Abstract
Background
The isoxazoline fluralaner is effective for prevention of Babesia canis transmission from infected Dermacentor reticulatus ticks to dogs for 84 days in a controlled environment. This study was designed to evaluate the effectiveness of fluralaner chewable tablets for sustained prevention of B. canis infection of dogs in endemic areas under natural conditions.
Methods
In Europe, privately owned, clinically healthy pet dogs were enrolled and randomized either to receive fluralaner at 25–56 mg/kg (Bravecto® chewable tablets) on days 0 and 84, or to remain untreated during the D. reticulatus season. Blood samples were collected to evaluate B. canis exposure: on days 0 and 21 (exposure before day 0), during the study and at the end of the tick season (dogs suspected of having become infected after day 0). Efficacy was determined by the percentage reduction in B. canis transmission risk based on the difference in B. canis-positive tests in fluralaner-treated dogs compared with untreated dogs. In addition, ticks collected at monthly intervals throughout the study were identified to species level and females tested for B. canis DNA.
Results
A total of 152 dogs were enrolled in the study, although nine dogs were excluded because they tested positive for B. canis DNA or antibodies within 21 days after enrollment. During the study period, no fluralaner-treated dog became positive for B. canis, resulting in calculated efficacy of 100%. However, babesiosis infection was diagnosed in five untreated control dogs (Fisher’s exact test, left-sided, P = 0.0312). Tick analyses revealed that one sample collected in Hungary was infected with B. canis.
Conclusion
Oral administration of Bravecto chewable tablets at the recommended dosage to dogs completely prevented B. canis transmission under field conditions in an endemic area for 12 weeks.
Graphical Abstract

Similar content being viewed by others
Background
Infections with tick-transmitted Babesia spp. are increasingly diagnosed in both animals and people [1,2,3,4,5]. In dogs, infections can remain asymptomatic or cause multi-organ failure, with a risk of death. The wide range of clinical signs include apathy, weakness, anorexia, pale mucous membranes, fever, enlarged lymph nodes and spleen, thrombocytopenia, jaundice, pigmenturia and anaemia that is caused by a combination of intravascular and extravascular hemolysis resulting from parasite-caused rupture of red blood cells and the activity of secondary immune-mediated processes [1, 6].
In Europe, reported infections with Babesia canis align with the expanding geographical distribution of its primary vector, the ornate dog tick Dermacentor reticulatus [4,5,6,7]. Tick distribution is not homogeneous and varies between countries and between regions in each country since it is largely dependent on the habitat [8]. In 2014, the estimated annual incidence of clinical babesiosis in the Western European dog population was 0.70% (95% confidence interval 0.69–0.71%). In Central Europe, canine babesiosis-associated mortality can be as high as 20%, whereas reports in the southwest of Europe, particularly Italy, France, Spain and Portugal, may be < 5%. These differences may result from genetic diversity and variable virulence of different B. canis strains [9].
Transmission of B. canis from a tick to a susceptible host is believed to occur around 48 h following attachment [10, 11]. Therefore, infection can be prevented if an acaricide is used that causes tick paralysis and death within 24 h of the onset of tick feeding. The isoxazoline compound fluralaner has demonstrated this rapid onset of efficacy and, by providing this efficacy against D. reticulatus for 12 weeks, prevented B. canis transmission by infected ticks throughout an 84-day period under a severe laboratory challenge [12, 13]. To validate these laboratory findings under field conditions, a study was designed to evaluate the effectiveness of oral fluralaner for preventing B. canis transmission to dogs. This negatively controlled, randomized, examiner-masked and multicenter study was conducted in areas known to be endemic for B. canis during the autumn/winter tick season when adult D. reticulatus are most active [4].
Methods
The field study was conducted in three countries: Albania (Tirana), France (Soulac-sur Mer) and Hungary (Sumeg) (Fig. 1). Study procedures adhered to those described in Good Clinical Practices, VICH (International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products) Guideline 9 [14], Statistical principles for clinical trials for veterinary medicinal products (pharmaceuticals) [15], Guideline for the Demonstration of Efficacy of Ectoparasiticides [16], and draft Guideline for Vector-Borne Diseases [17]. Sites were selected based on previous reports by local veterinarians of tick infestation and canine babesiosis transmission. Enrolled pet dogs were privately owned and clinically healthy, ≥ 8 weeks old, and weighed ≥ 2 kg. Dogs had access to outdoor areas, including regular walks in tick infested areas. Dogs were excluded for the following: pregnant or lactating; treated with acaricides or protozoicides within the labeled duration of those products prior to study enrollment; vaccinated against babesiosis; or already B. canis-infected.
Routine health procedures (e.g. vaccination) and medical care were continued during the study. However, owners were instructed to not use any acaricides, protozoicides or Babesia vaccines on their dog during the course of the study, and to keep the dog on its usual management food and water supply. Each owner provided a signed informed consent form.
Enrolled dogs were randomly allocated to study groups using computer-generated randomization lists. All dogs in one group were treated on days 0 and 84 with fluralaner (Bravecto® chewable tablets for dogs, MSD Animal Health) at the label dose (25–56 mg/kg), at or around the time of feeding [18]. Dogs were weighed on a calibrated scale prior to treatment, and the chewable tablets were selected according to the recommended body weight band. Dogs in the other group were not treated with acaricides and served as negative control. Animals were kept in their normal housing conditions at the time of treatment, and two dogs from one household could be in different study groups. Grooming and bathing were not restricted during the study.
It was determined that approximately 55 dogs per study group would be required in at least two different geographical locations to provide a representative picture of the exposed dog population, allow a meaningful statistical comparison of incidence rates and minimize the number of untreated control animals due to animal welfare. With an estimated drop-out rate of approximately 9%, targeted enrollment was 60 dogs per group divided across three different countries, and no country was permitted to enroll more than 40% of the total study population.
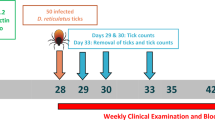
Allowing for a maximum of 21 days following infection for Babesia to become detectable, blood samples were collected on days 0 and 21 and tested for pre-existing B. canis infections, and any positive dogs were excluded [19, 20]. Additionally, throughout the study period, blood samples were taken from any dogs clinically suspected of being infected with B. canis and from all dogs 21 days after the end of tick season (determined by the complete absence of ticks on study dogs) in order to detect any evidence of B. canis infection. Blood samples were evaluated using two different methods: detection of B. canis antibodies in serum (commercial enzyme-linked immunoassay [ELISA] kit, Afosa GmbH, Blankenfelde-Mahlow, Germany) and polymerase chain reaction (PCR). PCR analysis was conducted on DNA extracted from blood samples (QIAamp DNA Mini Kit, Qiagen Hilden, Germany), and later the primers BJ1 5′–GTC TTG TAA TTG GAA TGA TGG–3′ and BN2 5′–TAG TTT ATG GTT AGG ACT ACG–3′ amplified a ~ 500-base-pair fragment of the 18S ribosomal RNA (rRNA) gene [21] for pathogen identification over a 1.5% agarose gel stained with ethidium bromide and visualized under ultraviolet light. Samples were analyzed at the Department of Parasitology and Zoology, University of Veterinary Medicine, Budapest, Hungary. The positive PCR products were purified and sequenced at the Hungarian Academy of Sciences, Biological Research Centre, Szeged, Hungary, and the sequences compared to the National Center for Biotechnology Information (NCBI) Nucleotide database.
All study assessments were completed by examiners masked to treatment group. Dogs were observed daily by their owners for abnormal clinical signs, and if any were observed, an examiner was informed immediately to visit, perform a complete physical exam and take a blood sample. Any dog testing positive for B. canis infection was removed from the study and administered rescue treatment.
Study areas were flagged monthly, and collected ticks were stored in a labeled tube containing 80% ethanol for later species, gender and life stage identification at the University of Veterinary Medicine in Budapest, Hungary. Each specimen was identified at species level based on its morphology [22, 23]. The same PCR method described previously for blood analysis was used on pooled female ticks (maximum 10 ticks per sample) collected at each site and time point to detect B. canis.
Treatment efficacy was determined from the percentage reduction of the risk of B. canis transmission based on the incidence rate in each treatment group:
where the incidence rate in each study group was the number of B. canis-infected dogs in relation to the total number of dogs. Treatment efficacy was considered to have been achieved if the percentage reduction of transmission risk was ≥ 90% [17].
The superiority of fluralaner treatment compared to the untreated control was investigated using Fisher’s exact test (one-sided) with the level of significance set to α = 0.025.
Reduction of transmission risk was determined from the incidence density rate in the untreated control group and the incidence density rate in the fluralaner group using the equation:
where the incidence rate in each study group was calculated on a monthly basis as follows:
Results and discussion
Study recruitment began in September 2019 with a total of 152 dogs enrolled: 52 in Hungary, 50 in Albania and 50 in France. Twenty dogs were subsequently excluded from the assessment: nine because of a positive B. canis antibody titer within the first 21 days (i.e. pre-study infection), three that died from dog bite wounds, seven that were lost to follow-up and one untreated control dog that had a concomitant infection manifested by lethargy, increased temperature and conjunctivitis. Of the nine dogs removed because of asymptomatic pre-study B. canis infection, three were from Albania, one was from France and five were from Hungary; confirming a history of canine babesiosis in these areas as informed by the local veterinarians, eight dogs were positive on day 0 tests, and one untreated dog was positive on day 21. There were no treatment-related adverse events and the study concluded in February 2020 in Hungary (day 168) and France (day 167), and in March 2020 in Albania (day 182).
Age (4 months to 13 years), body weight (4.6–51.3 kgs), and gender (approximately 65% males and 35% females) were similar between groups. The most common breed in the study was the Great Anglo-French Tricolor Hound (approximately 40% of dogs in each group), and 26.2% of dogs were described as mixed breed in the treated group and 14.9% in the control group.
All fluralaner-treated dogs remained free of B. canis infection throughout the study (Table 1). In October 2019, five control group dogs developed clinical signs of babesiosis, including anorexia, icterus, hemoglobinuria, increased body temperature and conjunctivitis. Each of these dogs was confirmed by PCR, based on the sequences, to be infected with B. canis, further confirmed by a positive antibody test, resulting in removal from the study. All affected dogs recovered clinically following treatment with imidocarb, dexamethasone and amoxicillin. All countries had B. canis-infected dogs prior to day 21, and all B. canis-positive tests after this date were from Hungary. All dogs sampled on the final day of the study were test-negative for B. canis.
Fluralaner treatment was 100% effective at reducing the risk of B. canis transmission at the end of the study period, and thus above the required ≥ 90% threshold established by the guideline for vector-borne disease [17]. Across the three countries, the low B. canis infection rate in the untreated control group (7.5%) resulted in a calculated P-value (Fisher’s exact test, left-sided, P = 0.0312) slightly above the predetermined superiority threshold (0.025). The efficacy of 100% did not allow a meaningful calculation of confidence limits for the difference in proportions.
In Hungary, the B. canis incidence rate in fluralaner-treated and untreated control dogs was 0% and 25%, respectively. The calculated P-value for this difference (Fisher’s exact test, left-sided, P = 0.0236) is significant (Table 1). Overall, the incidence density rate was 0.0 for fluralaner treatment and 1.4 for the untreated group, resulting in a 100% calculated risk reduction.
Dermacentor reticulatus ticks were collected from all three countries and were found in Hungary throughout the entire study period, with a peak in early October and November (Table 2), coinciding with the appearance of clinical B. canis infection in five untreated dogs. One tested tick sample from Hungary, collected in October, was positive for B. canis. It is likely that all ticks had some risk of carrying B. canis, and study dogs faced a constant risk of transmission. In the Czech Republic, 2.8% of ticks, and in Eastern Poland 3% of D. reticulatus ticks tested positive for B. canis, an infection frequency that confirms circulation of B. canis through the D. reticulatus population [18, 19]. The pathogen challenge level will vary under the natural conditions of a field study depending on the presence and quantity of the vector and the vector-borne pathogen. Despite the known incidence of B. canis in the selected areas of France and Albania among local practitioners, the enrolled dogs did not contract the infection during the study.
Seasonal data for D. reticulatus activity indicate that dog owners should be advised to protect their dogs with acaricides throughout the year [19]. The 12-week duration of acaricidal efficacy provided by fluralaner chewable tablets has been linked to improved owner compliance with acaricidal protection recommendations, effectively providing more months per year of coverage, relative to monthly administered acaricides [24]. The speed of tick kill and the duration of effect help to explain the 100% protection fluralaner provided against B. canis infection under the field conditions of this study.
In conclusion, the study demonstrated that B. canis is present in D. reticulatus ticks in Albania, France and Hungary, and that oral fluralaner administration results in 100% reduction of the risk of transmission of B. canis for 12 weeks.
Availability of data and materials
Data from this study are proprietary and are maintained by MSD Animal Health.
References
Irwin PJ. Canine babesiosis: from molecular taxonomy to control. Parasit Vectors. 2009;2:S4.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314.
Krause PJ. Human babesiosis. Int J Parasitol. 2019;49:165–74.
Leschnik M. Focus on common small animal vector-borne diseases in central and southeastern Europe. Acta Vet-Beograd. 2020;70:147–69.
Bajer A, Beck A, Beck R, Behnke JM, Dwużnik-Szarek D, Eichenberger RM, et al. Babesiosis in Southeastern, Central and Northeastern Europe: an emerging and re-emerging tick-borne disease of humans and animals. Microorganisms. 2022;10:945.
Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336.
Mathé A, Vörös K, Papp L, Reiczigel J. Clinical manifestations of canine babesiosis in Hungary (63 cases). Acta Vet Hung. 2006;54:367–85.
Halos L, Lebert I, Abrial D, Danlois F, Garzik K, Rodes D, et al. Questionnaire-based survey on the distribution and incidence of canine babesiosis in countries of Western Europe. Parasite. 2014;21:13.
Carcy B, Randazzo S, Depoix D, Adaszek L, Cardoso L, Baneth G, et al. Classification of Babesia canis strains in Europe based on polymorphism of the Bc28.1—gene from the Babesia canis Bc28 multigene family. Vet Parasitol. 2015;211:111–23.
Schein E, Mehlhorn H, Voigt WP. Electron microscopical studies on the development of Babesia canis (Sporozoa) in the salivary glands of the vector tick Dermacentor reticulatus. Acta Trop. 1979;36:229–41.
Little SE. Changing paradigms in understanding transmission of canine tick-borne diseases: the role of interrupted feeding and intrastadial transmission. In: 2nd Canine Vector-Borne Disease (CVBD) Symposium. Mazara del Vallo, Sicily, Italy. 2007. p. 30–4.
Taenzler J, Liebenberg J, Roepke RK, Heckeroth AR. Prevention of transmission of Babesia canis by Dermacentor reticulatus ticks to dogs after topical administration of fluralaner spot-on solution. Parasit Vectors. 2016;9:234.
Taenzler J, Liebenberg J, Roepke RK, Heckeroth AR. Prevention of transmission of Babesia canis by Dermacentor reticulatus ticks to dogs treated orally with fluralaner chewable tablets (Bravecto™). Parasit Vectors. 2015;8:305.
EMEA. VICH Topic GL9 (GCP). Guideline on Good Clinical Practices. The European Agency for the Evaluation of Medicinal Products (EMEA/CVMP/VICH/595/98-Final). 2000. https://www.ema.europa.eu/en/vich-gl9-good-clinical-practices. Accessed 5 Mar 2022.
European Medicines Agency. Guideline on statistical principles for veterinary clinical trials (EMA/CVMP/EWP/81976/2010). 2010. https://www.ema.europa.eu/en/statistical-principles-clinical-trials-veterinary-medicinal-products-pharmaceuticals#main-content. Accessed 5 Mar 2022.
European Medicines Agency. Demonstration of efficacy of ectoparasiticides (7AE17a). 1994. https://www.ema.europa.eu/en/demonstration-efficacy-ectoparasiticides. Accessed 5 Mar 2022.
European Medicines Agency. Guideline on data requirements for veterinary medicinal 5 products for the prevention of transmission of vector-borne diseases in dogs and cats 7 Draft. 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-data-requirements-veterinary-medicinal-products-prevention-transmission-vector-borne_en.pdf. Accessed 5 Nov 2022.
European Medicines Agency. Bravecto (fluralaner): an overview of Bravecto and why it is authorised in the EU. https://www.ema.europa.eu/en/documents/overview/bravecto-epar-medicine-overview_en.pdf. Accessed 6 Mar 2022.
Daněk O, Hrazdilová K, Kozderková D, Jirků D, Modrý D. The distribution of Dermacentor reticulatus in the Czech Republic re-assessed: citizen science approach to understanding the current distribution of the Babesia canis vector. Parasit Vectors. 2022;15:132.
Dwużnik-Szarek D, Mierzejewska EJ, Rodo A, Goździk K, Behnke-Borowczyk J, Kiewra D, et al. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasit Vectors. 2021;14:267.
Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65–70.
Hillyard PD. Ticks of north-west Europe. In: Barnes RSK, Crothers JH, editors. Synopses of the British Fauna (New Series), vol. 52. Shrewsbury: Field Studies Council; 1996.
Estrada-Peña A, Bouattour A, Camicas J-L, Walker AR. Ticks of domestic animals in the Mediterranean region: a guide to identification of species. Zaragoza: University of Zaragoza; 2004.
Lavan RP, Armstrong R, Newbury H, Normile D, Hubinois C. Flea and tick treatment satisfaction, preference, and adherence reported by cat owners in the US, UK, or France who treated their cats with transdermal fluralaner. Open Vet J. 2021;11:458–67.
Acknowledgements
The authors would like to thank Emmanuel Thomas, who retired before manuscript preparation, for the support during the study, and Sabrina Fuhrmann for conducting the data management.
Funding
The study was funded by MSD Animal Health.
Author information
Authors and Affiliations
Contributions
The study design, protocol and report of the study were prepared by RC, ET and EZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in compliance with local and national regulations. All dog owners (or delegates) were required to sign an informed consent form prior to any dog being enrolled into the study.
Consent for publication
Not applicable.
Competing interests
RC and EZ are employees of MSD Animal Health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chiummo, R., Zschiesche, E., Capári, B. et al. Field efficacy of fluralaner (Bravecto® chewable tablets) for preventing Babesia canis infection transmitted by Dermacentor reticulatus ticks to dogs. Parasites Vectors 16, 252 (2023). https://doi.org/10.1186/s13071-023-05820-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05820-2