Abstract
Background
Deer species play an important role in the enzootic cycles of several Anaplasma species. While in the Northern Hemisphere ticks of genus Ixodes are well recognized vectors of these intracellular bacteria, less is known regarding the biological cycles of Anaplasma spp. in South America.
Methods
Using PCR protocols and Sanger sequencing, we assessed the presence of Anaplasma spp. in blood and ticks collected on a native deer species (Pudu puda) from southern Chile.
Results
Based on phylogenetic analyses of the 16S rRNA, gltA and groEL genes and calculation of average sequence divergence for groEL, our results bring to light a novel genovariant of Anaplasma phagocytophilum (named strain “Patagonia”). The strain represents a novel ecotype within the A. phagocytophilum species complex and was detected in both P. puda and their ticks. Using a larger matrix, denser taxon sampling and outgroup, our maximum-likelihood- and Bayesian-inferred phylogenies for groEL provide an accurate picture of the topology of A. phagocytophilum ecotypes and their evolutionary relationships.
Conclusions
This is the first report of an ecotype of A. phagocytophilum in South America. Our results provide novel insight into the genetic diversity and ecology of this complex of bacterial lineages. Further studies should elucidate the enzootic cycle of A. phagocytophilum strain “Patagonia” and assess its pathogenic potential for pudues, domestic animals and humans in the region.
Graphical Abstract

Similar content being viewed by others
Background
Alphaproteobacteria in the genus Anaplasma are intracellular cocobacilli of mammal blood cells transmitted by ticks of genera Amblyomma, Dermacentor, Hyalomma, Ixodes and Rhipicephalus [1]. Anaplasma spp. are infectious agents that cause diseases ranging from harmless to fatal [2, 3]. Among five species and numerous genovariants that have been identified [1], Anaplasma phagocytophilum is of animal and public health relevance because of tick-borne fever in ruminants and granulocytic anaplasmosis in equines, canids, felids and humans in the Northern Hemisphere [4, 5].
The genetic diversity of Anaplasma spp. has been explored using the conserved 16S rRNA (rrs) gene [1]; however, due to its weak intraspecific discriminatory resolution [6], variable loci such as citrate synthase (gltA) and the heat-shock operon (groEL) have been selected as suitable markers for single-locus genetic analyses [1, 7, 8]. Based on these markers four ecotypes split into seven phylogenetic clusters have been proposed to compose the A. phagocytophilum complex in Europe, Asia and North America [1, 7, 8]. A bacterial ecotype is a monophyletic array of strains sharing a similar ecological niche [9, 10], for which the average sequence divergence among groups is significantly higher than the divergence within them for a given gene [9]. Anaplasma phagocytophilum ecotypes and clusters have been defined according to their genetics, geographic distribution, enzootic cycles, host preference and pathogenicity [7, 11]. For example, ticks of genus Ixodes and cervids constitute the ecological niche for A. phagocytophilum ecotypes I and II [1].
Cervids are reservoirs for Anaplasma spp. and are often parasitized by ticks of the genus Ixodes that transmit these bacteria [12]. For instance, in the Northern Hemisphere, Ixodes scapularis and Ixodes pacificus (USA), Ixodes ricinus (Europe), and Ixodes persulcatus (Eurasia) [13] are the known vectors of A. phagocytophilum. However, data on the epidemiology of Anaplasma spp. is vague in South American cervids [14,15,16,17,18,19,20], and restricted to few species from Brazil [14,15,16,17], Argentina [19] and Uruguay [18]. In Chile, temperate rainforests (roughly between 35º and 46º S) are the habitat for the pudu (Pudu puda), a deer species classified as near threatened [21], which is an important host of adults of the ticks Ixodes stilesi and Ixodes taglei [22]. Although the eco-epidemiological settings (i.e. Ixodes ticks and deer) for an ecotype of A. phagocytophilum to occur do exist in Chile, it is currently unknown whether the bacterium occupies this ecological niche in the country. In the present study, we analyzed blood and ticks collected directly from free-ranging pudues from southern Chile. Because only a few Anaplasma surveys performed in South American wild cervids have provided short sequences for the 16S rRNA locus (rrs) [14,15,16,17,18,19, 23], we performed genetic screenings with additional molecular markers to detect Anaplasma DNA to clarify inter- or intraspecific relationships.
Methods
Sample collection
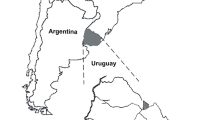
During a 5-year period (2017–2022), the blood (2–4 ml) of pudues admitted to any one of two wildlife rescue centers, Centro de Conservación Chiloé Silvestre (Nal Bajo, in Chiloé Island; − 41.839786, − 73.936015° W) and Cerefas Universidad San Sebastián (Puerto Montt; − 41.469628, − 72.907159), was collected from the cephalic or saphenous vein using an evacuated tube system (Vacutainer; Beckon, Dickson, and Company, Franklin Lakes, NJ, USA) on the day of admission (Fig. 1).
Map of Chile showing the origin of rescued pudues (black icons) within the Región de Los Lagos, Chile. Brown squares indicate the rehabilitation centers. Maps were constructed with QGIS 3.18.1-Zürich (https://www.gnu.org/licenses). QGIS, Quantum Geographic Information System
In addition to blood sampling, ticks were also removed with steel tweezers from various pudues. Blood samples and ectoparasites were kept in sterile tubes containing absolute ethanol and stored at − 80 °C until processing. The morphology of ticks was examined with a NexiusZoom (EVO) Stereo Microscope (Euromex Microscopen B.V., Arnhem, The Netherlands) and identified according to Nava et al. [22]. The identity of Anaplasma-positive ticks was further validated by sequencing a fragment of the tick mitochondrial (mt) 16S ribosomal RNA (rRNA) gene [22].
DNA isolation
Genomic DNA was extracted with the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol and eluted in 40 μl of buffer AE (10 mM Tris–Cl; 0.5 mM ethylenediaminetetraacetic acid [EDTA], pH 9.0). DNA was quantified with an Epoch™ Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) and assessed for quality at A260/A280 according to Khare et al. [24].
Gene amplification and sequencing
The suitability of the extracted DNA was checked by a conventional PCR (cPCR) assay targeting the mammalian glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the tick mt 16S rRNA genes, respectively. The primers and thermal conditions used in this study together with their references are shown in Table 1. Anaplasma detection was achieved by implementing different nested and hemi-nested PCR protocols targeting the rrs, gltA and groEL genes. DNA of Anaplasma platys (OQ155255) was used as the positive control and nuclease-free water was used as the negative control. All PCR reactions were performed in a thermal cycler (ProFlexTM Base 32 × 3; Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) in a final reaction volume of 25 μl (12.5 μl DreamTaq Green PCR Master Mix [Thermo Fisher Scientific], 1 μl of each primer (0.4 μM), 8.5 μl of ultra-pure water and 2 μl template DNA. The PCR products were stained with GelRed® (Biotum, Tehran, Iran), separated by electrophoresis in 2% agarose gels and then visualized using an ENDURO™ GDS UV transilluminator (Labnet International, Edison, NJ, USA). Amplicons with bands of the expected size were purified and Sanger-sequenced at Macrogen (Seoul, South Korea).
Assembly and sequence analyses
Amplicon sequences were quality-checked and edited with Geneious Prime® version (v) 2021.2.2 (www.geneious.com) to generate consensus sequences. Base calls with Phred values ≥ 20 were considered suitable for the analyses [35, 36]. The BLAST® tool (https://blast.ncbi.nlm.nih.gov) was employed to compare obtained nucleotide sequences and identify orthologous sequences.
Phylogenetic analyses
Orthologous sequences downloaded from GenBank (https://www.ncbi.nlm.nih.gov) and consensus sequences were used to build alignments with the MAFFT multiple sequence alignment program using default parameters [37]. The alignments were subsequently trimmed and filtered with Block Mapping and Gathering with Entropy (BMGE) using default parameters to map informative regions for phylogenetics inferences [38].
Phylogenetic trees were constructed with the Bayesian inference (BI [39, 40]) and maximum-likelihood (ML [41]) methods in MrBayes v 3.2.6 [42] and IQ-TREE v 1.6.12 [43], respectively. As protein-coding genes present different nucleotide exchange rates (heterogeneity) at the first, second and third codon positions [42, 44], datasets were partitioned into the three codon positions (position-1, position-2 and position-3) [42, 44,45,46]. Then, the Model Finder command “TESTNEWONLYMERGE -mrate G” was implemented to select the best-fit evolutionary models and best-partition scheme for protein-coding gene datasets [47]. The ML best evolutionary models for non-coding genes were calculated using the ModelFinder command “-m TESTNEWONLY -mrate G” [47]. We used rapid hill-climbing and stochastic disturbance methods with 1000 ultrafast bootstrapping pseudo-replicates to evaluate the inferred tree robustness. Bootstrap values < 70%, 70–94% and ≥ 95% were considered non-significant, medium and solid statistical support [48], respectively.
BI phylogenies were constructed based on nucleotide substitution models selected with the MrBayes command "lset nst = mixed rates = gamma" for the non-coding dataset [42, 49]. On the other hand, the best partition schemes computed by ModelFinder and the MrBayes command “lset = mixed rates = invgamma” were used to calculate the best models for protein-encoding datasets [42, 46, 49]. Two independent tests of 20 × 106 generations and four Markov chain Monte Carlo (MCMC) chains were implemented, sampling trees every 1000 generations and removing the first 25% as burn-in. Tracer v1.7.1 [50] was used to confirm the correlation and effective sample size of the MCMC. Bayesian posterior probabilities (BPP) with values > 0.70 in nodes were considered to indicate strong statistical support [51]. All best-fit models and partitions schemes were selected under the Bayesian Information Criterion (BIC) [52]. Trees were visualized and edited with FigTree v 1.4.1 (http://tree.bio.ed.ac.uk/software/figtree/) and Inkscape v 1.1 (https://inkscape.org/es/). Congruent topologies between ML and BI analyses were used to produce strict consensus trees in Geneious Prime with the Consensus Tree Builder tool, implementing a support threshold of 100%. The consensus phylogram included all monophyletic clades after comparing ML and BI topologies for each dataset.
Genetic distance analyses
To assess the corrected pairwise distance and determine the average sequence divergence within and among ecotypes, an alignment of 936 bp was constructed with default parameters in MAFFT, including 214 groEL sequences of A. phagocytophilum with > 70% coverage between them, using Anaplasma odocoilei and A. platys as outgroups. The corrected pairwise distance was assessed using raxmlGUI [53, 54] for RAxML v 8 [55] with the GTR + GAMMA + I substitution model.
Results
Tick identification and blood samples
A total of 26 hard ticks and 55 blood samples were collected from pudues. All ticks were morphologically identified as I. stilesi (17 females, 5 males, 4 nymphs). Amplicons of the expected size were obtained for the mt 16S rRNA gene by PCR in 20 of the 26 tick specimens, with negative results obtained for six ticks (4 females, 1 male, 1 nymph), which were subsequently excluded from the analysis. PCR targeting the GAPDH gene in pudu blood resulted in amplicons of the expected size, confirming successful DNA extractions in all cases (Table 2).
Anaplasma detection
Anaplasma DNA was amplified in 8/26 (30.8%) I. stilesi (1 nymph, 1 male, 6 females) and in 6/55 (10.9%) pudues (Table 2). Eleven identical sequences were obtained for rrs (1,212 bp), 12 for gltA (722 bp) and 13 for groEL (1,286 bp). Pairwise comparisons between generated sequences indicated one genotype for rrs, seven genotypes for gltA and 11 genotypes for groEL. A mitochondrial genotype of 429 bp retrieved for Anaplasma-positive ticks (OP750053) was 99.5% (428/430 bp, 100% query cover, 2 gaps, 0 E-value) identical with a previous sequence of I. stilesi from Chile (DQ061292) [56].
After BLASTn comparisons, the rrs genotype matched with 94.8% identity A. phagocytophilum isolate D2_2 (MK814406), detected in Canis lupus familiaris from South Africa [57]; the gltA genotypes showed an identity ranging from 82.9% to 83.1% with A. phagocytophilum strain Sheep (KP861639) detected in an Ixodes sp. collected on a Norwegian White Sheep [58]; and the groEL genotypes were 91.4–91.8% identical with A. phagocytophilum samc001 (LC496077) detected in Canis lupus familiaris from Japan [59].
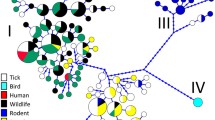
Phylogenies inferred for the three loci positioned Anaplasma genotypes retrieved from I. stilesi and pudu blood into the A. phagocytophilum clade, forming a monophyletic group (Figs. 2, 3, 4). In particular, the groEL phylogeny placed our genotypes in an independent clade related to ecotype III of A. phagocytophilum [1] (Fig. 4).
Maximum likelihood (ML) and Bayesian inference (BI) rrs gene consensus tree inferred for a subset of Anaplasma spp., using 41 sequences and an alignment of 1,382 bp. Best-fit evolutionary models calculated for the ML and BI methods were TPM3u + F + G4; and M90, M177, M85, M152, M179, M117, M195, respectively. Bootstrap values and Bayesian posterior probabilities (BPP) are indicated above or below each branch. The position of the strain of Anaplasma phagocytophilum characterized in the present study is highlighted in a gray box
ML and BI consensus tree inferred for a subset of Anaplasma spp., using 40 sequences of the gltA gene and an alignment of 1152 bp. Best-fit evolutionary models calculated for the ML and BI methods were GTR + F + I + G4 (position-1), GTR + F + G4 (position-2), HKY + F + I + G4 (position-3); and M64, M175, M173, M25, M171, M50, M125 (position-1); M80, M135, M164, M166, M145 (position-2); M90, M177, M152, M183, M136 (position-3), respectively. Bootstrap values and BPP are indicated above or below each branch. The position of the strain of A. phagocytophilum characterized in the present study is highlighted in a gray box
ML and BI consensus tree inferred for a subset of Anaplasma spp., using 226 sequences of the groEL gene, and an alignment length of 1224 bp. Best-fit evolutionary models calculated for the ML and BI methods were TIM + F + G4 (position-1); TN + F + G4 (position-2); and K3Pu + F + G4 (position-3); and M45, M136, M142, M130, M139, M185 (position-1); M81, M40 (position-2); M15, M50, M85, M122, M90 (position-3), respectively. Bootstrap values and BPP are indicated above or below each branch. Colors for ecotypes I, II, III and IV were assigned according to Jaarsma et al. [8]. The position of the strain of A. phagocytophilum characterized in the present study is highlighted in a green box
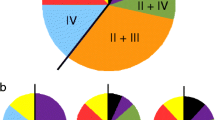
For the groEL gene, the average sequence divergence calculated within ecotypes was always less than the average sequence divergence calculated among them, including the ecotype characterized in this study (Table 3). Collectively, the genetic evidence provided by our study points to the finding of a fifth A. phagocytophilum ecotype, for which the name A. phagocytophilum strain “Patagonia” is proposed. GenBank accession numbers generated in this study are available in Additional file 1: Tables S1, S2).
Discussion
Tick-borne bacteria, including A. phagocytophilum, are geographically expanding, probably due to climate change and anthropogenic landscape perturbation, both factors that favor the spread of their vectors synergically [13, 60]. Although A. phagocytophilum was previously thought to be a single bacterial species [61], recent phylogenetic reconstructions have revealed a complex of lineages with different pathogeny, geographical distribution, reservoirs and vectors [1]; nevertheless, host range, zoonotic potential and transmission dynamics of this bacterium are still incompletely solved [1, 7, 8, 11].
Based on average divergence of partial groEL sequences (Table 3) and strongly supported phylogenies for rrs, gltA, and groEL, in this study we identified a novel genovariant of A. phagocytophilum associated with pudues, for which the name “Patagonia” is proposed (Figs. 2, 3, 4). Accordingly, this genovariant has been designated as the ecotype V (cluster 8) of A. phagocytophilum, which constitutes the first ecotype of this species complex described for South America. Variants of A. phagocytophilum are adapted to different hosts and vector species, therefore configuring different enzootic cycles [1, 13]. The fact that A. phagocytophilum strain “Patagonia” conforms an additional ecotype suggests that the eco-epidemiology of this novel strain differs from those of the northern latitudes.
Cervids such as roe deer (Capreolus capreolus), red deer (Cervus elaphus), white-tailed deer (Odocoileus virginianus), fallow deer (Dama dama), sika deer (Cervus nippon) and their associated ticks (I. ricinus and I. scapularis) are implicated in the maintenance of endemic cycles of some A. phagocytophilum variants (e.g. Ap-V1, B, J, S, W) in northern latitudes [13, 62,63,64,65]. In contrast, previous knowledge on A. phagocytophilum in South American deer species is vague, limited only to Brazil, and does not support its classification within any ecotype. For example, in their study on the brown brocket deer (Mazama gouazoubira), Silveira et al. [15] could not discriminate whether A. phagocytophilum or A. platys caused the infection using PCR and sequencing protocols. However, a posterior survey revealed that A. phagocytophilum would be circulating in brown brocket deer [23]. On the other hand, exposure to A. phagocytophilum in Brazilian marsh deer (Blastocerus dichotomus) has been reported using indirect immunofluorescence assays [14]. As far as we know, our study is the first multigenic detection of A. phagocytophilum DNA in pudu and I. stilesi.
Records of A. phagocytophilum in South American mammals include rodents (Cavia sp. and Calomys cerqueirai), peccary (Tayassu pecari and Pecari tajacu), sloths (Bradypus tridactylus) and coati (Nasua nasua) [17, 66, 67]. However, due to the use of short fragments of the rrs and groEL genes for identification, it is difficult to state whether the Anaplasma DNA detected in these mammals corresponded to A. phagocytophilum or not. While reports of A. phagocytophilum on South American cervids are few, other Anaplasma spp. have been recorded in deer in Brazil, such as Anaplasma bovis and Anaplasma sp. in red brocket deer (Mazama americana); A. bovis, Anaplasma marginale and A. platys in marsh deer; and A. marginale in brown brocket deer [14,15,16,17]. Likewise, the records in South America include A. platys, Anaplasma odocoilei, A. marginale and “Candidatus Anaplasma boleense” in marsh deer in Argentina [19], and Anaplasma sp. Mazama genotype in brown brocket deer in Uruguay [18].
In Chile, evidence of A. phagocytophilum is incipient. Indeed, infection by this bacterium has been reported in horses [68]. However, these results deserve further investigation, since the use of A. phagocytophilum-specific primers did not yield positive reactions, and the occurrence of a vector in the area where positive animals were detected is unknown. Further reports of Anaplasma spp. in Chile include A. platys in dogs, Andean foxes (Lycalopex culpaeus), the South American gray fox (Lycalopex griseus) [69] and hard ticks (Rhipicephalus sanguineus sensu lato). An Anaplasma-like agent has also been detected in seabird soft ticks (Ornithodoros spheniscus) [70]. Moreover, serological evidence of exposure to Anasplasma sp. has been recorded in dogs [71] and humans [71,72,73,74]. Our results thus expand current knowledge on vertebrate hosts of A. phagocytophilum in the continent.
There is no standardized approach for investigating the genetic diversity and population structure of Anaplasma species. Although the rrs, gltA and groEL markers used in this study are currently the most appropriate loci for the genetic characterization of Anaplasma spp. [1], rrs and groEL are conserved and do not have sufficient resolution to segregate some groups when short fragments are analyzed, even in different species of the genus. Therefore, the sequenced fragments must be long enough [1, 6]. Based on the above argument, our phylogenetic analyses did not include sequences shorter than 600 bp.
Previous studies found that the groEL gene may delimit lineages (ecotypes, clusters, groups) of A. phagocytophilum [1, 7, 8, 11]. Moreover, the discrimination capacity among lineages has improved due to the progressive increase in taxon sampling and the size of the sequences employed in the analyses [1]. Recently, a population study recovered ecotypes I, II, III and IV (mentioned by Jahfari et al. [7] and Jaarsma et al. [8]) as monophyletic but without statistical support for ecotypes I and II [1]. It is worth noting that ecotype IV was designated after including only one sequence in those analyses, and its monophyly was not assessed [1]. In addition, cluster 3 (paraphyletic within ecotype II) lacked statistical support (Electronic Supplementary Material Figure S4. in Rar et al. [1]). Thus, methodological factors, such as the inclusion of an outgroup [10, 75], longer alignments, denser taxon sampling [1, 11] and the application of phylogenetic inferences (BI, ML) [39,40,41], may circumscribe with higher confidence the monophyly and evolutionary relationships of ecotypes and subclades within A. phagocytophilum, as shown in our study.
Applying the above referred methods, ecotypes I, II and IV were depicted as monophyletic lineages with high statistical support (Fig. 4). In particular, ecotype II was only recovered with high support in ML analyses (92% of bootstrap), yet the cluster 3 (Europe) belonging to this ecotype represents a monophyletic group with confident support (0.94/89) (Fig. 4). Our results differ from those of other studies that described these monophyletic groups based on an eco-epidemiological approach without considering systematics [1, 7, 8, 11]. Undoubtedly, ecotype II and cluster 3 represent natural assemblages, but our study shows them now as also phylogenetically supported. Herein described ecotype V was moderately supported in the groEL-based ML inference (81% of ultrafast-bootstrap) and closely related to ecotype III (Fig. 4), which is integrated by variants of A. phagocytophilum related to small mammals and ticks (Additional file 1: Table S2) [1]. However, the phylogenetic position of the ecotypes should be re-evaluated as new members of the A. phagocytophilum complex are discovered.
The presence of A. phagocytophilum DNA does not conclusively confirm the role of pudues and I. stilesi in the epidemiology of this bacterium or any clinical impact on pudu health. However, the fact that P. puda is the sole deer that currently inhabits the areas from which positive animals for this bacterium were recorded [76] strengthens the notion that this cervid could be reservoir of A. phagocytophilum strain “Patagonia.” In addition, considering the role of Ixodes spp. as vectors of Anaplasma spp. in the Northern Hemisphere cervids [1], I. stilesi and I. taglei, two species that commonly parasitize pudues [22], represent potential vectors of A. phagocytophilum strain “Patagonia.” However, our hypotheses should be tested in experimental studies. Meanwhile, the epidemiological cycle of A. phagocytophilum strain “Patagonia” remains unknown.
Conclusions
We report the presence of and ecotype of A. phagocytophilum for the first time in South America. The genetic evidence showed conclusively that the A. phagocytophilum found in this study is a unique variant, and the name A. phagocytophilum strain “Patagonia” is tentatively proposed. The study of the enzootic cycle of A. phagocytophilum strain “Patagonia” is now essential to establish its zoonotic potential and health impact on pudues and further species, such as domestic ruminants. Furthermore, because some variants of A. phagocytophilum are infectious agents of public and veterinary health concern, the detection of this bacterium in Chile deserves further attention. Future research should define a standardized approach for genetically characterizing members of Anaplasma genus that would afford reliable comparisons, as recommended in Rar et al. [1]. Finally, these findings bring insight into the genetic diversity and ecology of A. phagocytophilum.
Availability of data and materials
GenBank accession numbers generated in this study are available in Additional files 1: Tables S1 and S2.
Abbreviations
- BI:
-
Bayesian inference
- BLAST:
-
Basic local alignment search tool
- gltA :
-
Citrate synthase gene
- GAPDH :
-
Glyceraldehyde-3-phosphate dehydrogenase gene
- groEL :
-
Heat-shock operon
- MAFFT:
-
Multiple alignment using fast Fourier transform
- ML:
-
Maximum likelihood
- MCMC:
-
Markov chain Monte Carlo
- rRNA:
-
Ribosomal ribonucleic acid
- rrs :
-
16S rRNA gene
References
Rar V, Tkachev S, Tikunova N. Genetic diversity of Anaplasma bacteria: twenty years later. Infect Genet Evol. 2021;91:104833.
Atif FA. Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology. 2016;143:659–85.
Battilani M, de Arcangeli S, Balboni A, Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infect Genet Evol. 2017;49:195–211.
Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–65.
Matei IA, Estrada-Peña A, Cutler SJ, Vayssier-Taussat M, Varela-Castro L, Potkonjak A, et al. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors. 2019;12:599.
Caudill MT, Brayton KA. The use and limitations of the 16S rRNA sequence for species classification of Anaplasma samples. Microorganisms. 2022;10:605.
Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Heylen D, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014;7:365.
Jaarsma RI, Sprong H, Takumi K, Kazimirova M, Silaghi C, Mysterud A, et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit Vectors. 2019;12:328.
Palys T, Nakamura LK, Cohan FM. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int J Syst Bacteriol. 1997;47:1145–56.
Cohan FM. Bacterial species and speciation. Syst Biol. 2001;50:513–24.
Rar V, Yakimenko V, Tikunov A, Makenov M, Epikhina T, Tancev A, et al. Genetic variability of Anaplasmataceae circulating in small mammals and ticks in an Ixodes persulcatus/Ixodes trianguliceps sympatric area in Russian Siberia. Ticks Tick Borne Dis. 2020;11:101499.
Remesar S, Prieto A, García-Dios D, López-Lorenzo G, Martínez-Calabuig N, Díaz-Cao JM, et al. Diversity of Anaplasma species and importance of mixed infections in roe deer from Spain. Transbound Emerg Dis. 2022;69:374–85.
Dugat T, Lagrée A-C, Maillard R, Boulouis H-J, Haddad N. Opening the black box of Anaplasma phagocytophilum diversity: current situation and future perspectives. Front Cell Infect Microbiol. 2015;5:61.
Sacchi ABV, Duarte JMB, André MR, Machado RZ. Prevalence and molecular characterization of Anaplasmataceae agents in free-ranging Brazilian marsh deer (Blastocerus dichotomus). Comp Immunol Microbiol Infect Dis. 2012;35:325–34.
Silveira JAG, Rabelo EML, Ribeiro MFB. Molecular detection of tick-borne pathogens of the family Anaplasmataceae in Brazilian brown brocket deer (Mazama gouazoubira, Fischer, 1814) and marsh deer (Blastocerus dichotomus, Illiger, 1815). Transbound Emerg Dis. 2012;59:353–60.
Mongruel ACB, Benevenute JL, André MR, de Carrasco AOT, Machado RZ, Seki MC. Molecular characterization of Anaplasma sp. in free-living gray brockets (Mazama gouazoubira). Vector Borne Zoonotic Dis. 2017;17:165–71.
Soares HS, Marcili A, Barbieri ARM, Minervino AHH, Malheiros AF, Gennari SM, et al. Novel Anaplasma and Ehrlichia organisms infecting the wildlife of two regions of the Brazilian Amazon. Acta Trop. 2017;174:82–7.
Félix ML, Armúa-Fernández MT, Parodi P, Bazzano V, Mangold AJ, Venzal JM. Detection of a putative novel genotype of Anaplasma in gray-brocket deer (Mazama gouazoubira) from Uruguay. Exp Appl Acarol. 2020;81:575–83.
Orozco MM, Argibay HD, Minatel L, Guillemi EC, Berra Y, Schapira A, et al. A participatory surveillance of marsh deer (Blastocerus dichotomus) morbidity and mortality in Argentina: first results. BMC Vet Res. 2020;16:321.
Hidalgo-Hermoso E, Cabello J, Novoa-Lozano I, Celis S, Ortiz C, Kemec I, et al. Molecular detection and characterization of hemoplasmas in the Pudu (Pudu puda), a native cervid from Chile. J Wildl Dis. 2022;58:8–14.
Silva-Rodríguez E, Pastore H, Jiménez J. Pudu puda. In: International Union for Conservation of Nature, editor. The IUCN Red List of threatened species. 2016. https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T18848A22164089.en.
Nava S, Venzal JM, González-Acuña D, Martins TF, Guglielmone AA. Ticks of the Southern Cone of America. London: American Press; 2017.
Silveira JAG, Rabelo EML, Lima PCS, Chaves BN, Ribeiro MFB. Post-mortem hemoparasite detection in free-living Brazilian brown brocket deer (Mazama gouazoubira, Fischer 1814). Rev Bras Parasitol Vet. 2014;23:206–15.
Khare P, Raj V, Chandra S, Agarwal S. Quantitative and qualitative assessment of DNA extracted from saliva for its use in forensic identification. J Forensic Dent Sci. 2014;6:81–5.
Birkenheuer AJ, Levy MG, Breitschwerdt EB. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. J Clin Microbiol. 2003;41:4172–7.
Mangold AJ, Bargues MD, Mas-Coma S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol Res. 1998;84:478–84.
Anderson BE, Dawson JE, Jones DC, Wilson KH. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–42.
Paddock CD, Sumner JW, Shore GM, Bartley DC, Elie RC, McQuade JG, et al. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–502.
Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, et al. Ultrastructure and phylogenetic analysis of “Candidatus Neoehrlichia mikurensis” in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol. 2004;54:1837–43.
Liz JS, Anderes L, Sumner JW, Massung RF, Gern L, Rutti B, et al. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J Clin Microbiol. 2000;38:1002–7.
Huang H, Unver A, Perez MJ, Orellana NG, Rikihisa Y. Prevalence and molecular analysis of Anaplasma platys in dogs in Lara, Venezuela. Braz J Microbiol. 2005;36:211–6.
Tabara K, Arai S, Kawabuchi T, Itagaki A, Ishihara C, Satoh H, et al. Molecular survey of Babesia microti, Ehrlichia species and Candidatus Neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol Immunol. 2007;51:359–67.
Gofton AW, Doggett S, Ratchford A, Ryan U, Irwin P. Phylogenetic characterisation of two novel Anaplasmataceae from Australian Ixodes holocyclus ticks: “Candidatus Neoehrlichia australis” and “Candidatus Neoehrlichia arcana”. Int J Syst Evol Microbiol. 2016;66:4256–61.
Inokuma H, Brouqui P, Drancourt M, Raoult D. Citrate synthase gene sequence: a new tool for phylogenetic analysis and identification of Ehrlichia. J Clin Microbiol. 2001;39:3031–9.
Ewing B, Green P. Base-calling of automated sequencer traces using Phred. II. Error probabilities. Genome Res. 1998;8:186–94.
Ewing B, Hillier LD, Wendl MC, Green P. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 1998;8:175–85.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80.
Criscuolo A, Gribaldo S. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol. 2010;10:210.
Yang Z, Rannala B. Bayesian phylogenetic inference using DNA sequences: a Markov chain Monte Carlo method. Mol Biol Evol. 1997;14:717–24.
Rannala B, Yang Z. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol. 1996;43:304–11.
Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–76.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42.
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74.
Yang Z. Maximum-likelihood models for combined analyses of multiple sequence data. J Mol Evol. 1996;42:587–96.
Kainer D, Lanfear R. The effects of partitioning on phylogenetic inference. Mol Biol Evol. 2015;32:1611–27.
Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29:1695–701.
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–9.
Minh BQ, Nguyen MAT, von Haeseler A. Ultrafast approximation for phylogenetic Bootstrap. Mol Biol Evol. 2013;30:1188–95.
Huelsenbeck JP. Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Mol Biol Evol. 2004;21:1123–33.
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol. 2018;67:901–4.
Huelsenbeck JP, Rannala B. Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Syst Biol. 2004;53:904–13.
Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–4.
Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12:335–7.
Edler D, Klein J, Antonelli A, Silvestro D. raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol Evol. 2021;12:373–7.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3.
Guglielmone AA, Venzal JM, González-Acuña D, Nava S, Hinojosa A, Mangold AJ. The phylogenetic position of Ixodes stilesi Neumann, 1911 (Acari: Ixodidae): morphological and preliminary molecular evidences from 16S rDNA sequences. Syst Parasitol. 2006;65:1–11.
Kolo AO, Collins NE, Brayton KA, Chaisi M, Blumberg L, Frean J, et al. Anaplasma phagocytophilum and other Anaplasma spp. in various hosts in the Mnisi community, Mpumalanga province, South Africa. Microorganisms. 2020;8:1812.
Alberdi P, Ayllón N, Cabezas-Cruz A, Bell-Sakyi L, Zweygarth E, Stuen S, et al. Infection of Ixodes spp. tick cells with different Anaplasma phagocytophilum isolates induces the inhibition of apoptotic cell death. Ticks Tick Borne Dis. 2015;6:758–67.
Fujii Y, Shoji Y, Kanda T, Kishida A, Asano M, Kishida K, et al. The first canine Anaplasma phagocytophilum infection in western Japan: a case report. J Anim Clin Med. 2019;28:100–4.
Rikihisa Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24:469–89.
Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013;3:31.
Massung RF, Mather TN, Levin ML. Reservoir competency of goats for the Ap-Variant 1 strain of Anaplasma phagocytophilum. Infect Immun. 2006;74:1373–5.
Silaghi C, Hamel D, Thiel C, Pfister K, Passos LMF, Rehbein S. Genetic variants of Anaplasma phagocytophilum in wild caprine and cervid ungulates from the Alps in Tyrol, Austria. Vector Borne Zoonotic Dis. 2011;11:355–62.
Overzier E, Pfister K, Thiel C, Herb I, Mahling M, Silaghi C. Anaplasma phagocytophilum in questing Ixodes ricinus ticks: comparison of prevalences and partial 16S rRNA gene variants in urban, pasture, and natural habitats. Appl Environ Microbiol. 2013;79:1730–4.
Overzier E, Pfister K, Herb I, Mahling M, Böck G, Silaghi C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), in questing ticks (Ixodes ricinus), and in ticks infesting roe deer in southern Germany. Ticks Tick Borne Dis. 2013;4:320–8.
Benevenute JL, Dumler JS, Ogrzewalska M, Roque ALR, Mello VVC, de Sousa KCM, et al. Assessment of a quantitative 5′ nuclease real-time polymerase chain reaction using groEL gene for Ehrlichia and Anaplasma species in rodents in Brazil. Ticks Tick Borne Dis. 2017;8:646–56.
de Sousa KCM, Calchi AC, Herrera HM, Dumler JS, Barros-Battesti DM, Machado RZ, et al. Anaplasmataceae agents among wild mammals and ectoparasites in Brazil. Epidemiol Infect. 2017;145:3424–37.
Hurtado C, Torres R, Pérez-Macchi S, Sagredo K, Uberti B, de Souza Zanatto DC, et al. Serological and molecular detection of Anaplasma phagocytophilum in thoroughbred horses from Chilean racecourses. Ticks Tick Borne Dis. 2020;11:101441.
di Cataldo S, Cevidanes A, Ulloa-Contreras C, Hidalgo-Hermoso E, Gargano V, Sacristán I, et al. Mapping the distribution and risk factors of Anaplasmataceae in wild and domestic canines in Chile and their association with Rhipicephalus sanguineus species complex lineages. Ticks Tick Borne Dis. 2021;12:101752.
Muñoz-Leal S, Lopes MG, Marcili A, Martins TF, González-Acuña D, Labruna MB. Anaplasmataceae, Borrelia and Hepatozoon agents in ticks (Acari: Argasidae, Ixodidae) from Chile. Acta Trop. 2019;192:91–103.
Acosta-Jamett G, Weitzel T, López J, Alvarado D, Abarca K. Prevalence and risk factors of antibodies to Anaplasma spp. in Chile: a household-based cross-sectional study in healthy adults and domestic dogs. Vector Borne Zoonotic Dis. 2020;20:572–9.
Abarca K, López J, Perret C, Guerrero J, Godoy P, Veloz A, et al. Anaplasma platys in dogs, Chile. Emerg Infect Dis. 2007;13:1392–5.
Weinborn AR, Zanelli GM, López SÓ, Pau VN, Valdés PF. Anticuerpos anti-Anaplasma spp en población de riesgo ocupacional de un hospital veterinario. Rev Investig Vet Peru. 2018;29:594–601.
Conejeros Ortiz C, Rodríguez Jorquera P. Diagnóstico serológico de Anaplasma phagocytophilum en caballos fina sangre de carrera pertenecientes al Valparaíso Sporting Club Viña del Mar. Saarbrücken: Editorial Académica Española; 2013.
Hennig W. Phylogenetic systematics. Annu Rev Entomol. 1965;10:97–116.
Iriarte A. Mamíferos de Chile. 1st edn. Santiago: Lynx Edicions; 2008.
Muñoz-Leal S, Silva-De-La-Fuente MC, Barros-Battesti DM, Guglielmone AA, Venzal JM, Nava S, et al. In memoriam: a eulogy for Daniel González-Acuña, 1963–2020. Rev Bras Parasitol Vet. 2021;30:e000821.
Acknowledgements
We thank Fidel Castro Reboredo and Lleretny Rodriguez Alvarez for their collaboration in the field and laboratory work. DM-A is grateful for Grant ANID/BASAL FB210006. This paper is dedicated to the memory of Daniel González-Acuña, who made significant contributions to the study of parasites and the conservation of wildlife in Chile [77].
Funding
This study was funded by the “Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT)” No. 11220177, and by the ANID BECAS/Scholarship Program/DOCTORADO NACIONAL/2019–21190078 and 2020–21200182. Funders had no role in the study design, data collection, analysis, preparation of the manuscript and decision to publish. The contribution of JEU was funded by the Atracción Talento de la Comunidad de Madrid Fellowship Program (REFF 2019-T2/AMB-13166).
Author information
Authors and Affiliations
Contributions
AS, RT, SM-L: material preparation, data collection, analysis, writing of the first draft. AS, RT, SR, JEU, CP-M, JC-S, FV-O, CV-S, DM-A, EH-H, SM-L contributed to the study conception and design and commented on initial versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to, and the appropriate ethical review committee approval has been received. Procedures performed in this study were verified and approved by the Bioethics Committee of the School of Veterinary Sciences, Universidad de Concepción (CBE-07-2022).
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
GenBank accession numbers of the sequences used for Anaplasma phagocytophilum rrs and gltA phylogenies. Sequences generated in this study are highlighted in bold. Table S2. GenBank accession numbers of the sequences used for Anaplasma phagocytophilum groEL phylogeny. Sequences generated in this study are highlighted in bold.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Santodomingo, A., Thomas, R., Robbiano, S. et al. Wild deer (Pudu puda) from Chile harbor a novel ecotype of Anaplasma phagocytophilum. Parasites Vectors 16, 38 (2023). https://doi.org/10.1186/s13071-023-05657-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05657-9