additional files 2, 3). This prevalence, however, is higher than the 16.4% estimated prevalence for 468 cats tested in 11 earlier Egyptian fecal surveys, which generally used either the sedimentation technique alone or in combination with the flotation technique using the saturated salt solution as the flotation fluid. In the present investigation, samples were tested using both sedimentation and flotation techniques, and a sensitive flotation protocol (modified Wisconsin sucrose flotation) was conducted utilizing centrifugal flotation with the Sheather sugar solution. This is the most effective method to isolate most helminth eggs and coccidial oocysts [24]. In addition, negative samples that had a lot of debris were re-examined.
A wide diverse of parasites was detected and Opisthorchis-like eggs were observed for the first time in cats from Egypt. The family Opisthorchiidae includes 33 genera [25], of which Opisthorchis felineus, Opisthorchis viverrini and Clonorchis sinensis are food-borne zoonotic trematodes common to infect fish eating mammals, e.g., dogs, cats and humans. Humans get infected through consumption of raw freshwater fish of the family Cyprinidae, and infected persons often develop fever, diarrhea, recurrent cholangitis, hepatic abscesses and acute pancreatitis [26]. Infections are endemic in Asia as well as in some European countries [27], and no infections have been reported in Africa or the Middle East. However, a case of C. sinensis infection has been reported in an Egyptian family, who often consumed imported fish, and diagnosis was based on the characteristic shape of eggs [28]. In Egypt, importing fish is common from various Asian countries to fill the gap in fish production. In the present study, Opisthorchis-like eggs were detected in only one out of 143 cats tested, probably that cat got infected through consumption of imported fish. To suggest Egypt as a new geographical range for the occurrence of Opisthorchis infections, a large-scale investigation on cats from various governorates is required, and should include PCR testing for Opisthorchis-positive samples if present. Regarding the other GIT parasites detected in the present study, results will be discussed in the following sections in the context of findings of the meta-analysis conducted to evaluate the parasitic zoonoses from cats in Egypt.
GIT helminths detected in cats from Egypt
Analysis of findings of 11 datasets that have determined the overall prevalence of GIT helminths in 1147 cats surveyed in Egypt, displayed a very high pooled prevalence (62.5%, 45.5 – 79.5%). Given that most of the sampled cats lived as strays, findings of this analysis confirm the widespread helminthic infections in stray cats likely due inadequate control measures as well as easy access to the intermediate hosts [3]. A wide diverse of GIT helminths have been found infecting cats from Egypt; of them, data on 6 were found suitable for conducting the meta-analysis.
Round worms
Toxocara cati is one of the most prevalent GIT parasites in cats worldwide [12]. Consistent with earlier surveys from Egypt, the parasite was the most frequently detected (30.0%) in 143 fecal samples from cats in Gharbia. Infections have been observed in cats from various Egyptian governorates; nonetheless, no T. cati has been found in 62 necropsied cats from Beni-Suef governorate [29]. Number of cats recruited varied among studies with the majority being from Cairo and Giza, which may represent a limitation for the meta-analysis conducted in the present study. Overall, the estimated pooled prevalence for T. cati infections in 1745 cats tested in 18 datasets from Egypt was higher (22.5%) than that reported globally (17.0%; [12]). The later included only 2 datasets from Egypt that have tested 283 cats with a prevalence of 8.5%. In Egypt, most cats are strays and can disseminate T. cati eggs everywhere in the environment; Toxocara eggs have been frequently recovered from soil in Egypt with a high burden up to 13–19 eggs/10 g of soil [30]. Therefore, the high Toxocara prevalence in the Egyptian cats suggests the underestimated role of T. cati in human toxocarosis in Egypt, where the disease is quite common [13].
The genus Toxascaris comprises a single species “T. leonina” that can infect both dogs and cats with limited pathologies in comparison to members of the genus Toxocara. In contrary to earlier surveys from Egypt, an unexplained high prevalence (22.4%) of T. leonina eggs was detected in 143 fecal samples tested from cats in Gharbia. Overall, the pooled prevalence estimated for this parasite in 1128 cats from Egypt (9.5%) was approximately 3 times higher than that estimated globally (3.4%), but coincided with that has been estimated for cats in the Eastern Mediterranean region (10.0%) where Egypt is located [31]. Because T. leonina has a limited zoonotic potential, its high prevalence in cats has a little significance for humans.
Likewise, the 143 fecal samples tested from cats in Gharbia has a higher prevalence of hookworm eggs (8.4%) than that have been reported from cats in the other Egyptian governorates (see Additional file 2). Hookworms are common to infect humans worldwide with various routes of transmission, which emphasizes the significance of our findings. The estimated pooled prevalence of hookworm infections in cats from Egypt was 3.2%. Variable prevalences have been reported from cats worldwide, and a recent study have determined a high pooled prevalence (26.0%) in cats from Asia [32]. Cats can be infected with various hookworms including Ancylostoma tubaeforme, Ancylostoma braziliense, Ancylostoma ceylanicum and Uncinaria stenocephala, with the former is the globally predominant species in cats [33]. A few Ancylostoma caninum (the most predominant dog species) cases have been reported from cats worldwide [34]. However, A. caninum was proposed as the species present in most studies on cats from Egypt (see Additional file 2).
Cestodes
Dipylidium caninum is a ubiquitous cestode that can infect dogs and cats. In the present study, a single sample (0.7%) out of the 143 tested in Gharbia had D. caninum egg capsules, which can be explained by infrequent discharge of egg capsules outside D. caninum gravid proglottids resulting in underestimated infections determined via the routine microscopic examination of feces [19]. This was also evidenced when the subgroup analysis was conducted to detect the variation in D. caninum prevalence in cats from Egypt according to the detection method used. Cats that were tested via the intestinal necropsy to detect the adult worms had significantly higher infections (p-value = 0.0012) than those tested via the fecal examination (Table 3). In total, high D. caninum pooled prevalence (26.7%, 18.4 – 34.9%) was detected in 1254 cats tested in 13 datasets from various Egyptian governorates, which identify cats as a major risk for D. caninum infections in humans from Egypt. However, the parasite displayed low prevalence in children in Egypt [35]. Likewise, a few human cases, mostly in children, have been reported worldwide [36].
Contrastingly to D. caninum, Taenia spp. eggs are commonly expelled outside the gravid proglottids during animal defecation [19]. This strengthens the validity of fecal examination to detect taeniid eggs, which were observed in 4.2% (6) of the 143 samples tested in Gharbia. The overall pooled prevalence of Taenia spp. detected in 1162 cats from various Egyptian governorates was 14.4% (8.9 – 20.0%). Taenia taeniaeformis is the only known species that can infect cats, and the parasite has been frequently detected in earlier surveys from Egypt (see Additional file 2). Taenia taeniaeformis larvae (strobilocercus fasciolaris) have been detected in 11 out of 120 rats from Egypt [37]. On the other hand, a report by El-Bakrey [38] is interesting. The author examined intestinal contents of 35 stray cats in Alexandria governorate, and Echinococcus spp. have been detected in 3 cats. It is well known that cats cannot serve as definitive hosts for E. granulosus, but Echinococcus multilocularis, the etiological agent of alveolar echinococcosis in humans. Echinococcus multilocularis is widespread in the northern hemisphere, and no E. multilocularis infections have been reported from Egypt; however, the parasite has been reported in other neighbouring countries in North Africa including Tunisia and Morocco [39].
Trematodes
Heterophyids are common global zoonoses from dogs and cats. No Heterophyid eggs were noticed in any of the 143 cat fecal samples tested in Gharbia; however, Heterophyes heterophyes has been observed in cats from various Egyptian governorates and displayed a considerably high pooled prevalence (13.1%, 5.8 – 20.3%). A high prevalence of heterophyid metacercariae (32.0%) has been detected in 100 Tilapia fishes from Northern Egypt, and heterophyid eggs have been observed in stools of 10 (13.3%) out of 75 residents from this region [40].
GIT protozoa detected in cats from Egypt
Various GIT protozoa have been detected in cats from Egypt including Toxoplasma gondii, the most important zoonotic protozoan transmissible from cats. The parasite can cause serious disease in humans particularly immunocompromised patients and unborn infants [41], and T. gondii infections appear highly prevalent in animals and humans from Egypt [42]. Infected cats shed millions of T. gondii oocysts in feces for relatively short time (1–3 weeks) during their life, then become immune and seldom to shed T. gondii oocysts again [41]. This explains the scarce detection of T. gondii oocysts in feces of cats. In a recent survey on 24,000 cats from Europe, T. gondii-like oocysts have been observed in only 0.2% [43]. However, some earlier reports on cats from Egypt stated very high prevalences up to 50.0% (see Additional file 3). Results of the meta-analysis conducted in the present study for T. gondii oocysts in cats from Egypt revealed a 5 times higher pooled prevalence (11.9%) than that estimated for cats worldwide (2.6%, [44]). Nonetheless, the estimated pooled prevalence for T. gondii antibodies in sera of cats from Egypt (49.7%) is not far from that estimated for cats globally (37.5%, [44]), which suggests the overestimation of T. gondii oocysts in cats from Egypt. To resolve this debate, feces of 143 stray cats from Gharbia, Egypt were carefully examined in the present study, and a few (3–5) T. gondii-like oocysts were observed in only 2 (1.4%) samples. Oocysts of Toxoplasma, Hammondia and Besnoitia in cat feces are quite similar morphologically, and the differential diagnosis requires further procedures particularly mouse inoculation assays [41], that were not conducted in the present study. It is worthy mentioned that many oocysts as smaller as 12 µm were observed in 10 out of the 143 samples tested. These oocysts had micropyles and belonged to the genus Eimeria, thus were identified as spurious parasites. In many rural areas in Egypt, household keeping of birds is common, and residents usually slaughter and eviscerate those birds in-home, and the viscera are thrown in the streets to feed stary cats and dogs. The existence of Eimeria oocysts in feces of cats is a possible cause for the overestimation of T. gondii oocysts. Noteworthy, Toxoplasma gondii oocysts were not detected in rectal contents of 158 cats that were tested in Dr Dubey Lab at USDA to detect T. gondii genotypes in various tissues of stray cats from Giza, Egypt [45].
Many reports documented Cystoisospora (formerly Isospora) infections in a total of 1220 cats from Egypt, with an estimated pooled prevalence of 14.1% (8.3 – 19.8%), which is much lower than that has been determined in the only report published from Africa on 103 cats in Kenya (43.7%, [7]). Variable prevalences (occasionally up to 84.0%) have been documented in cats worldwide [46]. Cats can be infected with 2 Cystoisospora spp., C. felis and C. rivolta, and the latter is more pathogenic and can cause diarrhea associated with villar atrophy and cryptitis in newborn kittens [46]. In Egypt, an outbreak of diarrhea in 3–5 weeks old kittens infected with Cystoisospora spp. has been documented [47]. However, the present study is the first to report C. rivolta in cats from Egypt; the parasite was detected in 4.9% out of 143 fecal samples from cats in Gharbia governorate. On the other hand, cats serve as definitive hosts for many Sarcocystis spp. that utilize various herbivores as intermediate hosts [48]. Of the 143 cats tested in Gharbia, sporocysts of Sarcocystis spp. were detected in 10 (7.0%). This prevalence is high when compared to that determined in 2 earlier reports from Egypt (see Additional file 3). However, sarcocystosis is common among ruminants in Egypt and Sarcocystis fusiformis, which circulates in a cat-buffalo cycle, is prevalent among water buffaloes in Egypt causing significant economic losses [49]. Scarce reports on some other protozoal infections (e.g., Cryptosporidium and Giardia) are also available from cats in Egypt. It seems that cats have a minimized role in the zoonotic cycles of both protozoa [50, 51].
Conclusion
High prevalence rates of zoonotic GIT parasites of cats in Egypt are alarming since the majority of cats live as strays in this country, which highlights the urgent need for implementing effective control strategies. Toxocariosis is likely the most important parasitic zoonosis from cats in Egypt due to their high prevalence and frequent detections. Toxoplasma gondii prevalence in cats is questionable and the high prevalence may be attributed to misdiagnosis with other protozoan oocysts of relatively small sizes. Opisthorchis-like eggs detected in this study may indicate zoonotic hazards of imported raw fish. A possible bias in results of the meta-analysis conducted in the present study may come from the high heterogeneity between the included studies, which have not also covered some governorates particularly the southern ones.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article (and its additional files).
References
Dantas-Torres F, Otranto D. Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors. 2014;7:1–25.
Symeonidou I, Gelasakis AI, Arsenopoulos K, Angelou A, Beugnet F, Papadopoulos E. Feline gastrointestinal parasitism in Greece: emergent zoonotic species and associated risk factors. Parasit Vectors. 2018;11:1–13.
Millán J, Casanova JC. High prevalence of helminth parasites in feral cats in Majorca Island (Spain). Parasitol Res. 2009;106:183–8.
Beugnet F, Bourdeau P, Chalvet-Monfray K, Cozma V, Farkas R, Guillot J, Halos L, Joachim A, Losson B, Miró G, Otranto D. Parasites of domestic owned cats in Europe: co-infestations and risk factors. Parasit Vectors. 2014;7:1–13.
Giannelli A, Capelli G, Joachim A, Hinney B, Losson B, Kirkova Z, René-Martellet M, Papadopoulos E, Farkas R, Napoli E, Brianti E. Lungworms and gastrointestinal parasites of domestic cats: a European perspective. Int J Parasitol. 2017;47:517–28.
P Overgaauw R Nijsse Prevalence of patent Toxocara spp. infections in dogs and cats in Europe from, 1994 to 2019 Adv Parasitol 2020 109 779 800
Njuguna NA, Kagira JM, Karanja MS, Ngotho M, Mutharia L, Maina WN. Prevalence of Toxoplasma gondii and other gastrointestinal parasites in domestic cats from households in Thika region. Kenya Biomed Res Int. 2017. https://doi.org/10.1155/2017/7615810.
Serpell JA. Domestication and history of the cat. The domestic cat: The biology of its behavior. Cambridge University Press, Cambridge, United Kingdom; 2000.
Faure E, Kitchener AC. An archaeological and historical review of the relationships between felids and people. Anthrozoos. 2009;22:221–38.
Poulsen CS, Skov S, Yoshida A, Skallerup P, Maruyama H, Thamsborg SM, Nejsum P. Differential serodiagnostics of Toxocara canis and Toxocara cati–is it possible? Parasite Immunol. 2015;37:204–7.
Rostami A, Riahi SM, Hofmann A, Ma G, Wang T, Behniafar H, Taghipour A, Fakhri Y, Spotin A, Chang BCH, Macpherson CNL, Hotez PJ, Gasser RB. Global prevalence of Toxocara infection in dogs. Adv Parasitol. 2020;109:561–83.
Rostami A, Sepidarkish M, Ma G, Wang T, Ebrahimi M, Fakhri Y, Mirjalali H, Hofmann A, Macpherson CN, Hotez PJ, Gasser RB. Global prevalence of Toxocara infection in cats. Adv Parasitol. 2020;109:615–39.
Adeel AA. Seroepidemiology of human toxocariasis in North Africa. Adv Parasitol. 2020;109:501–34. https://doi.org/10.1016/bs.apar.2020.01.023.
Ismail MAM, Khalafallah O. Toxocara canis and chronic urticaria in Egyptian patients. J Egypt Soc Parasitol. 2005;35:833–40.
Nashwan MS, Shahid S, Abd RN. Unidirectional trends in annual and seasonal climate and extremes in Egypt. Theor Appl Climatol. 2019;136:457–73.
Fouad E, Elnouby M, Saied M. Variability and Trend Analysis of Temperature in Egypt. Egypt J Phys. 2022;50:47–58.
Faust EC. A critical study of clinical laboratory technics for the diagnosis of protozoan cysts and helminth eggs in feces. I. Preliminary communication. Am J Trop Med Hyg. 1938;18:169–83.
Cox DD, Todd AC. Survey of gastrointestinal parasitism in Wisconsin dairy cattle. J Am Vet Med Assoc. 1962;1:16–20.
Bowman DD, Hendrix CM, Lindsay DS, Barr SC. Feline clinical parasitology. Iowa: Iowa State University Press; 2002.
Zajac AM, Conboy GA. Veterinary clinical parasitology, 8th ed., John Wiley and Sons, incorporation. 2012.
Bowman DD. Diagnostic parasitology. In: Bowman DD, editor. Georgi’s parasitology for veterinarians. 10th ed. St-Louis: Elsevier. 2014.
Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15.
Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903.
Broussard JD. Optimal fecal assessment. Clin Tech Small Anim Pract. 2003;18:218–30. https://doi.org/10.1016/S1096-2867(03)00076-8.
King S, Scholz T. Trematodes of the family Opisthorchiidae: a minireview. Korean J Parasitol. 2001;39:209.
Traverso A, Repetto E, Magnani S, Meloni T, Natrella M, Marchisio P, Giacomazzi C, Bernardi P, Gatti S, Gomez Morales MA, Pozio E. A large outbreak of Opisthorchis felineus in Italy suggests that opisthorchiasis develops as a febrile eosinophilic syndrome with cholestasis rather than a hepatitis-like syndrome. Eur J Clin Microbiol Infect Dis. 2012;31:1089–93.
Sithithaworn P, Yongvanit P, Tesana S, Pairojkul C. Liver flukes. In: Murrell, K.D., Fried, B. (Eds.), Food-borne parasitic zoonoses. Fish and plant-borne parasites. Springer, New York. 2007.
Morsy AT, Al-Mathal EM. Clonorchis sinensis a new report in Egyptian employees returning back from Saudi Arabia. J Egypt Soc Parasitol. 2011;41:221–5.
El-Dakhly KM, Aboshinaf AM, El-Nahass ES, Gharib AETF. A preliminary study on the helminth fauna in necropsied stray cats (Felis catus) in Beni-Suef. Egypt J Adv Vet Res. 2017;7:87–92.
El-Shazly AM, Mohammed RM, El-Beshbishi SN, Azab MS, El-Ghareeb AS, Abdeltawab AH, Zalook TK. Soil-parasites particularly Toxocara eggs in Egypt. J Egypt Soc Parasitol. 2009;39:151–62.
Rostami A, Riahi SM, Omrani FV, Wang T, Hofmann A, Mirzapour A, Foroutan M, Fakhri Y, Macpherson CN, Gasser RB. Global prevalence estimates of Toxascaris leonina infection in dogs and cats. Pathogens. 2020;9:503.
Zibaei M, Nosrati MRC, Shadnoosh F, Houshmand E, Karami MF, Rafsanjani MK, Majidiani H, Ghaffarifar F, Cortes HCE, Dalvand S, Badri M. Insights into hookworm prevalence in Asia: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2020;114:141–54. https://doi.org/10.1093/trstmh/trz115.
Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1–19.
Liu Y, Zheng G, Alsarakibi M, Zhang X, Hu W, Lu P, Lin L, Tan L, Luo Q, Li G. Molecular identification of Ancylostoma caninum isolated from cats in southern China based on complete ITS sequence. Biomed Res Int. 2013. https://doi.org/10.1155/2013/868050.
Elmonir W, Elaadli H, Amer A, El-Sharkawy H, Bessat M, Mahmoud SF, Atta MS, El-Tras WF. Prevalence of intestinal parasitic infections and their associated risk factors among preschool and school children in Egypt. PLoS ONE. 2021;16: e0258037. https://doi.org/10.1371/journal.pone.0258037.
Rousseau J, Castro A, Novo T, Maia C. Dipylidium caninum in the twenty-first century: epidemiological studies and reported cases in companion animals and humans. Parasit Vectors. 2022;15:131. https://doi.org/10.1186/s13071-022-05243-5.
Hussien LS. Role of rats in transmission of some helminths in Beni-suef governorate. Assiut Vet Med J. 2009;55:1–12. https://doi.org/10.21608/AVMJ.2009.174198.
El-Bakrey KM. Investigation on some internal parasites affecting stray dogs and cats. Alexandria J Vet Sci. 2012;35:211–9.
Dakkak A. Echinococcosis/hydatidosis: a severe threat in Mediterranean countries. Vet Parasitol. 2010;174:2–11. https://doi.org/10.1016/j.vetpar.2010.08.009.
Lobna SM, Metawea YF, Elsheikha HM. Prevalence of heterophyiosis in Tilapia fish and humans in Northern Egypt. Parasitol Res. 2010;107:1029–34. https://doi.org/10.1007/s00436-010-1976-x.
Dubey JP. Toxoplasmosis in animals and man. 2nd ed. Boca Raton: CRC Press; 2010.
Abbas IE, Villena I, Dubey JP. A review on toxoplasmosis in humans and animals from Egypt. Parasitology. 2020;147:135–59.
Schares G, Vrhovec MG, Pantchev N, Herrmann DC, Conraths FJ. Occurrence of Toxoplasma gondii and Hammondia hammondi oocysts in the faeces of cats from Germany and other European countries. Vet Parasitol. 2008;152:34–45.
Hatam-Nahavandi K, Calero-Bernal R, Rahimi MT, Pagheh AS, Zarean M, Dezhkam A, Ahmadpour E. Toxoplasma gondii infection in domestic and wild felids as public health concerns: a systematic review and meta-analysis. Sci Rep. 2021;11:9509. https://doi.org/10.1038/s41598-021-89031-8.
Al-Kappany YM, Rajendran C, Ferreira LR, Kwok OCH, Abu-Elwafa SA, Hilali M, Dubey JP. High prevalence of toxoplasmosis in cats from Egypt: isolation of viable Toxoplasma gondii, tissue distribution, and isolate designation. J Parasitol. 2010;96:1115–8.
Dubey JP. A review of Cystoisospora felis and C. rivolta-induced coccidiosis in cats. Vet Parasitol. 2018;263:34–48.
Abdel Halim M, Youssef HM. Treatment of Isospora felis in kittens. Vet Med J. 1986;34:183–8.
Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R. Sarcocystosis of animals and humans 2nd Edition. CRC Press. 2015.
Abu-Elwafa SA, Al-Araby MA, Abbas IE. Sarcocystis fusiformis (Railliet, 1897) infecting water buffaloes (Bubalus bubalis) in Dakahlia Province. Egypt Mansoura Vet Med J. 2016;17:1–11. https://doi.org/10.21608/mvmj.2016.130393.
Lucio-Forster A, Griffiths JK, Cama VA, Xiao L, Bowman DD. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010;26:174–9.
Ballweber LR, Xiao L, Bowman DD, Kahn G, Cama VA. Giardiasis in dogs and cats: update on epidemiology and public health significance. Trends Parasitol. 2010;26:180–9.
Acknowledgements
This research is published through the Transformative Agreement between Springer Nature and Science, Technology & Innovation Funding Authority (STDF) in cooperation with Egyptian Knowledge Bank (EKB).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
IA, EE: conceptualization. EE, MA, BE, IA: performed the experiments. IA, EE, BE: papers collection, testing for eligibility and data extraction. IA: data analysis. IA and EE: drafted the manuscript. IA and EE: manuscript revisions and editions. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee, Faculty of Veterinary Medicine, Mansoura University, according to the ethical principles of animal research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Patterns of the mixed GIT parasitic infections detected in 143 faecal samples of stray cats from Gharbia governorate, Egypt.
Additional file 2:
Table S2. Reports on prevalence of GIT helminths in cats from various governorates in Egypt.
Additional file 3:
Table S3. Reports on GIT protozoa detected in feces of cats from various governorates in Egypt.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abbas, I., Al-Araby, M., Elmishmishy, B. et al. Gastrointestinal parasites of cats in Egypt: high prevalence high zoonotic risk. BMC Vet Res 18, 420 (2022). https://doi.org/10.1186/s12917-022-03520-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03520-0