Abstract
Background
The subgenus Pholeoixodes contains Ixodes species typically associated with birds that nest in cavities or with carnivorous mammals that are burrow-dwelling. Among ticks infesting the latter, Ixodes rugicollis is regarded as the rarest species in the western Palearctic. Despite the unique morphology of this species, its identification (especially of subadult stages) is difficult, and molecular-phylogenetic data to offer other diagnostic methods and a better understanding of its taxonomy are not available.
Methods
In this study, a female and a male of I. rugicollis were collected in Romania. The female was compared morphologically to another female of this species collected in France and to the lectotype of Ixodes cornutus (from Tajikistan), which has similar morphology and host association. Following DNA extraction, two mitochondrial (cytochrome c oxidase subunit I: cox1 and the 16S rRNA gene) and two nuclear genetic markers (18S and 28S rRNA genes) of I. rugicollis were amplified and analyzed in a phylogenetic context.
Results
Females of I. rugicollis and I. cornutus differed in the shape of their palps, scutum and areae porosae and the size of peritremes, but they were similar in palpal setal length, dental formula and arrangement of anal setae. Measurements of two I. rugicollis females examined were not less different from each other than from I. cornutus. Phylogenetically, I. rugicollis clustered with other members of its subgenus. The topology of all trees showed the position of bat-associated tick species of the subgenus Eschatocephalus among Pholeoixodes species.
Conclusions
For the first time to our knowledge, this study provides high-resolution digital pictures of male and female I. rugicollis as well as corresponding molecular data. Morphological comparison of this species with I. cornutus could not resolve uncertainties in the validity of the latter species, which can only be accomplished after collecting new specimens of I. cornutus and consequent molecular comparisons. This study includes the first comprehensive molecular-phylogenetic analysis of western Palearctic Pholeoixodes species based on both nuclear and mitochondrial genetic markers and including I. rugicollis. The results of these confirm the phylogenetic position of subgenus Eschatocephalus within Pholeoixodes, justifying the need to merge them to comply with the taxonomic criterion of monophyly.
Graphical Abstract

Similar content being viewed by others
Background
Among hard ticks (Acari: Ixodidae), the genus Ixodes Latreille, 1795, contains the highest number of valid species [1], and as many as eight of its subgenera occur in the western Palearctic [2]. The subgenus Pholeoixodes Schulze, 1942, was established based on common morphological features of its members [3], as exemplified by the relatively short palps, absence of auriculae and subapical dorsal hump on the tarsi [4, 5]. Pholeoixodes species also share ecological traits as they are usually associated with burrow-dwelling mammals and terrestrial birds that nest in cavities (tree holes or burrows). Species of this subgenus infesting mammals, particularly carnivores (mainly Canidae, Mustelidae) and hedgehogs (Erinaceidae), in the western Palearctic include Ixodes canisuga Johnston, 1849, I. kaiseri Arthur, 1957, I. crenulatus Koch, 1844, I. hexagonus Leach, 1815, and I. rugicollis Schulze & Schlottke, 1929. Although during the past few years morphological keys were published to differentiate adults and developmental stages of this subgenus [6, 7], identification of western Palearctic Pholeoixodes species remains a difficult task for even experienced taxonomists.
This is well reflected by diagnostic uncertainties related to the most scarcely collected and least studied species of this subgenus in Europe, i.e. I. rugicollis. This species was reported from stone martens (Martes foina) and red foxes (Vulpes vulpes) in France [8], pine martens (Martes martes) in Germany [9], dogs and cats in Poland [10], stone martens (M. foina) in Switzerland [11] and Austria [12] and European polecat (Mustela putorius) in Romania [13]. Historically, even I. rugicollis adults were problematic to recognize, because in the original illustration [9] and later drawings [14], the characteristic frontal projections are not shown, and its proposed synonymy with I. cornutus Lotozky, 1956 [15], also led to misidentification [16]. Regarding developmental stages, when I. rugicollis was reported and probably correctly identified in Austria [12], the criteria of morphological recognition were not mentioned, and the picture provided by the authors does not show the most important diagnostic feature of nymphs, i.e. that the sides of basis capituli are parallel [8, 17]. These difficulties could be prevented if molecular identification of I. rugicollis were to become possible, but until this study there has been no sequence of this species available in GenBank.
The phylogenetic relationships of western Palearctic Pholeoixodes species were also reported, and it was shown that this subgenus is only monophyletic if containing bat-associated ticks of the subgenus Eschatocephalus [6]. However, these results were based exclusively on mitochondrial markers and did not include the rare species I. rugicollis. Therefore, considering the above, the aims of the present study were threefold: (i) to obtain a barcoding sequence of I. rugicollis and thus to prevent further difficulties in its morphological identification, especially in case of subadult stages; (ii) to provide and to evaluate the most complex phylogeny of western Palearctic Pholeoixodes ticks based on two mitochondrial and two nuclear markers, now including I. rugicollis; (iii) to investigate the morphology of the latter species compared with I. cornutus.
Methods
Sample collection and morphological identification
The most important sample that served as the initiative for this study, I. rugicollis (mating female and male), were collected in Fersig, Romania, from a stone (or beech) marten (M. foina) in January 2018. In addition, measurements of an I. rugicollis female from the collection of P. C. Morel were also performed (removed from M. foina in Dommartin, France, on March 27, 1973). Further Pholeoixodes ticks used for DNA extraction and cox1 (cytochrome c oxidase subunit I), 16S, 18S and 28S rRNA gene PCR analyses were as follows: Ixodes arboricola (nymph collected from Parus major in Ócsa, Hungary) and I. lividus (female collected from Riparia riparia in Ócsa, Hungary). In the latter analyses two further species were also included: Ixodes ricinus (female collected from the vegetation in Sümeg, Hungary) and I. trianguliceps (larva collected from Myodes glareolus in the Leningrad region, Russia).
All ticks were stored in 96% ethanol, and their species were morphologically identified according to standard keys (I. rugicollis female: [8]; I. rugicollis male: [18]; other species: [19]). In addition, the morphology of the syntype (female) of I. rugicollis was also studied previously by the authors [6], and this was considered here for its morphological identification. Pictures and measurements of I. rugicollis were made with a VHX-5000 digital microscope (Keyence Co., Osaka, Japan) while ensuring the appropriate angle of view, i.e. perpendicular to the surface under evaluation. In addition, the type specimen (lectotype) of I. cornutus (female collected from Mustela erminea in Tajikistan) was studied with an Altami B151060063 binocular microscope (OOO Altami, Russia) and a Levenhuk M 1400 plus digital camera (Levenhuk, Inc., USA) at the Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia. The description of this species was translated, and the drawings are taken from Filippova [20] (Additional File 1).
DNA extraction
Tick surfaces were disinfected with sequential washing in 10% sodium-hypochlorite, tap water and distilled water. DNA was extracted with the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions, including an overnight digestion in tissue lysis buffer and Proteinase K at 56 °C. An extraction control (tissue lysis buffer) was also processed in each set of tick samples to monitor cross-contamination. Additional DNA extracts from previous studies used for 18S and 28S rRNA gene PCRs and phylogenetic analyses are as follows: Ixodes vespertilionis and I. ariadnae (collected from Rhinolophus ferrumequinum and cave wall in Pilis Mountains, respectively, in Hungary: [21]) as well as I. frontalis and I. acuminatus (collected from birds, Erithacus rubecula and Anthus pratensis, respectively, in Malta: [22]).
Molecular taxonomic analyses
An approximately 710-bp-long fragment of the cox1 gene was amplified with a conventional PCR modified from Folmer et al. [23]. The primers HCO2198 (5ʹ-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) and LCO1490 (5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′) were used in a reaction volume of 25 µl, containing 1 U (0.2 µl) HotStarTaq Plus DNA polymerase, 2.5 µl 10 × CoralLoad Reaction buffer (including 15 mM MgCl2), 0.5 µl PCR nucleotide mix (0.2 mM each), 0.5 µl (1 µM final concentration) of each primer, 15.8 µl ddH2O and 5 µl template DNA. During the amplification, the initial denaturation step at 95 °C for 5 min was followed by 40 cycles of denaturation at 94 °C for 40 s, annealing at 48 °C for 1 min and extension at 72 °C for 1 min. Final extension was performed at 72 °C for 10 min.
Another PCR was used to amplify an approximately 460-bp fragment of the 16S rDNA gene of Ixodidae [24], with the primers 16S + 1 (5′-CTG CTC AAT GAT TTT TTA AAT TGC TGT GG-3ʹ) and 16S-1 (5ʹ-CCG GTC TGA ACT CAG ATC AAG T-3′). Reaction components and cycling conditions were the same as above, except for annealing at 51 °C. In addition, two nuclear genetic markers were also amplified: an approximately 1700-bp-long fragment of the 18S rRNA gene with the primers NS1 (5′-GTA GTC ATA TGC TTG TCT C-3′) and NS4a (5′-GCC CTT CCG TCA ATT CCT TTA AG-3′) [25] as well as an approximately 700-bp-long fragment of the 28S rRNA gene with the primers 28ScF (5′-GTG GTA GCC AAA TGC CTC GTC ATC-3′) and 28SR (5′-GAA TTC TGC TTC ACA ATG ATA GGA AGA GCC-3′) as reported [26].
PCR controls, sequencing and phylogenetic analyses
In all PCRs, non-template reaction mixture served as negative control. Extraction and negative controls remained PCR negative in all tests. Purification and sequencing of the PCR products were done by Biomi Ltd. (Gödöllő, Hungary). Quality control and trimming of sequences were performed with the BioEdit program. Obtained sequences were compared to GenBank data by the nucleotide BLASTN program (https://blast.ncbi.nlm.nih.gov). New sequences were submitted to GenBank (cox1 gene: OP997945 for I. rugicollis and OP997946 for I. arboricola, 16S rRNA gene: OP998019 for I. rugicollis, 18S rRNA gene: OP998033-OP998044, 28S rRNA gene: OP998050-OP998063). Sequences from other studies (retrieved from GenBank) included in the phylogenetic analyses had nearly 100% coverage with sequences from this study. In the cox1 and 16S rRNA gene phylogenetic analyses, unrooted trees were made for evaluating interspecific relationships. For this purpose, all sequences of Ixodes canisuga, I. kaiseri and I. hexagonus were used from our previous study [6]. In the phylogenetic analyses of nuclear markers (18S and 28S rRNA genes) Ixodes (Ceratixodes) uriae and I. (Exopalpiger) trianguliceps were used as outgroups. Sequence datasets were resampled 1000 times to generate bootstrap values. Initial phylogenetic analyses were conducted with the neighbor-joining method using p-distances and maximum likelihood method with Jukes-Cantor model by the MEGA version 7.0 program [27].
Results
Morphology of I. rugicollis and its comparison with I. cornutus
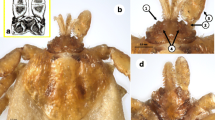
The female tick from M. foina was morphologically identified as I. rugicollis according to the frontal projections and small, well-separated porose areas on the basis capituli as well as the uniformly wrinkled (rugose) surface of the scutum and palps, the latter with a narrow “stalk” (Fig. 1). The male mating with this female was also identified as I. rugicollis based on the wrinkled surface of palps, small external spur on each coxa and the presence of short setae below the auricular ridge ventrally on the basis capituli (Fig. 2).
Key morphological characters of Ixodes rugicollis female, dry mounting: A dorsal view of basis capituli, hypostome and palp (asterisk and blue arrow mark the frontal protuberance); B dorsal view of scutum and basis capituli (dashed line marks maximum width of the scutum); C ventral view of basis capituli, hypostome and palp (dashed line separates the apical quarter of hypostome with dental formula higher than 2/2); D coxae. Numbers 1–8 mark structures of diagnostic importance described in Table 1
Key morphological characters of Ixodes rugicollis male, A wet and B–D dry mounting: A habitus, dorsal view; B ventral view of coxae (yellow arrows mark short internal spur on coxae I); C dorsal view of basis capituli and palps (yellow arrow indicates wrinkled surface of palp); D ventral view of basis capituli, hypostome and palps (yellow arrow indicates short hairs behind the ridge replacing auriculae)
The female of I. rugicollis and the lectotype of I. cornutus were also compared morphologically. In several characters relevant to recognize the subgenus Pholeoixodes in general, and its two members in particular, they were different (as exemplified by the shape of the palps, scutum and areae porosae), but at the same time other diagnostic characters, such as the shortness of palpal setae, dental formula and arrangement of anal setae, were similar for both (Table 1; Additional file 2, 3). Regarding measurements of the two I. rugicollis females and the lectotype of I. cornutus, the length, width and index (length-to-width ratio) of the scutum, basis capituli and palps anterior to their “stalk” showed minor differences between the two species, but also between the two conspecific females (Additional file 4). On the other hand, the peritremes of I. rugicollis females were < 200 μm (Additional file 3B), in contrast to those of I. cornutus measuring nearly 300 μm (Additional file 1).
Molecular-phylogenetic relationships of I. rugicollis and other western Palearctic Pholeoixodes species
The amplified part of the cox1 gene was identical between the female and male of I. rugicollis, showing the highest but only 85.6% (482/563 bp) sequence identity to that of I. vespertilionis reported from France (KR902757). Among Palearctic Pholeoixodes species, the 16S rRNA gene of I. rugicollis had the highest sequence identity, 89.1% (361/405 bp), to I. hexagonus from Croatia available in GenBank (KY962076). The 18S rRNA sequence of I. rugicollis differed from that of I. hexagonus (JN018307) in seven positions but only in six positions when compared to I. ricinus from France (GU074648) (meaning 1026/1033 or 1027/1033 bp, i.e. 99.3 or 99.4% identities, respectively). The 28S rRNA sequence of I. rugicollis was most similar to that of I. hexagonus (JN018404: 599/600 bp = 99.8% identity), followed by I. simplex (KY457498: 596/600 bp = 99.3% identity).
In the phylogenetic analyses of both mitochondrial (Fig. 3) and nuclear markers (Additional file 5), I. rugicollis clustered with other representatives of the subgenus Pholeoixodes. In the cox1 analysis I. rugicollis was a sister species to I. hexagonus and I. kaiseri (Fig. 3A), in the 28S rRNA tree to I. simplex (Additional file 5B), but in none of the four phylogenetic trees to I. canisuga. The topology of all trees showed the position of bat-associated tick species of the subgenus Eschatocephalus among Pholeoixodes species. Interestingly, in the 16S rRNA analysis, the subgenus Eschatocephalus was paraphyletic (Fig. 3B), and in the 28S rRNA phylogenetic tree, although with low support, it was also not monophyletic (Additional file 5B). In the 18S rRNA phylogenetic analysis, I. rugicollis belonged to a clade including Trichotoixodes species and members of the I. ricinus complex (Additional file 5A) and this was relatively well supported (86%).
Phylogenetic tree of Ixodes species based on A cox1 and B 16S rRNA gene sequences. In each row of individual sequences, the country of origin and GenBank accession number are shown after the species name. For Pholeoixodes species of carnivores and I. vespertilionis, all sequences from [6], were included, and their branches are shown collapsed with separate color and triangle at the end. Ixodes rugicollis is marked with a red branch and all sequences from this study with red fonts and maroon accession numbers. The subgenus Pholeoixodes is surrounded by blue dashed line, whereas Eschatocephalus species are marked with a green dot on their branch. The evolutionary history was inferred by using the maximum likelihood method based on the Jukes-Cantor model. The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying neighbor-join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 59 and 43 nucleotide sequences for the cox1 and 16S rRNA genes, and there were a total of 564 and 363 positions in the final dataset, respectively. All positions containing gaps and missing data were eliminated
Discussion
This study provides high-resolution digital pictures of I. rugicollis and corresponding molecular data. The importance of this can only be assessed when considering that I. rugicollis is the most scarcely collected and least studied western Palearctic Pholeoixodes species. Males of I. rugicollis are especially rare, missing from the collections listed in the redescription of female and developmental stages of this species from France and other countries [8]. Accordingly, the male was described elsewhere [18]. This study also contains, for the first time to our knowledge, high-resolution pictures of the male of I. rugicollis and thus can hopefully aid the appropriate recognition of this sex in future studies.
The frontal projections of the basis capituli are regarded as the most important characters of I. rugicollis females. However, the capitulum of another western Palearctic species of its subgenus, I. canisuga, may also resemble this character, because margins of the flattened, plateau-like frons around the hypostomal base can be elevated [16]. In addition, the wrinkling of the lateral fields of the scutum was also reported in I. canisuga [6], confirming the need for molecular barcoding of I. rugicollis for which the present study provided sequences of four genetic markers. This is even more desirable for the identification of subadult stages to prevent uncertainties in their reports [12].
Ixodes rugicollis is known to occur exclusively in Europe. However, the presence of frontal projections on the basis capituli of females of I. rugicollis is shared with I. cornutus reported from central Asia. The description and illustration of I. cornutus are based on a female lectotype collected from Mustela erminea in Tajikistan ([20]). Although the synonymy of I. rugicollis and I. cornutus was proposed [15], most taxonomical sources maintained the status of the latter species as provisionally valid [1, 28, 29]. Here, for the first time to our knowledge, the morphology of these two species was compared in detail. Based on the results, I. rugicollis and I. cornutus share several diagnostically important features, including the frontal projections, wrinkled (rugose) surface of the scutum and palps, hypostome dentition and arrangement of anal valve setae. Although the latter character is frequently neglected in the differential diagnosis of ixodid species, it was shown to be different between I. rugicollis and the closely related species I. crenulatus [14].
At the same time, other characters are apparently different between these two species, as exemplified by the shape of palps (changing if not perpendicularly viewed), areae porose (which are difficult to see because of the high degree of sclerotization) and scutal index. However, the latter two characters are strongly influenced by (1) individual variation, as shown here between two I. rugicollis females, (2) the existence of different morphotypes within the same Pholeoixodes species [6] and (3) the angle of view (sometimes difficult to assess) and (4) even the state of engorgement in case of the scutal index [30]. Unfortunately, no better quality pictures of I. cornutus lectotype could be made, and its further morphological examination is currently not possible. These uncertainties do not allow to draw a final conclusion on the validity of I. cornutus as a separate species, and a molecular comparison with I. rugicollis will be inevitable in the future when access to suitable, recently collected material for such investigation is possible.
The existence of two morphologically similar tick species with overlapping host spectra (i.e. Mustelidae for both I. rugicollis and I. cornutus) probably cannot be explained by association with different host subfamilies (Martinae vs. Mustelinae, respectively), particularly because I. rugicollis was also reported from Canidae, Felidae and Mustelinae. Another member of its subgenus (Pholeoixodes), the fox tick (I. kaiseri), was reported to be genetically very similar between distant continental regions of central Europe and central Asia [31], probably because fox populations are confluent between these regions [32]. The only known host of I. cornutus, the stoat (M. erminea), has a broad geographical range in Eurasia. Although they are sympatric with other mustelids, such as the common weasel (M. nivalis), they typically occupy different habitats (wetlands vs. grasslands and forests: [33]). Therefore, more data on the host spectrum and geographical range of I. cornutus are necessary to elucidate whether it occurs in sympatry with I. rugicollis and whether their host associations are habitat-dependent.
This study provides the first comprehensive molecular and phylogenetic analyses of western Palearctic Pholeoixodes species based on both nuclear and mitochondrial genetic markers and including the rare species I. rugicollis. Prior to this study only one 18S and two 28S rRNA sequences of western Palearctic Pholeoixodes species were available for comparison in GenBank, and now this is extended to all valid species except I. crenulatus. Based on the mitochondrial cox1 and 16S rRNA genes, it was already proposed that this subgenus is not monophyletic unless including bat-associated tick species of the subgenus Eschatocephalus [6]. This was confirmed here using nuclear genetic markers. In addition, it is interesting to note that in none of the four phylogenetic trees was I. rugicollis a sister species to I. canisuga, which appears to be the most closely related Pholeoixodes species in both its morphology and host range.
Although both mitochondrial and nuclear markers chosen in this study for molecular and phylogenetic analyses are well established and widely used genetic markers in tick systematics [25, 34], the relationships within the subgenus Pholeoixodes were only supported by low to moderately high bootstrap values. Thus, the results also justify the future need to investigate more resolutive molecular markers, for example the whole mitogenome [35], in combination with nuclear markers.
Availability of data and materials
The sequences obtained during this study are deposited in GenBank under the following accession numbers: cox1 gene: OP997945 for I. rugicollis and OP997946 for I. arboricola, 16S rRNA gene: OP998019 for I. rugicollis and 18S rRNA gene: OP998033-OP998044, 28S rRNA gene: OP998050-OP998063. All other relevant data are included in the manuscript and the references or are available upon request by the corresponding author.
Abbreviations
- ZIN-RAS:
-
Zoological Institute of the Russian Academy of Sciences
- cox1:
-
Cytochrome c oxidase subunit I
References
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG. The hard ticks of the world. Dordrecht: Springer; 2014. p. 738.
Estrada-Peña A, Pfäffle M, Baneth G, Kleinerman G, Petney TN. Ixodoidea of the western palaearctic: a review of available literature for identification of species. Ticks Tick Borne Dis. 2017;8:512–25.
Schulze P. Die morphologische bedeutung des afters und seiner umgebung bei den zecken. Zschr Morphol Ökol Tiere. 1942;38:630–58.
Clifford CM, Sonenshine DE, Keirans JE, Kohls GM. Systematics of the subfamily ixodinae (Acarina: Ixodidae). 1. The subgenera of Ixodes. Ann Entomol Soc Am. 1973;66:489–500. https://doi.org/10.1093/aesa/66.3.489.
Pérez-Eid C. Les tiques Identification, biologie, importance médicale et vétérinaire. Coll. Monographies de microbiologie. Paris: Lavoisier; 2007.
Hornok S, Sándor AD, Beck R, Farkas R, Beati L, Kontschán J, et al. Contributions to the phylogeny of Ixodes (Pholeoixodes) canisuga, I. (Ph.) kaiseri, I. (Ph.) hexagonus and a simple pictorial key for the identification of their females. Parasit Vectors. 2017;10:545. https://doi.org/10.1186/s13071-017-2424-x.
Hornok S, Meyer-Kayser E, Kontschán J, Takács N, Plantard O, Cullen S, et al. Morphology of Pholeoixodes species associated with carnivores in the western palearctic: pictorial key based on molecularly identified Ixodes (Ph.) canisuga, I. (Ph.) hexagonus and I. (Ph.) kaiseri males, nymphs and larvae. Ticks Tick Borne Dis. 2021;12:101715. https://doi.org/10.1016/j.ttbdis.2021.101715.
Morel PC, Aubert MFA. Contribution à la connaissance de Pholeoixodes rugicollis (Schulze et Schlottke, 1929) (Acariens, Ixodina). Cahiers ORSTOM Série Entomologie Médicale et Parasitologie. 1975;13:99–109.
Schulze P, Schlottke E. Kleinhohlenbewohnende deutsche Zecken mit Beschreibung dreiel neuer Bauhohlenbruter und einer Bestimmungstabelle der deutschen Ixodes. Sitz Bey Abhandl NafwJ Ges Rostock. 1929;1927–1929:95–110.
Siuda K, Nowak M, Gierczak M. Confirmation of the occurrence of Ixodes (Pholeoixodes) rugicollis Schule et Schlottke, 1929 (Acari: Ixodidae) in Poland, including the morphological description and diagnostic features of this species. Wiad Parazytol. 2010;56:77–80.
Zimmerli J. Etude des parasites de la fouine (Martes foina) dans le canton de Vaud durant la periode 1980–1981. Schweiz Arch Tierheilk. 1982;124:419–22.
Visser M, Messner C, Rehbein S. Massive infestation with fur mites (Lynxacarus mustelae) of a stone marten (Martes foina) from Tyrol. Wien Klin Wochenschr. 2011;123:36–42. https://doi.org/10.1007/s00508-011-0005-0.
Feider Z. O nouă contribuţie la cunoaşterea ixodidelor din R.P.R., studii și cercetări ştiinţifice. Filiala Iaşi A Academiei RPR. 1959;10:29–38.
Feider Z. Fauna republicii populare romane. arachnida, vol. 5, fasc. 2. acaromorpha, suprafamilie ixodoidea (capuse). academiei republicii populare romane, bucuresti. Amsterdam: Elsevier; 1965.
Camicas JL, Hervy JP, Adam F, Morel PC. Les tiques du monde Nomenclature, stades décrits, hôtes, répartition (Acarida, Ixodida). Paris: Orstom; 1998.
Hornok S, Trauttwein K, Takács N, Hodžić A, Duscher GG, Kontschán J. Molecular analysis of Ixodes rugicollis, Candidatus Neoehrlichia sp. (FU98) and a novel Babesia genotype from a European badger (Meles meles). Ticks Tick Borne Dis. 2017;8:41–4. https://doi.org/10.1016/j.ttbdis.2016.09.014.
Aubert MFA, Cordonnier JL. Comparative scanning electron microscope studies on Pholeoixodes rugicollis (Schulze et Schlottke, 1929). Folia Parasitol. 1979;26:179–80.
Aubert MFA. Description du mâle de Pholeoixodes rugicollis (Schulze et Schlottke, 1929) (Acariens, Ixodina). Ann Parasitol Hum Comp. 1977;52:481–90.
Estrada-Peña A, Mihalca AD, Petney TN. Ticks of Europe and North Africa: a guide to species identification. New York: Springer International Publishing; 2017.
Filippova NA. 1977 Ixodes cornutus. In: Ixodid Ticks of the Subfamily Ixodinae. Fauna SSSR, Paukoobraznye 4. Nauka, Leningrad.
Hornok S, Szőke K, Kováts D, Estók P, Görföl T, Boldogh SA, et al. DNA of piroplasms of ruminants and dogs in ixodid bat ticks. PLoS ONE. 2016;11:e0167735. https://doi.org/10.1371/journal.pone.0167735.
Hornok S, Cutajar B, Takács N, Galea N, Attard D, Coleiro C, et al. On the way between Africa and Europe: molecular taxonomy of ticks collected from birds in Malta. Ticks Tick Borne Dis. 2022;13:102001. https://doi.org/10.1016/j.ttbdis.2022.102001.
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mel Marine Biol Biot. 1994;3:294–9.
Black WC, Piesman J. Phylogeny of hard and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Nat Acad Sci USA. 1994;91:10034–8.
Dobson SJ, Barker SC. Phylogeny of the hard ticks (Ixodidae) inferred from 18S rRNA indicates that the genus Aponomma is paraphyletic. Mol Phylogenet Evol. 1999;11:288–95. https://doi.org/10.1006/mpev.1998.0565.
Burger TD, Shao R, Beati L, Miller H, Barker SC. Phylogenetic analysis of ticks (Acari: Ixodida) using mitochondrial genomes and nuclear rRNA genes indicates that the genus Amblyomma is polyphyletic. Mol Phylogenet Evol. 2012;64:45–55. https://doi.org/10.1016/j.ympev.2012.03.004.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. https://doi.org/10.1093/molbev/msw054.
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, et al. The argasidae, ixodidae and nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa. 2010;2528:1–28.
Guglielmone AA, Nava S. Names for Ixodidae (Acari: Ixodoidea): valid, synonyms, incertae sedis, nomina dubia, nomina nuda, lapsus, incorrect and suppressed names—with notes on confusions and misidentifications. Zootaxa. 2014;3767:1–256. https://doi.org/10.11646/zootaxa.3767.1.1.
Meiners T, Hammer B, Göbel UB, Kahl O. Determining the tick scutal index allows assessment of tick feeding duration and estimation of infection risk with Borrelia burgdorferi sensu lato in a person bitten by an Ixodes ricinus nymph. Int J Med Microbiol. 2006;296:103–7. https://doi.org/10.1016/j.ijmm.2006.01.048.
Zhao S, Yang M, Jiang M, Yan B, Zhao S, Yuan W, et al. Rickettsia raoultii and Rickettsia sibirica in ticks from the long-tailed ground squirrel near the China-Kazakhstan border. Exp Appl Acarol. 2019;77:425–33. https://doi.org/10.1007/s10493-019-00349-5.
Statham MJ, Murdoch J, Janecka J, Aubry KB, Edwards CJ, Soulsbury CD, et al. Range-wide multilocus phylogeography of the red fox reveals ancient continental divergence, minimal genomic exchange and distinct demographic histories. Mol Ecol. 2014;23:4813–30. https://doi.org/10.1111/mec.12898.
Hernández A, Zaldívar P. Ecology of stoats Mustela erminea in a valley of the cantabrian mountains, northwestern Spain. Vert Zool. 2016;66:225–38.
Lv J, Wu S, Zhang Y, Chen Y, Feng C, Yuan X, et al. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida). Parasit Vectors. 2014;7:93. https://doi.org/10.1186/1756-3305-7-93.
Kelava S, Mans BJ, Shao R, Moustafa MAM, Matsuno K, Takano A, et al. Phylogenies from mitochondrial genomes of 120 species of ticks: insights into the evolution of the families of ticks and of the genus Amblyomma. Ticks Tick Borne Dis. 2021;12:101577. https://doi.org/10.1016/j.ttbdis.2020.101577.
Acknowledgements
The authors are grateful for the help from Alex Lintu Viskontene (ZIN-RAS, St. Petersburg, Russia), Tibor Csörgő (Bird Ringing Station, Ócsa, Hungary) and Gergő Keve (Department of Parasitology and Zoology, University of Veterinary Medicine, Budapest, Hungary). The authors also appreciate the access to Morel’s specimen of the I. rugicollis female kindly provided by Laurence Vial (CIRAD, UMR ASTRE, Montpellier, France).
Funding
Open access funding provided by University of Veterinary Medicine. The study was funded by the Office for Supported Research Groups, Eötvös Loránd Research Network (ELKH), Hungary (project no. 1500107). SH, NT and ADS were supported by OTKA K-132794 of the National Research, Development and Innovation Office.
Author information
Authors and Affiliations
Contributions
SH: conceptualization, study design, species identification, DNA extraction, manuscript writing. ADM: study design, supervision. JK: phylogenetic analyses, digital photography. NT: PCR tests, sequencing. DF: morphological analysis. OP: study design, manuscript writing. ADS: study design, sample collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ixodes rugicollis was found on a road-killed stone marten collected in the frame of project MySMIS120009 (National Mammal Monitoring). For removing I. lividus and I. arboricola from birds at Ócsa Bird Ringing Station (Hungary) the license was provided by the National Inspectorate for Environment and Nature (no. 14/3858-9/2012). For the collection of I. trianguliceps, permission was provided by the Committee for the Protection, Control and Regulation of the Use of Wildlife Specimens, in the Leningrad Region (no. И-3729/2020). Ethical approval for the collection of all other ticks which were used for DNA extraction, or of which the DNA extracts were used, are included in relevant studies cited in the text.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Description and drawings of Ixodes cornutus by Filippova [20].
Additional file 2:
Low-resolution pictures of Ixodes cornutus lectotype (female) stored at ZIN-RAS (St. Petersburg, Russia): (A) dorsal view of scutum and basis capituli in dry mount (dashed line marks maximum width of the scutum); (B) ventral view of anterior idiosoma in wet mount. Numbers between 1 and 8 mark structures of diagnostic importance described in Table 1.
Additional file 3:
Morphological characters of Ixodes rugicollis female: (A) scutum and basis capituli in wet mount shown in a slightly posterior view (the dark arrow indicates concave posterolateral margin of the scutum); (B) respiratory opening and (C) anal valves with five pairs of hair (marginated white circles indicate the base of these hairs to highlight their arrangement).
Additional file 4:
Measurements of Ixodes rugicollis and I. cornutus.
Additional file 5:
Phylogenetic tree of ixodid ticks based on (A) 18S and (B) 28S rRNA gene sequences. In each row of individual sequences, the country of origin and the GenBank accession number are shown after the species name. Ixodes rugicollis is marked with red branch (A) and all sequences from this study with red fonts and maroon accession numbers. The subgenus Pholeoixodes is surrounded by a blue dashed line, and Eschatocephalus species are marked with a green dot on the branch. The evolutionary history was inferred by using the (A) maximum likelihood method based on the Jukes-Cantor model or (B) neighbor-joining method and p-distance model. The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 21 and 17 nucleotide sequences for the 18S and 28S rRNA genes, and there were a total of 1023 and 586 positions in the final dataset, respectively. All positions containing gaps and missing data were eliminated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hornok, S., Mihalca, A.D., Kontschán, J. et al. Phylogenetic analyses of Ixodes rugicollis with notes on its morphology in comparison with Ixodes cornutus. Parasites Vectors 16, 106 (2023). https://doi.org/10.1186/s13071-023-05718-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05718-z