Abstract
Background
Nematodes of the family Physalopteridae (Spirurida: Physalopteroidea) commonly parasitize the alimentary canal of all major vertebrate groups. However, many physalopterid species are not adequately described, especially regarding the detailed morphology of the cephalic end. The current genetic database for Physaloptera species is still very limited, which seriously hampers molecular-based species identification. Additionally, the systematic status of some genera and the evolutionary relationships of the subfamilies in the Physalopteridae remain under debate.
Methods
New morphological data for Physaloptera sibirica was gathered using light and scanning electron microscopy based on newly collected specimens from the hog badger Arctonyx collaris Cuvier (Carnivora: Mustelidae) in China. Six different genetic markers, including nuclear small ribosomal DNA (18S), large ribosomal DNA (28S) and internal transcribed spacer (ITS), mitochondrial cytochrome c oxidase subunit 1 (cox1) and subunit 2 (cox2), and the 12S small subunit ribosomal RNA gene of P. sibirica were sequenced and analyzed for the first time to our knowledge. Additionally, to construct a basic molecular phylogenetic framework for the Physalopteridae, phylogenetic analyses were performed based on the cox1 and 18S + cox1 genes using maximum likelihood (ML) and Bayesian inference (BI) methods.
Results
Scanning electron microscopy (SEM) observation displayed the details of the cephalic structures, deirids, excretory pore, caudal papillae, vulva, phasmids and egg of P. sibirica for the first time to our knowledge. Pairwise comparison of the sequences obtained for P. sibirica did not reveal intraspecific divergence regarding the 18S, 28S, cox1 and 12S genetic markers and a low level of divergence in the ITS (0.16%) and cox2 (2.39%) regions. Maximum likelihood and Bayesian inference analyses showed that the representatives of Physalopteridae formed two major clades (species of Physalopterinae + Thubunaeinae parasitic in terrestrial vertebrates and Proleptinae only occurring in marine or freshwater fishes). Turgida turgida was found nested among representatives of Physaloptera. Physaloptera sibirica clustered together with P. rara. Physalopteroides sp. (Thubunaeinae) formed a sister relationship to the physalopterine Abbreviata caucasica.
Conclusions
Physaloptera sibirica was redescribed, which is the fourth nematode parasite reported from the hog badger A. collaris, and A. collaris represents a new host for P. sibirica. The phylogenetic results challenged the validity of the subfamily Thubunaeinae and of the genus Turgida and supported dividing the family Physalopteridae into two subfamilies, Physalopterinae and Proleptinae. However, we do not make any immediate systematic changes in the Physalopteridae, because a more rigorous study with broader representation of the Physalopteridae is required. These present findings contribute to morphologically identifying P. sibirica more accurately and provide new insights into the systematics of the Physalopteridae.
Graphical Abstract

Similar content being viewed by others
Background
Nematodes of the family Physalopteridae (Spirurida: Physalopteroidea) commonly parasitize the alimentary canal of all major vertebrate groups [1,2,3,4,5]. According to the current classification, Physalopteridae is divided into three subfamilies, Physalopterinae, Proleptinae and Thubunaeinae [6]. Chabaud & Bain (1994) [7] speculated that the Proleptinae are morphologically closer to the Physalopterinae based on the cephalic structures; however, the evolutionary relationships of the representatives of these subfamilies remain unclear.
The genus Physaloptera, with over 100 nominal species reported from various amphibians, reptiles, birds and mammals worldwide, is the largest group in the Physalopteridae [8,9,10]. However, many species of Physaloptera are not adequately described, especially regarding the detailed morphology of the cephalic structure. Moreover, the current genetic database for Physaloptera species is still very limited. In Physaloptera, only 11 species have been genetically sequenced (many of these genetic data are still unpublished), which seriously hampers the molecular-based species identification and phylogeny of this group.
The greater hog badger Arctonyx collaris Cuvier (Carnivora: Mustelidae) is a terrestrial mammal, mainly distributed in Central and Southeast Asia, including Thailand, India, Bangladesh and P.R. China. This species is listed as vulnerable in the IUCN Red List of Threatened Species (https://www.iucnredlist.org/species/70205537/45209459), because the global population is thought to be declining because of over-hunting and the destruction of its habitat [11]. There is currently very little information on the nematode fauna of A. collaris. To date, only three nematode species have been reported from it, including Uncinaria stenocephala (Railliet, 1884) (Rhabditida: Ancylostomatidae), Toxocara vajrasthirae Sprent, 1972 (Ascaridida: Ascarididae) and Tetragomphius arctonycis Jansen, 1968 (Rhabditida: Ancylostomatidae) [12,13,14].
In the present study, some physalopterid nematodes were collected from A. collaris in China. The detailed morphology of these nematode specimens was studied using light and scanning electron microscopy, and the molecular characterization of the nuclear small subunit ribosomal DNA (18S), internal transcribed spacer (ITS) and large subunit ribosomal DNA (28S), and mitochondrial cytochrome c oxidase subunit 1 (cox1) and subunit 2 (cox2) and 12S regions were sequeced and analyzed to accurately identify them to species level. Additionally, to clarify the evolutionary relationships of the representatives of the Physalopterinae, Proleptinae and Thubunaeinae in the Physalopteridae and construct a basic molecular phylogenetic framework for this group, phylogenetic analyses based on the 18S + cox1 and cox1 sequence data using maximum likelihood (ML) and Bayesian inference (BI) were performed.
Methods
Parasite collection
A road-killed hog badger A. collaris Cuvier (Carnivora: Mustelidae) in Huangniupu (106.786E, 34.246N), Baoji city, Shaanxi Province, was opportunistically examined for parasites. The hog badger was a male with 50–60 cm body length. Only some nematode parasites (26 specimens) were isolated from the stomach of this host and were stored in 80% ethanol for further study.
Morphological observation
For light microscopical studies, nematodes were cleared in glycerine. Drawings were made with a Nikon microscope drawing attachment. For scanning electron microscopy (SEM), the cephalic and posterior ends of nematodes were fixed in 4% formaldehyde solution, post-fixed in 1% OsO4, dehydrated via an ethanol series and acetone, and then critical point dried. Samples were coated with gold and examined using a Hitachi S-4800 scanning electron microscope at an accelerating voltage of 20 kV. Measurements (range followed by mean in parentheses) are given in millimeters (mm) unless otherwise stated. Voucher specimens were deposited at the College of Life Sciences, Hebei Normal University, Hebei Province, China.
Molecular procedures
Three nematode specimens (1 male and 2 females) were randomly chosen for molecular analyses. Genomic DNA from each sample was extracted using a Column Genomic DNA Isolation Kit (Shanghai Sangon, China) according to the manufacturer’s instructions. The primers used for amplifying different target regions by polymerase chain reaction (PCR) in the present study are provided in Table 1. The cycling conditions were as described previously [15]. PCR products were checked on GoldView-stained 1.5% agarose gels and purified with Column PCR Product Purification Kit (Shanghai Sangon, China). Sequencing for each sample was carried out for both strands. The DNA sequences obtained herein using the PCR primers were aligned using ClustalW2 and compared (using the algorithm BLASTn) with those available in the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov). The 18S, 28S, ITS, cox1, cox2 and 12S sequence data obtained herein were deposited in the GenBank database (http://www.ncbi.nlm.nih.gov).
Phylogenetic analyses
Phylogenetic reconstructions were performed based on the 18S + cox1 and cox1 sequence data using the maximum likelihood (ML) and Bayesian inference (BI) criteria implemented within IQ-TREE and MrBayes [16, 17], respectively. Gnathostoma turgidum Stossich, 1902 (Spirurida: Gnathostomatidae) was treated as the outgroup according to the previous studies [18, 19]. The ingroup included 16 physalopterid species representing seven genera belonging to three subfamilies, Physalopterinae, Proleptinae and Thubunaeinae. Detailed information on nematode species included in the phylogenetic analyses is provided in Table 2. We used a built-in function in IQTREE to select a best-fitting substitution model for the sequences according to the Bayesian information criterion [20]. The TIM3 + F + I + G4 model was identified as the optimal nucleotide substitution model for both 18S + cox1 and cox1 sequence data. Reliabilities for maximum likelihood inference were tested using 1000 bootstrap replications, and Bayesian information criterion analysis was run for 1 × 107 MCMC generations, sampling a tree at every 1000 generations. The first 25% trees were treated as “burn-in.” Reliabilities for ML tree were tested using 1000 bootstrap replications, and BI tree was tested using 10 million generations. In the ML tree, bootstrap support (BS) values ≥ 80 were considered to constitute strong branch support, whereas BS values ≥ 50 and < 80 were considered to constitute moderate branch support. In the BI tree, Bayesian posterior probabilities (BPP) ≥ 0.98 were considered to constitute strong branch support, whereas BPP values ≥ 0.95 and < 0.98 were considered to constitute moderate branch support.
Results
Order Spirurida Railliet, 1914
Superfamily Physalopteroidea Railliet, 1893
Family Physalopteridae Railliet, 1893
Genus Physaloptera Rudolphi, 1819
Physaloptera sibirica Petrow & Gorbunow, 1931
Host: The hog badger Arctonyx collaris Cuvier (Carnivora: Mustelidae).
Locality: Shaanxi Province, China.
Site of infection: Stomach.
Specimen deposition: 5 males, 10 females (HBNU-N-2022M1120-CL); deposited at the College of Life Sciences, Hebei Normal University, Hebei Province, China.
Representative DNA sequences: Representative nuclear ribosomal and mitochondrial DNA sequences were deposited in the GenBank database under accession nos. OQ846900–OQ846902 (18S), OQ846911–OQ846913 (ITS), OQ846907–OQ846909 (28S), OQ852731–OQ852733 (cox1), OQ867994–OQ867996 (cox2) and OQ846904–OQ846906 (12S).
Description (Figs. 1, 2, 3)
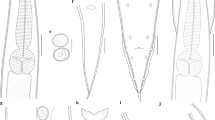
Physaloptera sibirica collected from Arctonyx collaris Cuvier (Carnivora: Mustelidae) in China. a Anterior part of male, ventral view. b Anterior end of male, ventral view. c Anterior end of male, lateral view. d Region of vulva, lateral view. e Posterior end of male, ventral view. f Spicules. g Egg, embryonated. h Egg, unembryonated. i Posterior end of female, lateral view. Scale bars: a 1000 μm; b, c, f: 200 μm; d, e, i: 500 μm; g, h: 30 μm
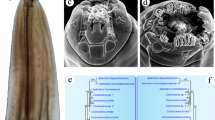
Scanning electron micrographs of Physaloptera sibirica collected from Arctonyx collaris Cuvier (Carnivora: Mustelidae) in China, female. a Anterior part of body (deirids indicated by white arrows, excretory pore indicated by black arrow), ventral view. b Magnified image of vulva. c Tail (phasmids arrowed), ventral view. d Magnified image of phasmid. e Magnified image of deirid. f Magnified image of excretory pore. g Magnified image of egg
Scanning electron micrographs of Physaloptera sibirica collected from Arctonyx collaris Cuvier (Carnivora: Mustelidae) in China, male. a Cephalic extremity (longitudinal grooves present at internal base of pseudolabium indicated by arrows), subapical view. b Magnified image of lateral pseudolabium, apical view. c Magnified image of cloacal region, ventral view. d Posterior end of body (4 pairs of precloacal and postcloacal pedunculate papillae indicated by white arrows, 3 pairs of postcloacal ventral sessile papillae indicated by black arrows), ventral view. e Magnified image of phasmid. f Magnified image of second pair of precloacal pedunculate papilla. g Magnified image of median sessile papilla at anterior margin of cloaca. h Magnified image of first pair of postcloacal ventral sessile papilla. i Magnified image of third pair of postcloacal ventral sessile papilla. cp, submedian cephalic papillae; am, amphid; st, simple tooth; tt, tripartite tooth; da, depressed areas on each pseudolabium; cr, cuticular ridges; pp1–3, first to third pairs of sessile papillae at anterior margin of cloaca; ps1–4, first to fourth pairs of sessile papillae at posterior margin of cloaca; pf, papillose formations on posterior cloacal lip; ph, phasmids; sp, spicule
General
Medium-sized whitish nematodes. Cuticle thick with fine transverse striations. Cephalic collarette (vesicle) present, extending posteriorly to the level of deirids (Figs. 1a‒c, 2a). Cephalic end dome-shaped, oral aperture dorsoventrally elongate, surrounded by two lateral pseudolabia (Figs. 1a‒c, 2a, 3a, b). Each pseudolabium bearing two large submedian (dorsolateral and ventrolateral) cephalic papillae and one small amphid situated at nearly the middle of pseudolabium (Fig. 3a, b). Inner margin of each pseudolabium with one simple median conical tooth, one median tripartite tooth and two lateral cuticular ridges (Fig. 3a, b). One pair of short longitudinal grooves present at internal base of pseudolabium. Two oval depressed areas present on each pseudolabium (Fig. 3a, b). Oesophagus divided into anterior short muscular portion and long glandular portion (Fig. 1a). Nerve-ring encircles posterior muscular oesophagus (Fig. 1a‒c). Deirids well developed, spine-shaped, slightly posterior to nerve ring (Figs. 1a‒c, 2a, e). Excretory pore situated slightly posterior to junction of muscular and glandular oesophagus (Figs. 1c, 2a, f). Posterior end of body sexually dimorphic.
Male (based on 5 specimens)
Body 19.6–32.7 (27.8) long; maximum width 0.73–1.12 (0.94). Pseudolabia 0.08–0.12 (0.10) long, 0.14–0.19 (0.17) wide. Cephalic collarette (vesicle) 0.46–0.81 (0.65) long, 0.20–0.25 (0.23) wide. Entire oesophagus 3.46–5.37 (4.62) long, representing 14.4–18.9 (16.8) % of body length; muscular oesophagus 0.49–0.71 (0.55) long, 0.15–0.32 (0.22) in maximum width; glandular oesophagus 3.00–4.88 (4.07) long, 0.29–0.39 (0.34) in maximum width; length ratio of two parts of oesophagus 1: 6.15–9.52 (1: 7.45). Nerve ring, deirids and excretory pore 0.44–0.56 (0.50), 0.51–0.73 (0.63) and 0.76–0.93 (0.83) from cephalic extremity, respectively. Spicules similar in shape, with subpointed distal end, unequal in length; left spicule relatively long, 0.54–1.27 (1.00) in length, representing 2.38–3.97 (3.28) % of body length; right spicule short, 0.44–1.22 (0.87) long, representing 1.94–3.82 (2.83) % of body length; spicule (right: left) ratio 1/1.04–1.25 (1/1.18) (Fig. 1f). Gubernaculum absent. Posterior end of body spirally coiled ventrally. Posterior expansion of cuticle forming spade-like caudal bursa, ornamented ventrally with numerous longitudinal cuticular tubercles (Fig. 3d) and supported by four pairs of subventral pedunculate papillae (2 pairs precloacal, 2 pairs postcloacal) (Figs. 1e, 3f). Anterior margin of cloaca with three sessile papillae (median papilla tabular, usually larger than lateral papillae), posterior margin of cloaca with two pairs of sessile papillae (Figs. 1e, 3c, g). Three additional pairs of postcloacal ventral sessile papillae present (Figs. 1e, 3d, h, i). Tail 0.88–1.93 (1.44) long, with rounded tip (Figs. 1e, 3d). Phasmids situated between second and third pairs of postcloacal ventral papillae (Figs. 1e, 3d, e).
Female (based on 10 specimens)
Body 25.0–51.5 (37.7) long; maximum width 1.00–1.59 (1.26). Pseudolabia 0.09–0.14 (0.12) long, 0.15–0.21 (0.18) wide. Cephalic collarette (vesicle) 0.44–0.83 (0.60) long, 0.26–0.39 (0.32) wide. Entire oesophagus 4.39–7.32 (5.67) long, representing 12.2–17.6 (15.4) % of body length; muscular oesophagus 0.49–0.73 (0.56) long, 0.15–0.27 (0.20) in maximum width; glandular oesophagus 3.90–6.59 (5.11) long, 0.34–0.49 (0.42) in maximum width; length ratio of two parts of oesophagus 1: 8.00–10.5 (1: 9.05). Nerve ring, deirids and excretory pore 0.46–0.63 (0.53), 0.51–0.85 (0.68) and 0.63–1.10 (0.85) from anterior extremity, respectively. Vulva slightly protruding, situated 4.10–9.81 (6.46) from cephalic extremity, at 13.9–21.4 (17.4) % of body length (Figs. 1d, 2b). Vagina long, muscular, initially directed posteriorly from vulva and then oriented anteriorly; uteri didelphic with two uterine branches (Fig. 1d). Eggs oval, unembryonated or embryonated, thick-shelled, with smooth surface, 0.04–0.05 (0.05) × 0.02–0.04 (0.03) (n = 20) (Figs. 1g, h, 2g). Posterior end of body almost straight, not spirally coiled ventrally. Tail 0.37–0.73 (0.49) long, with roughly rounded tip (Figs. 1i, 2c). A pair of small lateral phasmids present at base of tail tip (Figs. 1i, 2c, d).
Remarks
Petrow & Gorbunow (1931) [21] described P. sibirica from the Eurasian badgers Meles meles amurensis (originally as Nyctereutes amurensis) and red fox Vulpes vulpes from the Far East, Siberia, Uzbekistan and Tatarstan. Subsequently, P. sibirica was reported from various mammals in the Palaearctic region [22,23,24,25,26,27,28,29,30]. However, only a few previous studies [2, 24, 25] provided the morphological characters of this species after the original description.
The morphology and measurements of our material are almost identical to the original description of P. sibirica and some other studies [2, 21, 24, 25] regarding several features, including the morphology of the pseudolabia, position of the nerve ring and excretory pore, length of the oesophagus (including muscular and glandular portions), morphology and length of the spicules and tail, number and arrangement of caudal papillae, position of the vulva and size of the eggs (see Table 3 for details). Therefore, we consider our newly collected material conspecific with P. sibirica. However, the body length of males in the original description [21] and Li & Zhu’s (1980) [25] material is distinctly smaller than in our specimens and Quentin & Biocca’s (1976) [24] description. Moreover, the ratio of the left and right spicules in Quentin & Biocca’s (1976) [24] material is larger than that of our specimens. The above-mentioned morphometric differences should be considered as intraspecific variability, possibly owing to the different hosts, geographical locations or infection levels. Petrow & Gorbunow (1931) [21] stated that there were 4–5 pairs of pedunculate papillae in the original description, and Skrjabin & Sobolev (1964) [2] reported only five pairs of pedunculate papillae in their redesciption, but they both considered the presence of six pairs of postcloacal sessile papillae in their material. However, the subsequent [24, 25] and present study all observed only four pairs of pedunculate papillae (2 pairs precloacal, 2 pairs postcloacal). Additionally, these two previous studies [2, 21] both erroneously treated the phasmids as one pair of postcloacal sessile papillae. The present SEM observation clearly showed the details of the cephalic structures, deirids, excretory pore, caudal papillae, vulva, phasmids and egg for the first time to our knowledge, which are helpful for the specific diagnosis of this species. Physaloptera sibirica is the fourth nematode parasite reported from A. collaris, which also represents a new host record for P. sibirica.
In the genus Physaloptera, there are 22 species with two uterine branches in females parasitic in mammals in the Palaearctic region, including P. apodemi Wang & Zhang, 2020; P. clausa Rudolphi, 1819; P. anomala Molin, 1860; P. bedfordi Ortlepp, 1932; P. brevispiculum Linstow, 1906; P. brevivaginata Seurat, 1917; P. canis Monnig, 1929; P. dispar Linstow, 1904; P. getula Seurat, 1917; P. hispida Schell, 1950; P. lumsdeni Yeh, 1957; P. limbata Leidy, 1856; P. maxillaris Molin, 1860; P. murisbrasiliensis Diesing, 1861; P. massino Schulz, 1926; P. praeputiale Linstow, 1889; P. peramelis Johnston & Mawson, 1939; P. rara Hall & Wigdor, 1918; P. semilanceolata Molin, 1860; P. seurati Isaitschikov, 1926, P. sibirica and P. terdentata Molin, 1860 [1, 2, 31‒35]. Physaloptera sibirica can be easily distinguished from P. apodemi, P. anomala, P. brevispiculum, P. brevivaginata, P. dispar, P. getula, P. limbata, P. lumsdeni, P. maxillaris, P. massino, P. murisbrasiliensis, P. peramelis, P. praeputiale, P. rara and P. terdentata by having unequal spicules (0.40–1.50 mm and right spicule < 1.7 times the left one in length) without striated sheaths at their proximal end and only four pairs of pedunculated caudal papillae. Physaloptera sibirica differs from P. clausa, P. canis and P. bedfordi by the position of the vulva (vulva from cephalic extremity representing about 1/7–1/5 of body length in P. sibirica vs. about 1/3–1/2 of the body length in the last three species). Physaloptera sibirica is different from P. hispida and P. seurati by having relatively longer spicules (spicules representing about 2.0‒4.0% of body length in P. sibirica vs. about 1.0% of body length in the latter two species). With two pairs of precloacal and two pairs of postcloacal pedunculated papillae, P. sibirica differs from P. semilanceolata (3 pairs precloacal and 1 pair postcloacal pedunculated papillae).
Molecular characterization
Partial 18S region
Three 18S sequences of P. sibirica obtained herein are all 1710 bp in length, with no nucleotide polymorphism detected. In the genus Physaloptera, the 18S sequence data are available in GenBank for P. alata (AY702703), P. amazonica (MK312472), P. apivori (EU004817), P. bispiculata (KT894817), P. mirandai (KT894815, KT894816), P. praeputialis (MW410927), P. rara (MH938367), P. retusa (KT894814), P. thalacomys (JF934734) and P. tupinambae (MT810006). Pairwise comparison of the 18S sequences of P. sibirica with those of Physaloptera spp. available in GenBank displayed 0.11% (P. rara) to 2.10% (P. alata) of nucleotide divergence.
Partial ITS region
Three ITS sequences of P. sibirica obtained herein are 1272–1274 bp in length and represent two different genotypes, which showed 0.16% nucleotide divergence. In the genus Physaloptera, the ITS sequence data are available in GenBank only for P. alata (only 5.8S + ITS2 region, AY702694) and P. tupinambae (MT809124). Pairwise comparison of the ITS sequences of P. sibirica with those of Physaloptera spp. available in GenBank displayed 47.6% (P. alata) to 48.7% (P. tupinambae) nucleotide divergence.
Partial 28S region
Three 28S sequences of P. sibirica obtained herein are all 783 bp in length, with no nucleotide polymorphism detected. In the genus Physaloptera, the 28S sequence data are available in GenBank only for Physaloptera sp. (MG808041). Pairwise comparison of the 28S sequences of P. sibirica with that of Physaloptera sp. available in GenBank displayed 13.1% nucleotide divergence.
Partial cox1 region
Three cox1 sequences of P. sibirica obtained herein are all 892 bp in length, with no nucleotide polymorphism detected. In the genus Physaloptera, the cox1 sequence data are available in GenBank for P. alata (MZ391893), P. amazonica (MK309356), P. bispiculata (KT894806), P. hispida (MH782844, MH782845), P. mirandai (KP981418, KT894804, KT894805), P. rara (MH931178) and P. retusa (KT894803). Pairwise comparison of the cox1 sequences of P. sibirica with those of Physaloptera spp. available in GenBank displayed 15.2% (P. rara) to 19.5% (P. bispiculata) nucleotide divergence.
Partial cox2 region
Three cox2 sequences of P. sibirica obtained herein are all 376 bp in length and represent two different genotypes, which showed 2.39% nucleotide divergence. In the genus Physaloptera, the cox2 sequence data are available in GenBank only for P. rara (MH931178). Pairwise comparison of the cox2 sequences of P. sibirica with that of P. rara available in GenBank displayed 15.7% to 16.8% nucleotide divergence.
Partial 12S region
Three 12S sequences of P. sibirica obtained herein are all 472 bp in length, with no nucleotide polymorphism detected. In the genus Physaloptera, the 12S sequence data are available in GenBank only for P. rara (MH931178). Pairwise comparison of the 12S sequences of P. sibirica with that of P. rara available in GenBank displayed 14.8% nucleotide divergence.
Phylogenetic analyses (Figs. 4, 5)
Maximum likelihood (ML) inference and Bayesian inference (BI) based on the 18S + cox1 sequence data showing the phylogenetic relationships of representatives of Physalopteridae. Gnathostoma turgidum Stossich, 1902 (Spirurida: Gnathostomatidae), was chosen as the outgroup. Bootstrap support (BS) values ≥ 50 in ML tree and Bayesian posterior probabilities (BPP) ≥ 0.95 in BI tree are shown
Maximum likelihood (ML) inference and Bayesian inference (BI) based on the cox1 sequence data showing the phylogenetic relationships of representatives of Physalopteridae. Gnathostoma turgidum Stossich, 1902 (Spirurida: Gnathostomatidae), was chosen as the outgroup. Bootstrap support (BS) values ≥ 50 in ML tree and Bayesian posterior probabilities (BPP) ≥ 0.95 in BI tree are shown
Phylogenetic trees constructed from the 18S + cox1 and cox1 sequence data using the ML and BI methods had almost identical topologies (Figs. 4–5). The representatives of the Physalopteridae were divided into two major clades. Clade I included species of Physaloptera, Turgida and Abbreviara (Physalopterinae), and Physalopteroides (Thubunaeinae). Clade II contained representatives of Heliconema, Paraleptus and Proleptus (Proleptinae). In Clade I, P. alata + (P. rara + P. sibirica) formed a separated branch with moderate support in ML tree, but weak support in BI tree (Figs. 4, 5). Turgida turgida clustered together with P. amazonica + P. bispiculata + P. mirandai + P. retusa. Physalopteroides sp. (Thubunaeinae) formed a sister relationship to Abbreviata caucasica (Physalopterinae).
Discussion
Skrjabin (1964, 1969) [2, 31] assigned the genus Thubunaea into the subfamily Proleptinae as a tribe Thubunaeinea. Later, Chabaud (1975) [6] treated Thubunaeinea as a separated subfamily Thubunaeinae, including only two genera, Thubunaea and Physalopteroides. Chabaud & Bain (1994) [7] speculated that in the family Physalopteridae, the Physalopterinae and Proleptinae had closer relationships than the Thubunaeinae based on the cephalic structures. However, our phylogenetic results challenged the validity of the subfamily Thubunaeinae because of the single representative of Thubunaeinae (Physalopteroides sp.) clustered together with species of Abbreviata (member of Physalopterinae) and supported the classification of Physalopteridae comprising only two subfamilies, Physalopterinae and Proleptinae, proposed by Skrjabin & Sobolev (1964) [2], which conflicted with the above-mentioned traditional opinions [6, 7]. We considered that the features of cephalic collarette and caudal bursa used as the main criterion for differentiating the Thubunaeinae from Physalopterinae and Proleptinae by Chabaud (1975) [6] are rather vulnerable and questionable. The division of Physalopteridae into Physalopterinae and Proleptinae can be easily understood when we consider the host range and geographical distribution of the species in Physalopterinae, Proleptinae and Thubunaeinae (species of Physalopterinae and Thubunaeinae are both parasitic in terrestrial vertebrates vs. species of Proleptinae occur only in teleosts and elasmobranchs). Wason & Johnson (1977) [36] erected a subfamily Mirzalopterinae for the genus Mirzaloptera (type species M. barbari Wason & Johnson, 1977, collected from bat in India) in the Physalopteridae. However, the Mirzalopterinae has received little attention since its inception, and only the compilations of Jones & Gibson (1987) [37] and Gibbons (2010) [38] included it. The systematic status of Mirzalopterinae has been unclear.
The present molecular analyses also challenged the validity of the genus Turgida, because T. turgida nested among representatives of Physaloptera, which are accordant with previous phylogenetic studies [18, 19, 39]. In fact, Ortlepp (1922) [1] and Yorke & Maplestone (1927) [40] both suspected the validity of Turgida. Although Travassos (1920) [41] used the uterus with 10 uterine branches as a generic criterion in separating Turgida from the other genera in the Physalopterinae, the number of uterine branches in the type species of Turgida, T. turgida seems to be extremely variable. Gray & Anderson (1982) [42] observed 7 to 10 uterine branches in their specimens collected from opossum Didelphis virginiana. Ortlepp (1922) [1] reported that there were up to 14 uterine branches in female of T. turgida. Meanwhile, species of Physaloptera have also been reported to exhibit a broad range of variability in a number of uterine branches among different species, for example, P. ackerti and P. aduensis possessing six to nine uterine branches; P. amazonica and P. goytaca having four or five uterine branches; P. clausa and P. apodemi having two uterine branches [1, 4, 18, 32, 33, 43‒45]. The variability in the number of uterine branches among different individuals of T. turgida and different species of Physaloptera brings into question the reliability of the number of uterine branches as a generic criterion. We considered that the number of uterine branches should be treated as a specific character. The present phylogenetic results indicated that the number of uterine branches of Physaloptera species is not in relation to the relationships of species in the phylogenetic trees, because T. turgida with 7‒14 uterine branches and P. amazonica with 4 uterine branches are scattered into the other Physaloptera species with ony two uterine branches (including P. bispiculata, P. mirandai, P. retusa, P. alata, P. rara and sibirica). In contrast, our results showed that the phylogenetic relationships of Physaloptera species seem to be associated with their geographical distribution, because all species from the Neotropical region (Brazil) (including P. amazonica, P. bispiculata, P. mirandai, P. retusa and T. turgida) formed a monophyletic subclade, which displayed a sister to the other subclade constituted by the species from the Palearctic region (P. alata and P. sibirica) and Palearctic + Nearctic region (P. rara).
The present molecular phylogenies reinforced the limited knowledge pertaining to the evolution of physalopterid nematodes. However, we do not make any immediate systematic changes in the Physalopteridae, and care must be taken in using the preliminary phylogenetic results, because only a limited number of physalopterid representatives were included in the phylogeny, and many of their genetic data have not been critically evaluated. Overall, a more rigorous molecular phylogeny including species of Thubunaea and broader representatives of Turgida, Physaloptera and Physalopteroides is required to further test the systematic status of Thubunaeinae and Turgida and clarify the phylogeographic pattern of Physaloptera.
Pairwise comparison of the sequences obtained for P. sibirica revealed no genetic divergence regarding the 18S, 28S, cox1 and 12S genetic markers and a low level of divergence in the ITS (0.16%) and cox2 (2.39%) regions. The high genetic divergence observed between the present specimens and other species of Physaloptera further confirmed their distinct specific identity. The genetic data presented here will contribute to the molecular identification, population genetics and phylogenetics of physalopterid nematodes.
Conclusions
The detailed morphology of P. sibirica was further studied using light and scanning electron microscopy based on newly collected specimens from the hog badger A. collaris in China. Physaloptera sibirica is the fourth nematode parasite reported from the hog badger A. collaris, and A. collaris represents a new host for P. sibirica. The characterization of the nuclear 18S, 28S and ITS and mitochondrial cox1, cox2 and 12S sequences of P. sibirica were provided for the first time. The phylogenetic results indicated that there could be issues with the current understanding of the systematic status of the subfamily Thubunaeinae and the genus Turgida within the Physalopteridae, and more genetic data are required across the species and genera that do not yet have molecular information to further clarify the phylogenetic relationships of the three subfamilies Thubunaeinae, Physalopterinae and Proleptinae. These present findings contribute to morphologically recognizing P. sibirica more accurately and provide new insights into the systematics of the family Physalopteridae.
Availability of data and materials
The nuclear and mitochondrial DNA sequences of Physaloptera sibirica obtained in the present study were deposited in GenBank database under the accession numbers OQ846900–OQ846902 (18S), OQ846911–OQ846913 (ITS), OQ846907–OQ846909 (28S), OQ852731–OQ852733 (cox1), OQ867994–OQ867996 (cox2) and OQ846904–OQ846906 (12S). Specimens of Physaloptera sibirica were deposited in the College of Life Sciences, Hebei Normal University, Hebei Province, China, under accession number HBNU–N-2022M1120-CL.
Abbreviations
- SEM:
-
Scanning electron microscopy
- PCR:
-
Polymerase chain reaction
- ML:
-
Maximum likelihood
- BI:
-
Bayesian inference
- BIC:
-
Bayesian information criterion
- 18S:
-
Small ribosomal subunit
- 28S:
-
Large ribosomal subunit
- ITS:
-
Internal transcribed spacer
- cox1:
-
Cytochrome c oxidase subunit 1
- cox2:
-
Cytochrome c oxidase subunit 2
- 12S:
-
12S small subunit ribosomal RNA gene
References
Ortlepp RJ. The nematode genus Physaloptera Rudolphi. Proc Zool Soc London. 1922;4:999–1107.
Skrjabin KI, Sobolev AA. Principles of nematology. Vol. XII. Spirurates of animal and man and the diseases caused by them. Part II. Physalopteroidea. Izdat, AN SSSR, Moscow, Russia. 1964. p. 334.
Pereira FB, Alves PV, Rocha BM, de Souza LS, Luque JL. Physaloptera bainae n. sp. (Nematoda: Physalopteridae) parasitic in Salvator merianae (Squamata: Teiidae), with a key to Physaloptera species parasitizing reptiles from Brazil. J Parasitol. 2014;100:221–7.
Ederli NB, Gallo SSM, Oliveira LC, DeOliveira FCR. Description of a new species Physaloptera goytaca n. sp. (Nematoda, Physalopteridae) from Cerradomys goytaca Tavares, Pessôa & Goncaves, 2011 (Rodentia, Cricetidae) from Brazil. Parasitol Res. 2018;117:2757–66.
Alves PV, Couto JV, Pereira FB. Redescription of the two most recorded Physaloptera (Nematoda: Physalopteridae) parasitizing lizards in the Americas: first step towards a robust species identification framework. Syst Parasitol. 2022;99:63–81.
Chabaud AG. Keys to genera of the Order Spirurida. No. 3, Part 1. Camallanoidea; Dracunculoidea, Gnathostomatoidea. In: Anderson RC, Chabaud AG, Wilmont S (Eds). CHI keys to the nematode parasites of vertebrates. Commonweatlh Agricultural Bureaux, Famham Royal, U.K. 1975;1–27.
Chabaud AG, Bain O. The evolutionary expansion of the Spirurida. Int J Parasitol. 1994;24:1179–201.
Pereira FB, Alves PV, Rocha BM, Souza Lima S, Luque JL. A new Physaloptera (Nematoda: Physalopteridae) parasite of Tupinambis merianae (Squamata: Teiidae) from southeastern Brazil. J Parasitol. 2012;98:1227–35.
Kalyanasundaram A, Henry C, Brym MZ, Kendall RJ. Molecular identification of Physaloptera sp. from wild northern bobwhite (Colinus virginianus) in the Rolling Plains ecoregion of Texas. Parasitol Res. 2018;117:2963–9.
Matias CSL, Morais DH, Ávila RW. Physaloptera nordestina n. sp. (Nematoda: Physalopteridae) parasitizing snakes from Northeastern Brazil. Zootaxa. 2020;4766:173–80.
Helgen KM, Lim NTL, Helgen LE. The hog badger is not an edentate: systematics and evolution of the genus Arctonyx (Mammalia: Mustelidae). Zool J Linn Soc. 2008;154:353–85.
Jansen J Jr. Tetragomphius arctonycis n. sp. from the pancreatic ducts of a mustelid Arctonyx collaris. J Helminthol. 1968;42:53–6.
Sprent JFA. Toxocara vajrasthirae sp. nov. from the hog-badger (Arctonyx collaris) of Thailand. Parasitology. 1972;65:491–8.
Wu J, Chen D-M, Ma F-H. Study on nematode of Uncinaria, parasiting in Arctonyx collaris and Vulpes ferrilata. Chin J W. 1982;3:53–5 (In Chinese).
Li L, Lü L, Nadler SA, Gibson DI, Zhang L-P, Chen H-X, et al. Molecular phylogeny and dating reveal a terrestrial origin in the early carboniferous for ascaridoid nematodes. Syst Biol. 2018;67:888–900.
Ronquist F, Teslenko M, Mark PVD, Ayres DL, Darling A, Hӧhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across across a large model space. Syst Biol. 2012;61:539–42.
Nguyen LT, Schmidt HA, Haeseler A, Minh BQ. IQ-tree: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74.
Maldonado Jr A, Simões RO, Luiz JS, Costa-Neto SF, Vilela RV. A new species of Physaloptera (Nematoda: Spirurida) from Proechimys gardneri (Rodentia: Echimyidae) from the Amazon rainforest and molecular phylogenetic analyses of the genus. J Helminthol. 2019;1–11.
Maharana BR, Snehil G, Surbhi G, Ganguly A, Binod K, Chandratre GA, et al. First report of molecular and phylogenetic analysis of Physaloptera praeputialis in naturally infected stray cats from India. Parasitol Res. 2021;120:2047–56.
Posada D, Crandall KA. Selecting the best-fit model of nucleotide substitution. Syst Biol. 2001;50:580–601.
Petrow AM, Gorbunow EI. New parasite of the fox and raccoon (Physaloptera sibirica n. sp.). Soiuzpushnina. 1931;17–19:45–6 (in Russian).
Delyanova RS. Helminth fauna of dogs in the Uzbek SSR. Uzbek Biol Zh. 1958;5:47–57 (in Russian).
Tenora F, Barus V. Occasional findings of Helminths in some domestic and free living mammals in Afghanistan. Acta Univ Agric. 1968;16:327–36.
Quentin JC, Biocca E. Presence du nematode Physaloptera sibirica Petrow et Gorbunow, 1931, parasite de carnivores chez lelerot Eliomys quercinus L. Dans les Alps. Ann Parasitol Hum Comp. 1976;51:255–62.
Li Z-H, Zhu Y-P. Physaloptera sibirica Petrow & Gorbunow, 1931, a new record in China, parasitizing in the commercial animal Nyctereutes procyonoides. China Vet Sci. 1980;3:13–5 (In Chinese).
Miquel J, Segovia JM, Feliu C, Torres J. On Physaloptera sibirica Petrow et Gorbunow, 1931 (Nematoda: Physalopteridae) parasitizing Iberian mammals. Wiad Parazytol. 1996;42:435–42.
Torres J, Miquel J, Motjé M. Helminth parasites of the Eurasian badger (Meles meles L.) in Spain: a biogeographic approach. Parasitol Res. 2001;87:259–63.
Segovia JM, Torres J, Miquel J. Helminth parasites of the red fox (Vulpes vulpes L., 1758) in the Iberian Peninsula: an ecological study. Acta Parasitol. 2004;49:67–79.
Segovia JM, Torres J, Miquel J, Sospedra E, Guerrero R, Feliu C. Analysis of helminth communities of the pine marten, Martes martes, in Spain: mainland and insular data. Acta Parasitol. 2007;52:156–64.
Ferroglio E, Ragagli C, Trisciuoglio A. Physaloptera sibirica in foxes and badgers from the Western Alps (Italy). Vet Parasitol. 2009;163:164–6.
Skrjabin KI. Key to parasitic nematodes. Volume 1. Spirurata and Filariata. Jerusalem: IPST Press Binding. 1969. p. 497.
Chen H-X, Ju H-D, Li Y, Li L. Further study on Physaloptera clausa Rudolphi, 1819 (Spirurida: Physalopteridae) from the amur hedgehog Erinaceus amurensis Schrenk (Eulipotyphla: Erinaceidae). Acta Parasitol. 2017;62:846–52.
Wang Y-H, Zhang L-P. Physaloptera apodemi sp. nov. (Nematoda: Physalopteridae) from Apodemus sylvaticus (Linnaeus, 1758) (Rodentia: Muridae) from Tianjin, China. Zool Syst. 2020;45:259–65.
Yeh L-S. On Physaloptera lumsdeni n. sp. from a bush-baby in Tanganyika, with a note on Abbreviata caucasica. J Helminthol. 1957;31:29–32.
Esteban JG, Botella P, Toledo R. Redescription of Physaloptera brevivaginata Seurat, 1917 (Nematoda: Physalopteridae) from the bat Myotis blythii (Tomes) (Chiroptera: Vespertilionidae) in Spain. Syst Parasitol. 1995;32:107–12.
Wason A, Johnson S. A new physalopterid nematode from the small bat Rhinopoma microphyllum. Rev Brasil Biol. 1977;37:563–5.
Jones MES, Gibson DI. A list of old and recently erected genus-group names not included in the ‘CIH Keys’ to nematode parasites of vertebrates and invertebrates. Syst Parasitol. 1987;9:125–36.
Gibbons LM. Keys to the nematode parasites of vertebrates: supplementary volume. Cambridge: CUP; 2010. p. 416.
Lopes-Torresa EJ, Girard-Diasd W, Melloa WN, Simõesd RO, Pintoc IS, Maldonadod A, et al. Taxonomy of Physaloptera mirandai (Nematoda: Physalopteroidea) based in three-dimensional microscopy and phylogenetic positioning. Acta Trop. 2019;195:115–26.
Yorke W, Maplestone PA. The nematode parasites of vertebrates. Philadelphia Blakiston. 1927. p. 536.
Travassos L. Contribuigces para o conhecimento de fauna helmintolojica brazileira X. Sobre as especies do genero Turgida. Mem Inst Oswaldo Cruz. 1920;12:73–7.
Gray JB, Anderson RC. Development of Turgida turgida (Rudolphi, 1819) (Nematoda: Physalopteroidea) in the opossum (Didelphis virginiana). Can J Zool. 1982;60:1265–74.
Baylis HA. On a collection of nematodes from Nigerian mammals (chiefly rodents). Parasitology. 1928;20:280–304.
Canavan W. Nematode parasites of vertebrates in the Philadelphia Zoological Gardens and vicinity Part I. Parasitology. 1929;21:63–102.
Hill WC. The genus Physaloptera Rudolphi, 1819 (Nematoda: Physalopteridae). Wasmann Club Collect. 1940;4:60–70.
Floyd RM, Rogers AD, Lambshead PJD, Smith CR. Nematode-specific PCR primers for the 18S small subunit rRNA gene. Mol Ecol Notes. 2005;5:611–2.
Nadler SA, Hudspeth DSS. Ribosomal DNA and phylogeny of the Ascaridoidea (Nemata: Secernentea): implications for morphological evolution and classification. Mol Phylogenet Evol. 1998;10:221–36.
Zhu X-Q, Gasser RB, Jacobs DE, Hung G-C, Chilton NB. Relationships among some ascaridoid nematodes based on ribosomal DNA sequence data. Parasitol Res. 2000;86:738–44.
Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103.
Nadler SA, Hudspeth DSS. Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: Hypotheses of structural and sequence evolution. J Parasitol. 2000;86:380–93.
Li Y, Niu L-L, Wang Q, Zhang Z-H, Chen Z-G, Gu X-B, et al. Molecular characterization and phylogenetic analysis of ascarid nematodes from twenty-one species of captive wild mammals based on mitochondrial and nuclear sequences. Parasitology. 2012;139:1329–38.
Laidoudi Y, Medkour H, Latrofa MS, Davoust B, Diatta G, Sokhna C, et al. Zoonotic Abbreviata caucasica in Wild Chimpanzees (Pan troglodytes verus) from Senegal. Pathogens. 2020;9:517.
Thompson AT, Cleveland CA, Koser TM, Wyckoff ST, Yabsley MJ. The Occurrence of Physaloptera hispida and a Mastophorus sp. in pulmonary vessels of hispid cotton rats (Sigmodon hispidus) from Georgia, U.S.A. J Parasitol. 2019;105:718–23.
Smythe AB, Sanderson MJ, Nadler SA. Nematode small subunit phylogeny correlates with alignment parameters. Syst Biol. 2006;55:972–92.
Prosser SWJ, Velarde-Aguilar MG, León-Règagnon V, Hebert PDN. Advancing nematode barcoding: a primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes. Mol Ecol Resour. 2013;13:1108–15.
Moravec F. Some aspects of the taxonomy and biology of adult spirurine nematodes parasitic in fishes: a review. Folia Parasitol (Praha). 2007;54:239–57.
Park J-K, Sultana T, Lee S-H, Kang S, Kim HK, Min G-S, et al. Monophyly of clade III nematodes is not supported by phylogenetic analysis of complete mitochondrial genome sequences. BMC Genomics. 2011;12:392.
Tang L-S, Gu X-H, Wang J-H, Ni X-F, Zhou K-F, Li L. Morphological and molecular characterization of Paraleptus chiloscyllii Yin & Zhang, 1983 (Nematoda: Physalopteridae) from the brownbanded bambooshark Chiloscyllium punctatum Müller & Henle (Elasmobranchii: Orectolobiformes). Parasitol Int. 2022;87:e102511.
Silva C, Veríssimo A, Cardoso P, Cable J, Xavier R. Infection of the lesser spotted dogfish with Proleptus obtusus Dujardin, 1845 (Nematoda: Spirurida) reflects ontogenetic feeding behaviour and seasonal differences in prey availability. Acta Parasitol. 2017;62:471–6.
Goswami U, Chaudhary A, Verma C, Singh HS. Molecular and ultrastructure characterization of two nematodes (Thelandros scleratus and Physalopteroides dactyluris) based on ribosomal and mitochondrial DNA sequences. Helminthologia. 2016;53:165–71.
Luiz JS, Simões RO, Torres EL, Barbosa HS, Santos JN, Giese EG, et al. A new species of Physaloptera (Nematoda: Physalopteridae) from Cerradomys subflavus (Rodentia: Sigmodontinae) in the cerrado biome. Brazil Neotrop Helminthol. 2015;9:301–12.
Acknowledgements
The authors are grateful to Dr. Felipe Bisaggio Pereira (Universidade Federal de Mato Grosso do Sul, Brazil), David Gibson (Natural History Museum, UK), Professor Vitaliy Kharchenko (I. I. Schmalhausen Institute of Zoology, National Academy of Sciences of Ukraine, Ukraine) and Hideo Hasegawa (Oita University, Japan) for providing important literature.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 32170442) for Dr. Liang Li, the National Key R&D Program of China (2022YFC2601601) for Dr. Dong Zhang and the Natural Science Foundation of Hebei Province (C2021205018) and funded by Science and Technology Project of Hebei Education Department (QN2021085) for Dr. Hui-Xia Chen.
Author information
Authors and Affiliations
Contributions
HXC and LL contributed to the study design and identification of the nematode specimens. DZ and YYG provided the parasite specimens. HXC, JLZ, YL and LL analyzed morphological and genetic data. HXC and LL conducted the phylogenetic analyses and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted under the protocol of Hebei Normal University. All applicable national and international guidelines for the protection and use of animals were followed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, HX., Zeng, JL., Gao, YY. et al. Morphology and genetic characterization of Physaloptera sibirica Petrow & Gorbunov, 1931 (Spirurida: Physalopteridae), from the hog-badger Arctonyx collaris Cuvier (Carnivora: Mustelidae), with molecular phylogeny of Physalopteridae. Parasites Vectors 16, 227 (2023). https://doi.org/10.1186/s13071-023-05838-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05838-6