Abstract
Background
A close connection between a protozoan parasite and the balance of the other gut microbes of the host has been demonstrated. The calves may be naturally co-infected with many parasites, and the co-effects of parasites on other intestinal microbes of calves remain unclear. This study aims to preliminarily reveal the relationship between intestinal parasites and other intestinal microbes in calves.
Methods
Fecal samples were collected from four calves with bloody diarrhea, four calves with watery diarrhea, and seven normal calves, and the microbial flora of the samples were analyzed by whole-genome sequencing. Protozoal parasites were detected in the metagenome sequences and identified using polymerase chain reaction (PCR).
Results
Cryptosporidium, Eimeria, Giardia, Blastocystis, and Entamoeba were detected by metagenomic analysis, and the identified species were Giardia duodenalis assemblage E, Cryptosporidium bovis, Cryptosporidium ryanae, Eimeria bovis, Eimeria subspherica, Entamoeba bovis, and Blastocystis ST2 and ST10. Metagenomic analysis showed that the intestinal microbes of calves with diarrhea were disordered, especially in calves with bloody diarrhea. Furthermore, different parasites show distinct relationships with the intestinal microecology. Cryptosporidium, Eimeria, and Giardia were negatively correlated with various intestinal bacteria but positively correlated with some fungi. However, Blastocystis and Entamoeba were positively associated with other gut microbes. Twenty-seven biomarkers not only were significantly enriched in bloody diarrhea, watery diarrhea, and normal calves but were also associated with Eimeria, Cryptosporidium, and Giardia. Only Eimeria showed a distinct relationship with seven genera of bacteria, which were significantly enriched in the healthy calves. All 18 genera of fungi were positively correlated with Cryptosporidium, Eimeria, and Giardia, which were also significantly enriched in calves with bloody diarrhea. Functional genes related to parasites and diseases were found mainly in fungi.
Conclusions
This study revealed the relationship between intestinal protozoan parasites and the other calf gut microbiome. Different intestinal protozoan parasites have diametrically opposite effects on other gut microecology, which not only affects bacteria in the gut, but also is significantly related to fungi and archaea.
Graphical Abstract

Similar content being viewed by others
Background
Protozoan parasites are commonly found in the digestive tract of cattle, especially in the rumen and intestines, and have an important effect on their health. Ciliate species, mainly in the rumen, play an important role in rumen fermentation and the stability of rumen ecology [1]. On the contrary, protozoan parasites in the cattle gut are usually associated with intestinal diseases. Many intestinal protozoan parasites, such as Cryptosporidium, Eimeria, and Giardia, cause intestinal disease outbreaks in humans and animals [2, 3]. However, the controversial pathogenicity of some intestinal protozoan parasites, such as Blastocystis and nonpathogenic Entamoeba, is mainly observed in asymptomatic individuals [4]. Protozoan parasites interact with other gut microbes in symbiotic environments and co-evolve over time [5, 6]. At present, studies on the effects of parasites on calves mainly focus on the interaction between parasites and hosts, and there are few studies on the effects of parasites on the intestinal microflora of calves.
Intestinal microecology impacts cattle health and is correlated with nutrient metabolism, energy production, and the immune system [7]. Intestinal diseases are among the most important health problems in calves (0–1 year of age). Approximately 4–25% of calves in the United States die from diarrhea each year, causing tremendous economic losses to the cattle industry [8,9,10]. Although many factors cause calf diarrhea, disorders of the intestinal microbiome are the main manifestations of calf diarrhea [10,11,12]. Diverse gut microbiota prevent colonization by foreign pathogens and enhance the host immune system through interactions between antigens and immune cells during the early stages of life [8]. Studies have shown that restoring the intestinal microbial composition of diarrheal calves by fecal microbiota transplantation can ameliorate diarrhea in pre-weaning calves [13].
There are few effective drugs and vaccines for protozoan parasites such as Cryptosporidium and Giardia duodenalis [3, 14]. Changes in the intestinal bacteria may be related to various intestinal protozoan parasites, and understanding the effects of parasites on gut microbes could provide new insights into treating parasitic diseases [15,16,17]. In this study, parasites and other intestinal microecological elements in the posterior intestines of calves were characterized using metagenomic analysis. The association between intestinal protozoan parasites, other intestinal microbes, and calf diarrhea was also investigated, which provides a theoretical basis for maintaining calf intestinal health.
Methods
Animal management
Samples for this study were collected from a beef cattle farm in Henan Province, China. A total of 15 fresh stool samples were collected from four calves with bloody diarrhea (B1–B4), four calves with watery diarrhea (W1–W4), and seven calves with normal stools (N1–N7). All calves were 2–3 months old, and samples were collected by rectal sampling, stored in 2-ml cryotubes, transported on dry ice to the laboratory, and stored at −80 °C until use.
Sample preparation and Illumina sequencing
Total DNA from the fecal microbiota was extracted using the QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions (QIAamp Fast DNA Stool Mini Kit Handbook, www.qiagen.com/handbooks). The degree of degradation and potential contamination of DNA was analyzed by electrophoresis using 1% agarose gel. DNA purity was determined using a NanoPhotometer® spectrophotometer (IMPLEN, Westlake Village, CA, USA), and DNA concentration was measured using the Qubit® double-stranded DNA (dsDNA) Assay Kit on a Qubit® 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA). One microgram of the qualified DNA was used to construct the library. DNA samples were fragmented to 350 base pairs (bp) by sonication, and the DNA fragments were end-polished, A-tailed, and ligated with a full-length adaptor for Illumina sequencing with further polymerase chain reaction (PCR) amplification. The libraries were analyzed for size distribution using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and quantified via real-time PCR. Libraries were sequenced using the Illumina PE150 platform (Illumina, Inc., San Diego, CA, USA).
Identification of intestinal microorganisms
Metagenomic analysis was carried out following previously published methods [18, 19]. The host sequence was removed from the raw data using Bowtie 2 (v2.4.5) [20] and assembled using MEGAHIT (v1.2.9) [21]. The sequences (≥ 500 bp) were used to predict the open reading frame using MetaGeneMark (v3.38) [22] and eliminate redundancy using CD-HIT (v4.5.8) [23]. Clean data from each sample were mapped to the initial gene catalog using Bowtie2. The corresponding relative abundance of each gene was calculated based on the following formula: Ai = Ci/∑ni=1 Ci (where Ni represents the number of reads mapped to each gene and Li represents the length of each gene; Ci = Ni/Li) [24]. The obtained genes were used to BLAST the sequences for bacteria, fungi, archaea, viruses, and intestinal protozoan parasites, which were extracted from the National Center for Biotechnology Information (NCBI) non-redundant (NR) database (https://www.ncbi.nlm.nih.gov) using DIAMOND software (v2.0.14) [25]. We used the lowest common ancestor (LCA) algorithm to obtain the number of genes and abundance information for each sample in each taxonomic hierarchy (kingdom, phylum, class, order, family, genus, and species) [26]. DIAMOND software was used to annotate the unigenes using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.kegg.jp/kegg/).
PCR amplification and sequence analysis
Further specialization of parasites was accomplished using PCR. Giardia was identified based on the β-giardin (bg) gene [27], and Cryptosporidium [28], Eimeria [29], Blastocystis [30], and Entamoeba [31] were identified based on the small subunit (SSU) ribosomal RNA (rRNA) gene. After amplification, the DNA fragments were separated by agarose gel electrophoresis (1% agarose), stained with DNA Green (TIANDZ, Beijing, China), and observed using a Tanon 3500 gel imaging system (TANON, Shanghai, China). Amplified samples with the target band were selected as positive PCR production. Positive PCR amplicons with the target band were sequenced by SinoGenoMax (Beijing, China). Bidirectional sequencing was chosen to ensure the veracity of sequences. Phylogenetic analysis was conducted using MEGA 7.0 software (http://www.megasoftware.net/), choosing the maximum composite likelihood model, and bootstrap values were calculated by analyzing 1000 replicates.
Statistical analysis and visualization
Venn diagrams, alpha diversity (Chao1 and Shannon indices), and principal coordinates analysis (PCoA) based on the Bray–Curtis distance were calculated and plotted using Tutools (https://www.cloudtutu.com), and the ImageGP (https://416h86i955.zicp.fun/Cloud_Platform/front/#/) platform, and microbial features showing differential abundance were identified using linear discriminant analysis effect size (LEfSe) threshold criteria of linear discriminant analysis (LDA) score > 2 and P < 0.05 (http://huttenhower.sph.harvard.edu/galaxy/). To reveal the relationship between parasites and the microbial or KEGG ortholog group (KO), we calculated the pairwise Spearman’s rank correlation and removed coefficients below 0.7 with P > 0.05. We adjusted the P-value to avoid false positives using the Benjamin-Hochberg (BH) method from the ‘Hmisc’ package in R (v4.2.2) statistical software. Network analysis and visualization were conducted using Gephi (https://gephi.org/) and Cytoscape (https://cytoscape.org/).
Results
Species of intestinal protozoan parasites
The parasites identified using metagenomics belonged to the genera Giardia, Eimeria, Cryptosporidium, Blastocystis, and Entamoeba (Fig. 1A). Specific genetic information is shown in Additional file 3: Table S2. Not all positive samples were successfully amplified. Based on these results, the parasite species in calves were G. duodenalis assemblage E (Fig. 1B), Cryptosporidium bovis, Cryptosporidium ryanae (Fig. 1C), Eimeria bovis, Eimeria subspherica (Fig. 1D), Blastocystis (ST2 and ST10) (Fig. 1E), and Entamoeba bovis (Fig. 1F).
Intestinal parasitic protozoa detected in the calves. a Parasites annotated by metagenomic sequencing. b Phylogenetic relationships of Giardia duodenalis based on β-giardin (bg) nucleotide sequences. c Phylogenetic relationships of Eimeria based on the SSU rRNA gene. d Phylogenetic relationships of Cryptosporidium based on the SSU rRNA gene. e Phylogenetic relationships of Blastocystis based on the SSU rRNA gene. f Phylogenetic relationships of Entamoeba based on the SSU rRNA gene. Phylogenetic relationships were calculated using the maximum composite likelihood model. Percent bootstrap values greater than 50% from 1000 replicates are shown next to the branches. Triangles represent isolates detected in this study, and the name of samples which detected the parasite are written in parentheses
Intestinal microbial imbalance in diarrheal calves
Comparing calves with watery diarrhea and calves with normal stools, the major intestinal microbiota of calves with bloody diarrhea changed greatly at the phylum and genus levels. At the phylum level, Firmicutes, Bacteroidetes, and Proteobacteria were the main phyla in the gut of the calves. Firmicutes and Bacteroidetes were mainly found in calves with watery diarrhea and calves with normal stools, but Proteobacteria were more abundant in calves with bloody diarrhea (Fig. 2A). At the genus level, Bacteroides were mainly found in calves with watery diarrhea and calves with normal stools and were lower in calves with bloody diarrhea (Fig. 2B).
Comparison of gut microbes among calves with bloody diarrhea, watery diarrhea, and normal stools. a Relative abundance (%) at the phylum level. b Relative abundance (%) at the genus level. c PCoA of gut microbes based on the Bray–Curtis distance. d α-diversity (Chao1 and Shannon index) of gut microbes. Boxes of α-diversity denote the interquartile range (IQR) between the first and third quartiles (the 25th and 75th percentiles, respectively), and the line inside denotes the median. Whiskers denote the lowest and highest values within 1.5 times and the IQR from the first and third quartiles, respectively
The intestinal microbes of calves with diarrhea show disorders, especially calves with bloody diarrhea. The PCoA results show that calves with watery diarrhea and calves with normal stools have comparable intestinal microbiota compositions, which differ significantly from calves with bloody diarrhea (Fig. 2C). The diversity of microbes declined with an increase in the degree of diarrhea, while in calves with bloody diarrhea, it was significantly reduced (Fig. 2D).
The taxa that most likely explain the differences between calves with bloody diarrhea, calves with watery diarrhea, and calves with normal stools were defined by LEfSe. One hundred thirty-six biomarkers were detected at the genus level (LDA > 2, P < 0.05). Thirty-two biomarkers significantly enriched in calves with bloody diarrhea, including various fungi and opportunistic pathogens, such as Escherichia, Streptococcus, Salmonella, and Shigella. Thirty-two biomarkers in calves with watery diarrhea, mainly Bacteroidetes, Firmicutes, and Proteobacteria, and 71 biomarkers were significantly enriched in calves with normal stool and mainly belonged to Firmicutes (Additional file 1: Fig. S1).
Relationship between parasites, other microbes, and calf diarrhea
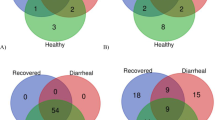
Cryptosporidium, Eimeria, and Giardia were distributed in both diarrheal and healthy calves and were more abundant in diarrheal cattle and lower in calves with normal stools. However, Blastocystis and Entamoeba were found only in calves with normal stools, with a higher abundance than that of the other parasites (Additional file 1: Fig. S2).
The network analysis results showed that the parasites were associated with various other gut microbes (Fig. 3). In total, five protozoan parasites formed two distinct networks, indicating that protozoan parasites have different mutual regulatory relationships with other intestinal microbes. Blastocystis and Entamoeba were related to the diversity of the intestinal bacteria, archaea, fungi, and viruses in calves. All 494 genera of intestinal microbes were positively associated with Blastocystis and Entamoeba; about 81.2% (401/494) were bacteria, and the others were archaea (41/494), fungi (44/494), and viruses (8/494). Interestingly, Blastocystis and Entamoeba were significantly positively correlated with various archaea, including many methanogens: Methanothermus, Methanothermococcus, Methanothermobacter, Methanosalsum, and Methanolacinia. One hundred sixty-one intestinal microbes were associated with Eimeria, Cryptosporidium, and Giardia, including 62 genera of bacteria, 97 fungi, and two viruses. Most of the bacteria were negatively correlated with Eimeria, Cryptosporidium, and Giardia, and all the fungi and viruses were positively correlated with them, meaning that these protozoan parasites may be related to the imbalance of other gut microbes.
Relationship between protozoan parasites and calf microbiome. Network relationships between protozoan parasites and gut microbes at the genus level. The relationship was calculated using the pairwise Spearman rank correlation, the removed coefficient was below 0.7, P > 0.05, and the P-value was adjusted to avoid false positives using the BH method
Key microbes associated with protozoan parasites and the health of calves
Twenty-seven biomarkers were not only significantly enriched in calves with bloody diarrhea, watery diarrhea, and normal stools, but were also associated with Eimeria, Cryptosporidium, and Giardia. In addition, all intestinal microorganisms associated with Blastocystis and Entamoeba were not significantly enriched in calves with varying degrees of diarrhea. Among the biomarkers, seven genera of bacteria were negatively correlated with Eimeria, which were mainly enriched in the intestines of calves with normal stools, namely, Dietzia, Flavonifractor, Gemmiger, Intestinimonas, Lachnoclostridium, Negativibacillus, and Ruthenibacterium. All 18 genera of fungi were positively correlated with Eimeria, Cryptosporidium, and Giardia were enriched in the intestines of calves with bloody diarrhea, including Blastocladiella, Diaporthe, Diplocarpon, Endocarpon, Fomitiporia, Gaeumannomyces, Gelatoporia, Hydnomerulius, Kalmanozyma, Leucosporidium, Magnaporthe, Magnaporthiopsis, Melampsora, Meyerozyma, Sclerotinia, Setosphaeria, Spiromyces, and Trichophyton. The two viruses, Alphaentomopoxvirus and Pandoravirus, were enriched in the intestines of calves with bloody diarrhea (Fig. 4).
Relationship between parasites and other gut microbial function
Functional annotation based on the KEGG database showed that the intestinal microorganisms of calves had abundant functional genes (Additional file 1: Fig. S3). The functional categories identified included Metabolism, Cellular Processes, Environmental Information Processing, Genetic Information Processing, Human Diseases, and Organismal Systems. PCoA results showed that, at the KO level, the samples of calves with watery diarrhea and normal stools showed a clear distance from calves with bloody diarrhea. This suggests that severe diarrhea could affect gut microbial function (Additional file 1: Fig. S4).
The functions of other gut microbes were related to intestinal parasites. A total of 611 KOs were found to be associated with parasites by calculating their correlation coefficients (Additional file 2: Table S1), and 87 KOs were disease-related (Fig. 5A). The genes annotated as 87 KOs were aligned with the NCBI NR database to trace the possible integration of the bacteria. Sixty-six KOs were successfully annotated, most of which were from fungi (Fig. 5B).
Discussion
Intestinal protozoan parasitic diseases are prevalent in ruminants, and infections with intestinal protozoan parasites in cattle are associated with outbreaks of diarrhea, mainly in calves, leading to economic losses for agricultural producers [32, 33]. However, the relationship between parasites and calf gut microbes remains unclear. Here, we have shown the relationship between parasites, gut microbes, and diarrhea.
The abundance of parasites found in this study was associated with calf diarrhea. Eimeria, Cryptosporidium, and Giardia were detected in both diarrheal and normal calves, but in higher abundance in diarrheal calves. Previous studies have shown similar results; the oocysts/cyst per gram of feces of Eimeria, Cryptosporidium, and Giardia in diarrheal animals were positively higher than in healthy animals [34,35,36]. Nevertheless, Blastocystis and Entamoeba were found only in healthy calves, which may indicate a positive association with intestinal health. The pathogenicity of Blastocystis and Entamoeba in cattle is controversial; however, Blastocystis and Entamoeba are highly prevalent in cattle and are mostly detected in healthy cattle [37, 38], which is consistent with the results of this study.
Calf diarrhea was often characterized by an imbalance in intestinal flora [10,11,12], and this study also revealed intestinal microbial imbalance in calves with diarrhea. Moreover, Eimeria, Cryptosporidium, and Giardia intestinalis were related to an imbalance in gut microbes. Previous studies have also shown negative effects of these parasites on gut microbes. For example, Eimeria damaged the chicken intestinal barrier and reduced the abundance of probiotic bacteria [16]. Cryptosporidium infection decreased bacterial diversity [39], and Giardia infection was associated with significant dysbiosis within the murine foregut and hindgut [15]. By contrast, Blastocystis and Entamoeba in this study were associated with the diversity of other intestinal microorganisms. The same phenomenon has been observed in humans, where it was found that Blastocystis was associated with both higher richness and higher evenness of the gut bacterial microbiota, whereas Entamoeba was associated only with higher richness, all of which have a role in maintaining intestinal health [17]. It is worth noting that Blastocystis and Entamoeba were significantly positively correlated with various methanogenic archaea. The possible use of methanogens as probiotics has received particular attention in humans [40, 41]; therefore, the positive relationship between Blastocystis, Entamoeba, and methanogenic archaea may be beneficial for gut health. The relationship between archaea and parasites in animals has received little attention, and more research is needed to uncover this phenomenon.
Little attention has been paid to the relationship between fungi and parasites in cattle. In this study, many fungi that were significantly enriched in calves with bloody diarrhea were positively correlated with Eimeria, Cryptosporidium, and Giardia. The parasites and fungi may play a synergistic role in intestinal disease in calves. Previous studies have found that higher fungal abundance is associated with intestinal disease, with a higher prevalence and abundance in patients with inflammatory bowel disease [42], which enhances inflammation by preventing intestinal healing [43, 44]. Fungi are neglected microorganisms that influence intestinal diseases in cattle, and further studies on the interactions between fungi and parasites are needed.
Conclusion
In conclusion, this study revealed the relationship between protozoan parasites and the calf microbiome. Eimeria, Cryptosporidium, and Giardia are associated with calf diarrhea and intestinal microbial disorders. By contrast, Blastocystis and Entamoeba play positive roles in maintaining intestinal health and microbial diversity. In addition, many fungi have a potential synergistic relationship with Eimeria, Cryptosporidium, and Giardia; on the contrary, archaea were only positively correlated with Blastocystis and Entamoeba. Due to the limited collection from the same farm, more extensive sampling in the future will clearly help produce a better association between protozoan parasites and intestinal health. As we described only the association of protozoan parasites, microbiome, and health of calves in this study, further work, including intervention studies, will be needed to fully elucidate the role of the protozoan parasites in the intestinal environment.
Availability of data and materials
The data generated during this study are available at the Sequence Read Archive under the BioProject accession number PRJNA924548.
Abbreviations
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- KO:
-
KEGG ortholog group
- LDA:
-
Linear discriminant analysis
- LEfSe:
-
Linear discriminant analysis effect size
- PCoA:
-
Principal coordinates analysis
- PCR:
-
Polymerase chain reaction
- NCBI:
-
National Center for Biotechnology Information
References
Li ZJ, Wang XN, Zhang Y, Yu ZT, Zhang TT, Dai XL, et al. Genomic insights into the phylogeny and biomass-degrading enzymes of rumen ciliates. Isme J. 2022;16:2775–87.
Zahedi A, Ryan U. Cryptosporidium - An update with an emphasis on foodborne and waterborne transmission. Res Vet Sci. 2020;132:500–12.
Ryan UM, Feng YY, Fayer R, Xiao LH. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia - a 50 year perspective (1971–2021). Int J Parasitol. 2021;51:1099–119.
Audebert C, Even G, Cian A, Loywick A, Merlin S, Viscogliosi E, et al. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci Rep-Uk. 2016;6:25255.
Huang J, Mullapudi N, Lancto CA, Scott M, Abrahamsen MS, Kissinger JC. Phylogenomic evidence supports past endosymbiosis, intracellular and horizontal gene transfer in Cryptosporidium parvum. Genome Biol. 2004;5:R88.
Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–5.
Correa PS, Jimenez CR, Mendes LW, Rymer C, Ray P, Gerdes L, et al. Taxonomy and functional diversity in the fecal microbiome of beef cattle reared in Brazilian traditional and semi-intensive production systems. Front Microbiol. 2021;12:768480.
Fan P, Kim M, Liu G, Zhai Y, Liu T, Driver JD, et al. The gut microbiota of newborn calves and influence of potential probiotics on reducing diarrheic disease by inhibition of pathogen colonization. Front Microbiol. 2021;12:772863.
Bartels CJ, Holzhauer M, Jorritsma R, Swart WA, Lam TJ. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev Vet Med. 2010;93:162–9.
McGuirk SM. Disease management of dairy calves and heifers. Vet Clin North Am Food Anim Pract. 2008;24:139–53.
Zeineldin M, Aldridge B, Lowe J. Dysbiosis of the fecal microbiota in feedlot cattle with hemorrhagic diarrhea. Microb Pathogenesis. 2018;115:123–30.
Gomez DE, Arroyo LG, Costa MC, Viel L, Weese JS. Characterization of the fecal bacterial microbiota of healthy and diarrheic dairy calves. J Vet Intern Med. 2017;31:928–39.
Kim HS, Whon TW, Sung H, Jeong YS, Jung ES, Shin NR, et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat Commun. 2021;12:161.
Olson ME, O’Handley RM, Ralston BJ, McAllister TA, Thompson RC. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 2004;20:185–91.
Barash NR, Maloney JG, Singer SM, Dawson SC. Giardia alters commensal microbial diversity throughout the murine gut. Infect Immun. 2017. https://doi.org/10.1128/IAI.00948-16.
Lu C, Yan Y, Jian F, Ning C. Coccidia-microbiota interactions and their effects on the host. Front Cell Infect Microbiol. 2021;11:751481.
Even G, Lokmer A, Rodrigues J, Audebert C, Viscogliosi E, Segurel L, et al. Changes in the human gut microbiota associated with colonization by Blastocystis sp. and Entamoeba spp. in non-industrialized populations. Front Cell Infect Microbiol. 2021;11:533528.
Liu Z, Luo G, Du R, Sun W, Li J, Lan H, et al. Effects of spaceflight on the composition and function of the human gut microbiota. Gut Microbes. 2020;11:807–19.
Zhou Y, Fu H, Yang H, Wu J, Chen Z, Jiang H, et al. Extensive metagenomic analysis of the porcine gut resistome to identify indicators reflecting antimicrobial resistance. Microbiome. 2022;10:39.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9.
Li D, Liu CM, Luo R, Sadakane K, Lam TW. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–6.
Oh J, Byrd AL, Deming C, Conlan S, Program NCS, Kong HH, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514:59–64.
Nielsen HB, Almeida M, Juncker AS, Rasmussen S, Li J, Sunagawa S, et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat Biotechnol. 2014;32:822–8.
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8.
Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59–60.
Huson DH, Mitra S, Ruscheweyh HJ, Weber N, Schuster SC. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011;21:1552–60.
Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Cacciò SM. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35:207–13.
Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J Clin Microbiol. 2003;41:2744–7.
Li G, Xiao S, Zhou R, Li W, Wadeh H. Molecular characterization of Cyclospora-like organism from dairy cattle. Parasitol Res. 2007;100:955–61.
Scicluna SM, Tawari B, Clark CG. DNA barcoding of blastocystis. Protist. 2006;157:77–85.
Bahrami F, Haghighi A, Zamini G, Khademerfan M. Differential detection of Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii in faecal samples using nested multiplex PCR in west of Iran. Epidemiol Infect. 2019;147:e96.
Santin M. Cryptosporidium and Giardia in ruminants. Vet Clin North Am Food Anim Pract. 2020;36:223–38.
Bangoura B, Bhuiya MAI, Kilpatrick M. Eimeria infections in domestic and wild ruminants with reference to control options in domestic ruminants. Parasitol Res. 2022;121:2207–32.
Bangoura B, Mundt HC, Schmaschke R, Westphal B, Daugschies A. Prevalence of Eimeria bovis and Eimeria zuernii in German cattle herds and factors influencing oocyst excretion. Parasitol Res. 2012;110:875–81.
Olson ME, Guselle NJ, O’Handley RM, Swift ML, McAllister TA, Jelinski MD, et al. Giardia and Cryptosporidium in dairy calves in British Columbia. Can Vet J. 1997;38:703–6.
Operario DJ, Bristol LS, Liotta J, Nydam DV, Houpt ER. Correlation between diarrhea severity and oocyst count via quantitative PCR or fluorescence microscopy in experimental cryptosporidiosis in calves. Am J Trop Med Hyg. 2015;92:45–9.
Shams M, Shamsi L, Sadrebazzaz A, Asghari A, Badali R, Omidian M, et al. A systematic review and meta-analysis on the global prevalence and subtypes distribution of Blastocystis sp. infection in cattle: a zoonotic concern. Comp Immunol Microbiol Infect Dis. 2021;76:101650.
Matsubayashi M, Matsuura Y, Nukata S, Daizi Y, Shibahara T, Teramoto I, et al. First detection and molecular identification of Entamoeba bovis from Japanese cattle. Parasitol Res. 2018;117:339–42.
Mammeri M, Obregon DA, Chevillot A, Polack B, Julien C, Pollet T, et al. Cryptosporidium parvum infection depletes butyrate producer bacteria in goat kid microbiome. Front Microbiol. 2020;11:548737.
Coker OO, Wu WKK, Wong SH, Sung JJY, Yu J. Altered gut archaea composition and interaction with bacteria are associated with colorectal cancer. Gastroenterology. 2020;159:1459–70.
Han SW, Zhuang J, Pan YF, Wu W, Ding KF. Different characteristics in gut microbiome between advanced adenoma patients and colorectal cancer patients by metagenomic analysis. Microbiol Spectr. 2022;10:e0159322.
Guzzo GL, Mittinty MN, Llamas B, Andrews JM, Weyrich LS. Individuals with Inflammatory bowel disease have an altered gut microbiome composition of fungi and protozoa. Microorganisms. 2022;10:1910.
Chiaro T, Round JL. Fungi prevent intestinal healing. Science. 2021;371:1102–3.
Jain U, Ver Heul AM, Xiong S, Gregory MH, Demers EG, Kern JT, et al. Debaryomyces is enriched in Crohn’s disease intestinal tissue and impairs healing in mice. Science. 2021;371:1154–9.
Acknowledgements
We are very grateful to the editors and reviewers for critically evaluating the manuscript and providing constructive comments for its improvement.
Funding
This work was supported by the Key Program of the National Natural Science Foundation of China–Henan Province Joint Fund (U1904203), the National Key Research and Development Program of China (2022YFD1800200), and the Leading Talents of Thousand Talents Program of Central China (19CZ0122).
Author information
Authors and Affiliations
Contributions
LZ designed the study. YF, KZ, MY, PD, and YC performed the experiments. YF, KZ, and MY analyzed the data. YF and KZ wrote the first draft of the manuscript and prepared the figures. MY, XL, JL, HX, and YC reviewed and edited the manuscript. All authors have read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Following the Chinese Laboratory Animal Administration Act of 1988, the research protocol was reviewed and approved by the Research Ethics Committee of Henan Agricultural University (approval no. IRB-HENAU-20180914-01). Appropriate permission was obtained from the farmers before fecal samples were collected, and no animals were harmed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
LEfSe analysis of gut microbes in calves with bloody diarrhea, watery diarrhea, and normal stools. Microbial features showing differential abundance were identified using the LEfSe threshold criteria of an LDA score > 2 and P < 0.05. The length of the bar column represents the LDA score. Figure S2. Abundance of protozoan parasites in calves with bloody diarrhea, watery diarrhea, and normal stools. Figure S3. KEGG pathway annotation. Figure S4. PCoA of gut microbial functions based on Bray–Curtis distance.
Additional file 2: Table S1.
Correlation coefficients between KEGG ortholog group and parasites.
Additional file 3: Table S2.
Annotated parasite genes and corresponding species information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, Y., Zhang, K., Yang, M. et al. Metagenomic analysis reveals the relationship between intestinal protozoan parasites and the intestinal microecological balance in calves. Parasites Vectors 16, 257 (2023). https://doi.org/10.1186/s13071-023-05877-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05877-z