Abstract
Background
In canine leishmaniosis (CanL) endemic areas, pathologists often receive skin biopsies for testing with histopathologic findings suggestive—but not conclusive for a definitive diagnosis—of CanL lesions. I the absence of data on the infective status of animals, the diagnosis can therefore be challenging. The aim of this retrospective study was to evaluate the ability of immunohistochemistry (IHC) and quantitative PCR (qPCR) methods to detect Leishmania infection in skin biopsies with a histopathologic diagnosis of lymphoplasmacytic/histiocytic and/or granulomatous dermatitis and to correlate the pattern, depth and severity of the histopathologic lesions with the parasite load detected by qPCR and IHC.
Methods
Thirty formalin-fixed, paraffin-embedded skin samples were evaluated by hematoxylin–eosin (H&E) staining, IHC, conventional PCR (cPCR) and qPCR. The severity, pattern and depth of the dermal inflammation and parasite load were graded.
Results
Leishmania was detected by H&E staining in 8/30 sections (26.66%) and by IHC in 14/30 samples (46.66%). Parasite DNA was detected in 14/30 samples (46.66%) by cPCR and in 21/30 samples (70%) by qPCR, with an extremely variable parasite load (1.32–62.700 copies). The level of agreement was fair between H&E staining and cPCR (κ = 0.32), and moderate between H&E staining and IHC (κ = 0.58). The level of agreement between IHC and cPCR was good (κ = 0.65); between IHC and qPCR, moderate (κ = 0.41); and between cPCR and qPCR, fair (κ = 0.28). A significant association was found between the severity of dermal inflammation and the parasitic skin load by IHC, although with weak linear correlation.
Conclusions
Our study underlines the difficulty of obtaining a definitive diagnosis of CanL cutaneous lesions, even with the most accurate diagnostic tests currently available. Based on our results, no single test is suitable on its own for the diagnosis of cutaneous lesions caused by Leishmania. However, in the presence of a moderate/severe lymphoplasmacytic/histiocytic and/or granulomatous dermatitis, we suggest performing IHC, as in our study this technique proved to be the method with the highest discriminatory power to estimate the role of the parasite in skin lesions. In mild lesions, IHC loses its discriminatory power and should be effectively combined with techniques such as qPCR.
Graphical Abstract

Similar content being viewed by others
Background
Most dogs living in endemic areas of canine leishmaniosis (CanL) are constantly exposed to being bitten by sand flies and thus to Leishmania infection. However, only relatively few animals develop the disease, depending on whether their immune system is adequate to control parasite multiplication and tissue invasion.
The skin is the organ where the first interaction between the parasite and the canine immune system occurs [1]. Skin lesions are thus frequently the most common finding (67–89% of cases) in dogs infected with Leishmania upon physical examination. Such lesions can appear in isolation or in various combinations with other clinicopathologic abnormalities [2, 3].
Cutaneous manifestations of CanL are extremely pleomorphic and may be grouped into typical and atypical forms [4, 5]. In the typical forms (e.g. exfoliative dermatitis, ulcerative dermatitis of pressure points, etc.), the clinical features are highly suggestive of CanL and, according to the guidelines suggested by the Canine Leishmaniasis Working Group (CLWG) [6], veterinarians should follow the diagnostic algorithm, including indirect tests [i.e. indirect fluorescence antibody test (IFAT), enzyme-linked immunosorbent assay (ELISA) and direct agglutination test (DAT)] and direct tests aimed at the direct visualization of the parasite or amplification of the parasitic DNA [7]. Skin biopsies and histopathologic examination can be carried out in these cases to confirm the clinical suspicion of CanL. However, in endemic areas, several atypical forms can also occur, such that the cutaneous lesions are not highly suspicious of CanL and indeed are similar to those of other skin diseases with a completely different pathogenesis (i.e. pemphigus foliaceus, etc.). In these cases, the referring veterinarians may be completely unaware of the possibility that Leishmania may cause the observed lesions, resulting in the possibility that the cutaneous biopsies can be sent to the pathologist without mention of a specific suspect cause and with no preliminary (direct and indirect) tests performed.
Histopathologic findings in skin biopsies are not specific for CanL and consist of varying degrees of perivascular to interstitial, and focal to nodular to diffuse inflammatory infiltrate in the dermis, with various combinations of macrophages, plasma cells, lymphocytes, mast cells and isolated neutrophils [8,9,10]. These patterns may be shared by other infectious and non-infectious/immune-mediated diseases (i.e. sterile granuloma and pyogranuloma syndrome, granulomatous forms of sebaceous adenitis, etc.) [11]. Therefore, the pathologist diagnosing a lymphoplasmacytic/histiocytic dermatitis (LHD) and/or granulomatous dermatitis (GD) in skin biopsies from dogs with cutaneous lesions compatible with CanL has to rule out Leishmania in the list of differential diagnoses.
Upon histopathology of the skin biopsies, a definitive diagnosis of Leishmania dermatitis should be based on the demonstration of the parasite in the inflammatory foci. However, examination of histological sections stained with hematoxylin–eosin (H&E) alone is frequently inconclusive due to (i) the possibility of there being an insufficient number of amastigotes of Leishmania; and (ii) the variable rate at which amastigotes can be seen in cases (14–80%) [5]. The development of the immunohistochemistry (IHC) technique has substantially improved diagnosis based on histopathology owing to the former’s higher sensitivity and specificity (18.2–100% of cases) [12, 13], together with its ability to correlate intralesional Leishmania amastigotes with the pathogenesis of lesions [14]. However, doubtful cases still emerge when the inflammatory infiltrate is compatible with Leishmania but neither H&E staining nor IHC are able to detect intralesional parasites. In practice, molecular methods (i.e. PCR) associated with histopathology are thus adopted as alternative or additional methods for diagnosing CanL cutaneous forms [15].
PCR on skin biopsies seems to be superior to other techniques in terms of sensitivity and specificity to detect Leishmania [2, 10, 15, 16]. However, the molecular identification of parasite DNA simply confirms the infection and that Leishmania is present in the skin; it does not necessarily indicate that the cutaneous lesions are the result of CanL [14].
Quantitative PCR (qPCR) overcomes some of the drawbacks of conventional PCR (cPCR) techniques, such as reducing the risk of contamination, decreasing the assay time and improving the detection limit [17,18,19]. It also gives a better correlation with the pathogenesis of the lesions alone or in association with IHC.
The aim of the retrospective study reported here was to evaluate the ability of IHC and qPCR methods to detect Leishmania infection in formalin-fixed and paraffin-embedded (FFPE) skin sections from dogs living in an endemic area for CanL and having a histopathologic diagnosis of LHD and/or GD without any previous suspicion of CanL (no suspicion or record of any preliminary tests in the history sheet). A detailed histopathologic examination was also conducted to demonstrate a possible correlation between the severity, pattern and depth of dermal inflammation and the parasite load as determined by qPCR and IHC. Identifying a pattern, depth and severity of dermal inflammation significantly associated with the parasite, and possibly the parasitic burden, would be useful to guide histopathologic diagnosis and choice of complementary tests to be performed or suggested on FFPE samples.
Methods
Skin sampling collection
The database repository of the Veterinary Pathology Service of the Veterinary Teaching Hospital (OVUD) of the Department of Veterinary Medicine of Perugia (central Italy) was searched to identify histopathology records of skin biopsies from dogs living in CanL endemic areas, namely those dogs recently estimated to be at medium–high risk (prevalence rates: 10–20%) for CanL [20].
The skin FFPE blocks of the dogs were included in the study if: (i) the histopathology previously performed by a board-certified (CB) veterinary pathologist confirmed features consistent with LHD and/or GD compatible with Leishmania infection; and (ii) an adequate amount of FFPE tissue (> 0.5 cm2) was stored in the biorepository at the time of the investigation. The present study, therefore, included 30 dogs whose samples has been collected between 2005 and 2020.
Histopathology and immunohistochemistry
Slides containing 4-µm-thick sections were prepared from the skin FFPE blocks and stained with H&E for histopathologic examination. H&E-stained slides were checked for lesions in the dermis as follows: inflammation (presence/absence); severity (mild, moderate, severe); pattern (perivascular, interstitial, band-like, nodular, diffuse); depth (superficial, mid dermis, deep); and relative percentage of inflammatory cells (macrophages, lymphocytes, plasma cells, neutrophils, multinucleated giant cells, eosinophils and mast cells). H&E staining was used to characterize amastigote forms of Leishmania according to their size, shape and location inside macrophages, and to estimate the parasite load. The number of microscopic fields that were positive for Leishmania amastigotes was counted, and the percentage of positive fields was calculated based on the average number of positive fields per skin sample. The grading system used for H&E-stained samples was: (i) negative (−, no amastigotes found); (ii) suspect [+/−, < 5% positive microscopic fields, at 400× magnification, field number (FN) = 22 mm], or samples where the detection of parasites was inconclusive or difficult and where differentiation from phagocytosed cell debris was necessary); (iii) positive [+, with mild parasite load (5–25% positive microscopic fields); ++, moderate parasite load (25–50% positive microscopic fields); +++, intense parasite load (> 50% positive microscopic fields)]. A minimum of 10 high-power fields at 400× magnification were evaluated.
For IHC, a protocol described by Tafuri et al. [13] was used, with slight modifications. Briefly, immunohistochemical labeling was performed on 4-μm-thick serial sections mounted on poly-L-lysine-coated slides. After deparaffination in xylene and rehydration in graded alcohols, antigen retrieval was performed in a microwave by immersion of slides in a pre-heated citrate solution (pH 6.0). The slides were then washed with Tris-buffered saline (TBS) buffer and incubated in 3% H2O2 for 10 min. After protein blocking (ab93677; Abcam, Cambridge, UK), the slides were incubated overnight in a humidified chamber with the serum of a dog naturally infected with Leishmania infantum (titre 1:320), at a 1:2000 dilution, which was applied as the primary antibody. As a secondary antibody, a horseradish peroxidase-conjugated goat polyclonal anti-dog antibody (ab112835; Abcam) was applied at 1:200 dilution and incubated for 1 h at room temperature. After the secondary antibody, immunolabeling was revealed with DAB (3,3′-diaminobenzidine; ab64238; Abcam), and Mayer’s hematoxylin was applied as a counterstain. A known positive skin sample from a sick dog due to CanL was used as a positive control. Negative controls were incubated with TBS, omitting the primary antibody. In addition, skin sections collected at necropsy from PCR-negative dogs from a non-endemic country were used as negative controls. The skin tissue parasite loads were semi-quantitatively analyzed by the presence of immunolabeled amastigote forms of Leishmania associated with the chronic inflammatory reaction in the dermis at 400× magnification; the grading system was the same as that described for the morphological (H&E) examination.
Molecular methods
Five 5-mm-thick sections of each FFPE skin tissue block were cut and handled with a new disposable razor blade and new gloves to prevent cross-contamination with Leishmania DNA. After each block was cut, the microtome blade, tweezers and entire cutting area were carefully cleaned with a 0.1 M solution of sodium hypochlorite to break down any potential contamination.
The sections were deparaffinized at room temperature by two consecutive immersion washes, 30 min each wash, in 1 ml of xylene and then rinsed twice, 5 min each rinse, with 1 ml of 100% ethanol. The samples were centrifuged at 10,000 g for 5 min, and the liquid was decanted between each change. Total genomic DNA was extracted using the ExgeneTM Clinic SV Mini Kit (GeneAll, Seoul, Korea), according to the manufacturer’s protocol. The extracted DNA was used in a cPCR protocol to amplify a final 120-bp fragment of conserved region of the Leishmania kinetoplastic (k) DNA minicircle, according to Francino et al. [21]. The Leishmania-specific primers used were NP13A (forward: 5′- AACTTTTCTGGTCCTCCGGG -3′) and NP13B (reverse: 5′- CCCCCAGTTTCCCGCCC -3′). Reaction mixtures were prepared in a total reaction volume of 50 μl that contained 25.0 μl of EconoTaq PLUS GREEN 2× Master mix (Lucigen Corporation, Middleton, WI, USA), 1 μM of sense primer, 1 μM of reverse primer and 1 μl of extracted DNA, ranging from 50 to 100 ng for each reaction. The conditions for the cPCR amplification were: initial denaturation at 94 °C for 5 min; denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 1 min, for 50 cycles; and a final extension at 72 °C for 5 min.
The reaction was carried out in a StepOnePlus™ instrument (Applied Biosystems, Thermo Fischer Scientific, Foster City, CA, USA). Each reaction included a negative (sterile water) and a positive control (DNA extracted from L. infantum-cultured promastigotes).
Samples (each sample: 15 μl) of the amplification products were electrophoresed in a 1.2% agar gel for 30 min at 100 V in TBE buffer (89 mM Tris borate, 2.0 mM EDTA, pH 8.3) with 5 ul of EuroSafe Nucleic Acid Stain (EuroClone S.p.A., Pero, Italy) and 10 ul of SharpMassTM 100 ready-to load DNA ladder (100 bp) (EuroClone S.p.A.) to determine the PCR fragment size. The gel was visualized under UV transillumination.
The amplicons obtained from cPCR were directly sequenced in both directions using a 16-capillary ABI PRISM 3130 × l Genetic Analyzer, assembled and edited with SeqScape software v 2.5 (all Applied Biosystems). The assembled sequences were compared to Leishmania spp. sequences available in GenBank using the Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/; accessed on 05 Feb 2022).
Detection and quantification of L. infantum DNA was performed using a real-time PCR commercial kit (TIB Molbiol, Genova, Italy), as described by Solano-Gallego et al. [22]. The kit was based on a couple of primers and a fluorescent resonance energy transfer (FRET) probe specific for L. infantum kinetoplast DNA minicircles. Real-time PCR was carried out using LightCycler FastStart DNA MasterPLUS Hybridization Probes (Roche, Mannheim, Germany) in a LightCycler II instrument (Roche); the composition of the reaction mix and thermal cycling conditions were according to the manufacturer’s protocol. A no-template control (water) and a negative control were included in the qPCR run in order to exclude the risk of contamination. Parasite load was quantified using the absolute quantification method. Serial tenfold dilutions of recombinant plasmid containing the target DNA with a known copy number (ranging from 1 × 108 to 1 × 100 copies/μl) were used to generate the qPCR standard curve. Results were expressed as target DNA copies per microliter (copies/μl) of extract.
A grading system based on the amount of target DNA copies per microliter of extract was used. Samples were arbitrarily classified as negative (−, no amplification), low (+, 1–100 copies/μl), moderate (++, 101–10,000 copies/μl) or intense (+++, > 10,000 copies/μl).
Statistical analysis
The frequencies of positive results obtained from all the skin samples through the diagnostic tests (e.g. H&E staining, IHC, cPCR and qPCR) with 95% confidence intervals (CI) were calculated and compared using the Chi-square test (χ2) test. An accepted level of significance was set at p < 0.05. In addition, the degree of agreement between the evaluated tests was determined by the Kappa coefficient (κ) value and interpreted as follows: κ = 0.01–0.20, scant agreement; κ = 0.21–0.4, fair agreement; κ = 0.41–0.60, moderate agreement; κ = 0.61–0.80, good agreement; and κ ≥ 0.80, almost total agreement.
A preliminary statistical descriptive analysis of the dependent variables was performed taking into consideration the severity, the depth and the pattern of dermal inflammation. The position indices (median and mode) were calculated in order to find the “central trend” of the variables, as well as to determine the value corresponding to the maximum observed absolute frequency. The associations between the histologic variables and grading of the parasitic load detected by H&E staining, IHC and qPCR were assessed using the χ2 test. To verify any correlations between variables, we used the Pearson test (Gaussian distribution data) and the Spearman test (non-normal data). Associations between histological variables and the presence of the parasite were assessed with the Mann–Whitney U-test. The calculations were carried out using the SPSS computer program for epidemiologist V.11.30 (IBM Corp., Armonk, NY, USA).
Results
The overall results for the positivity rates detected by H&E staining, IHC, cPCR and qPCR techniques are shown in Table 1.
The qPCR technique had the highest rate for detecting Leishmania, followed, in order of decreasing detection rate, by IHC and cPCR and H&E. The results of the severity, pattern and depth of the dermal inflammatory infiltrates as well as the parasite load graded using histologic, IHC and biomolecular methods are shown in Table 2.
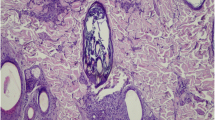
Amastigote forms of Leishmania in H&E-stained sections were visualized in 8/30 samples (positivity rate: 26.66%); five skin samples (16.66%) were considered suspicious (+/−) for Leishmania but the results were inconclusive. No amastigotes were detected in the remaining 17 skin samples (56.68%). The parasite load detected by H&E staining was mild (+) in 3.33% (1/30) of skin samples, moderate (++) in 10% (3/30) (Fig. 1a) and intense (+++) in 13.33% (4/30). The results of the IHC revealed that 14/30 (46.66%) of the skin samples were positive for Leishmania, while the findings for one (3.33%) skin sample was considered to be inconclusive for amastigote. In 15 skin samples (50.00%), no amastigotes were detected. The parasite load within lesions was considered to be mild (+) in seven cases (23.33%) (Fig. 1b), moderate (++) in three cases (10%) and intense (+++) in four cases (13.33%).
Morphological and immunohistochemical features of Leishmania dermatites. a An interstitial, multifocal image of coalescing, lymphoplasmacytioc amd histiocyitc dermatites in the superficial dermis. Insert: Amastigotes of Leishmania can be seen in the cytoplasm of macrophages (H&E, 40×; ID number: 3). b Immunohistochemistry showing positivity for anti-Leishmania antibody in the cytoplasm of macrophages, as indicated by the arrowheads (IHC, 40×; ID number: 6)
Parasite DNA was detected in the analyzed tissue in 14/30 samples (46.66%) by cPCR, and sequencing of amplicons confirmed that L. infantum was the species isolated; in comparison, 21/30 skin samples (70%) tested positive by qPCR (Table 2). The parasite load within lesions detected by qPCR was extremely variable, ranging from 1.32 to 62.700 copies. Depending on the grading system adopted, there was a low (+) parasitic load in seven samples (23.33%), a moderate (++) parasitic load in five samples (16.66%) and an intense parasitic load (+++)(30%) in nine tissue samples.
Of the 30 skin samples, 23 tested positive for Leishmania, as demonstrated by the results of the DNA amplification of the parasite and/or the presence of visible amastigotes by histologic or immunohistochemical assay. Of these 23 Leishmania-positive skin samples, six with predominantly moderate or severe parasitic loads were positive in all four tests, ten were positive in at least two diagnostic techniques and seven were only positive for one of the molecular techniques (cPCR or qPCR). The remaining seven samples were totally negative for all the tests applied (Table 2). Based on these results, we identified two groups: Group A, which consisted of samples that tested positive for the diagnosis of Leishmania dermatitis (n = 16 cases), with morphologic evidence of the parasite in the skin lesions and positive molecular tests supporting the morphologic results; and Group B, which consisted of skin samples that tested negative for the diagnosis of Leishmania dermatitis (n = 14 cases) and for which no parasite could be demonstrated by any of the tested techniques, and samples that, despite testing positive for parasite DNA amplification, the actual presence of the parasite in the skin lesions could not be demonstrated.
Regarding the main histopathologic findings observed in the skin biopsies, the most common dermal change found was a moderate (13/30, 43.3%), nodular (8/30, 26.6%) inflammation located in the mid or deep dermis (both 12/30, 40% each). However, considering Group A and Group B separately, there was a statistically significant difference in the degree of inflammation between the positive and negative samples (p < 0.037). In skin samples in Group A, the inflammatory infiltrate was predominantly moderate (7/16, 43.7%) or severe (8/16, 50%), whereas in those of Group B, dermal inflammation was predominantly mild (5/14, 35.7%) or moderate (6/14, 42.9%). Although not statistically significant, the positive group more often had a nodular (5/16, 31.3%), interstitial (5/16, 31.3%) or diffuse (3/16, 18.7%) distribution of inflammation, with a depth in the mid (8/16, 50%) or deep (7/16, 43.8%) dermis. On the other hand, the negative group more often had a perivascular (5/14, 35.7%) or nodular (3/14, 21.5%) distribution of inflammation, localized in the superficial (4/14, 28.6%) or mid (4/14, 28.6%) dermis. Overall, the severity of inflammation correlated with the depth of the inflammatory infiltrate (ρ = 0.741, p < 0.001).
Table 3 shows a comparison of the results obtained with the H&E staining, IHC and biomolecular techniques (i.e. cPCR and qPCR) and their relative agreement.
No statistical difference in the rates of positivity was observed between the H&E staining, cPCR and IHC techniques (p = 0.1), although a lower number of positive samples was detected with H&E staining than with the cPCR and IHC methods [8 vs 14 [for cPCR and IHC, respectively]). The level of agreement between H&E and cPCR was fair (66%, κ = 0.32), and that between H&E and IHC was moderate (80%, κ = 0.58). All the H&E-positive tissue samples were also positive by IHC. Of the five skin samples found to be suspicious but inconclusive by H&E staining, three were confirmed to be positive by cPCR and IHC, with a mild to moderate parasitic load. The agreement between IHC and cPCR was good (κ = 0.65), except in five samples where cPCR was positive and IHC was negative (2/5), or the other way round (3/5). The rate of positivity detected by IHC and cPCR was lower than that obtained by qPCR (14 vs 21), but the difference was not significant (p = 0.18). Moderate agreement (70%, κ = 0.41) was found between IHC and qPCR and fair agreement (63%, κ = 0.28) was found between cPCR and qPCR. Of the 14 positive IHC samples, 13 were also positive according to qPCR; however, qPCR detected six additional positive samples compared to IHC.
The rates of positivity detected by H&E staining and qPCR were statistically different (p < 0.001), with a higher number of positives (21 vs 8) detected by qPCR and a a fair degree of agreement (56%, κ = 0.27). All of the positive samples by H&E staining were also positive by qPCR. Four of the five skin samples found to be suspicious but inconclusive by H&E staining were confirmed to be positive by qPCR, with mainly a moderate parasitic load.
No association between any of the variables tested was found (p > 0.05), with the exception of a significant association between the severity of dermal inflammatory infiltrate and parasitic skin load detected by IHC (p = 0.029) (Table 4). However, a weak linear correlation was detected (r = 0.26, p = 0.099).
Discussion
In the present study we evaluated the ability of different techniques and methods, such as IHC and qPCR, to detect Leishmania infection in skin samples that have histologic findings compatible or suggestive of CanL. These techniques are often used, or at least have been proposed to be used in a histopathologic diagnostic setting due to the clinical-anamnestic data frequently being incomplete. Demonstrating the presence of Leishmania within the skin lesion is often essential to a diagnosis of CanL due to the histopathologic pattern not being specific and the potential for the parasite to be present in lesions caused by other infectious (GD caused by Toxoplasma spp., Histoplasma spp., Sporothrix spp., Blastomyces spp., etc.) and non-infectious processes (such as sterile granuloma and pyogranuloma syndrome, granulomatous form of sebaceous adenitis), and in healthy skin due to the role played by such tissue in the epidemiology of CanL [23, 24].
Selection of the tissue samples included in the study was based exclusively on morphologic (H&E) inclusion criteria and with the authors blinded to both the presence/absence of parasite in skin lesions and to the infection status of the animals. Since a gold test result was not available, the true sensitivity and specificity of the different techniques could not be evaluated, and we therefore exclusively tested their ability to guide the histopathologic diagnosis in cases of dermal inflammation compatible with CanL cutaneous lesions.
Our results highlight that there is an increase in the detection rate of Leishmania when highly sensitive techniques are used, as has been described earlier [25, 26]. However, of the 23 skin samples that tested positive for Leishmania, 17 showed discordant results with the four techniques used, thereby confirming that there is no single suitable test for the diagnosis of cutaneous lesions caused by Leishmania and that histological and molecular methods need to be combined.
The best diagnostic performance was obtained by qPCR followed, in decreasing order of diagnostic performance, by cPCR/IHC and H&E staining. This finding reinforces previous findings that the qPCR method detects the presence of Leishmania DNA better than other techniques in both the skin and in many other tissues (i.e. blood, lymph node, spleen and bone marrow), even when conducted on FFPE tissues, which might result in DNA degradation [27, 28]. Although qPCR is an excellent tool to assess the level of exposure of a canine population to Leishmania infection, on the basis of the present results it does not represent the technique of choice for a histopathologist to attribute a skin lesion to Leishmania since its results are not associated with either the characteristics or the severity of the lesions. In addition, the feasibility of using expensive molecular techniques, such as the qPCR, in laboratories with limited resources in developing countries must be considered.
Direct microscopic examination of parasites with H&E staining is considered to be a technique specific for this purpose and used in routine evaluation. However, H&E staining has a limited sensitivity and when few amastigotes are present, these amastigotes can be easily overlooked or they can be mistaken for cellular debris phagocytosed by macrophages in an inflammatory process [29]; in both cases, the result may be an inconclusive diagnosis. IHC has been confirmed to have an improved sensitivity relative to H&E staining since it provides a higher degree of contrast between parasites and host tissues, giving unequivocal results for diagnostic purposes, even when there is a low parasitic burden. Our results are in agreement with this finding, showing that while the results for several of the analyzed skin samples (16.66%) were considered inconclusive by H&E labeling, they were confirmed in 60% of cases using IHC, with mild to moderate parasite loads. cPCR [15, 30], especially when based on kinetoplast DNA amplification, as one used in the present study, is generally considered to be more sensitive than IHC, since it amplifies a multicopy genetic target present at high copies per cell [31, 32]. However, in our study, the discriminative power of cPCR was the same as that of IHC, and the rate of agreement with qPCR was higher for IHC than for cPCR (moderate vs fair, respectively). We cannot exclude that formalin fixation could result in DNA degradation, thus reducing the power of parasite detection by this technique; however we have to consider that the amplified codon was short and the target sequence is highly repeated.
Cutaneous lesions in CanL are very pleomorphic, both from a clinical and a histologic point of view [5, 33]. They can have a different pathogenesis, with inflammatory-mediated and immune-mediated changes being the most common. Each clinical presentation has been associated with particular histologic changes involving the dermis, epidermis, hair follicles and sebaceous glands [5]. A common trait for the different clinical presentations is the presence of an inflammatory infiltrate that predominantly consists of macrophages, lymphocytes and plasma cells. Dogs with less severe clinical stages of the disease (Stage I) are more frequently characterized by nodular to diffuse patterns of inflammation, a significant higher frequency of granuloma formation and a higher detection of amastigote DNA by qPCR than dogs with more severe clinical stages (Stage II–III) [3]. Also, the frequency and intensity of granuloma formation and of a monomorphic macrophage inflammatory infiltrate have been found to be significantly associated with skin parasitism [9]. Since the more severe dermal inflammation observed in Group A samples (positive for Leishmania dermatitis) was more frequently characterized by a nodular and diffuse pattern, we were able to partially confirm these results. Further studies with a larger cohort and clinical information are recommended.
Due to the retrospective nature of the study, no information was available on the clinical status of the animals and the staging of the disease. However, a significant difference was demonstrated between the severity of dermal inflammatory infiltrate between Groups A and B, and the severity of dermal inflammation was associated with the parasitic skin load detected by IHC, although with a weak linear correlation. This weak link may be due to the interaction between the parasite and host being quite enigmatic; in fact, host defense against parasitic infection depends on the activation response of macrophages to the stimuli and the receptors involved and, ultimately, on the anti-inflammatory or proinflammatory profile developed [34], which makes the distribution and intensity of the inflammatory response variable.
In clinically normal skin of Leishmania-infected dogs that show no clinical signs and are seronegative or mildly seropositive, no histological lesions and parasite detection by IHC have been demonstrated [10]; our results partially contradict these findings because Group B included seven samples that tested negative by IHC but for which variable degrees of inflammation associated with positive cPCR or qPCR were observed. We cannot rule out that the inflammation in these cases could be related to another skin disease since (i) biomolecular results should always be interpreted cautiously [4] and (ii) inflammatory infiltrates are a common histological finding in the skin of dogs from endemic CanL areas, irrespective of the presence of parasites or evidence of infection [9].
Conclusions
The recommendation arising from this work, and which will obviously need to be confirmed with a larger cohort of samples, is to first perform a histologic examination; once the presence of a moderate/severe LHD and/or GD has been identified, in absence of a direct demonstration of Leishmania, a immunohistochemical investigation should be carried out, as this test has proven to be the only technique capable of clearly defining the pathogenesis of the lesion. However, the case of mild lesions is more complex; IHC, although being able to visualize the parasite in the inflammatory cells, then loses its discriminatory power, possibly leading to an underestimation of the role of the parasite in the skin lesion. In these cases, qPCR could be of help, given its greater detection ability, although the results should be interpreted with caution and following the published guidelines for diagnosis of leishmaniosis in dogs (CLWG) [6]. In addition, in an endemic area, all history sheets of cutaneous biopsies from dogs should include the results of at least one quantitative serological test before the biopsy is sent to the pathologist.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CanL:
-
Canine leishmaniosis
- CLWG:
-
Canine Leishmaniasis Working Group
- cPCR:
-
Conventional PCR
- DAT:
-
Direct agglutination test
- ELISA:
-
Enzyme-linked immunosorbent assay
- FFPE:
-
Formalin-fixed and paraffin-embedded
- GD:
-
Granulomatous dermatitis
- H&E:
-
Hematoxylin and eosin
- IFAT:
-
Immunofluorescence antibody test
- IHC:
-
Immunohistochemistry
- LHD:
-
Lymphoplasmacytic/histiocytic dermatitis
- qPCR:
-
Quantitative PCR
References
Ciaramella P, Oliva G, De Luna R, Gradoni L, Ambrosio R, Cortese L, et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec. 1997;141:539–43.
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. https://doi.org/10.1186/1756-3305-4-86.
Ordeix L, Dalmau A, Osso M, Llull J, Montserrat-Sangrà S, Solano-Gallego L. Histological and parasitological distinctive findings in clinically-lesioned and normal-looking skin of dogs with different clinical stages of leishmaniosis. Parasit Vectors. 2017;10:1–8.
Solano-Gallego L, Koutinas A, Miró G, Cardoso L, Pennisi MG, Ferrer L, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol. 2009;165:1–18.
Saridomichelakis MN, Koutinas AF. Cutaneous involvement in canine leishmaniosis due to Leishmania infantum (syn. L. chagasi). Vet Dermatol. 2014;25:61–72.
Paltrinieri S, Solano-gallego L, Fondati A, Lubas G, Gradoni L, Castagnaro M, et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1184–91.
Mohammadiha A, Haghighi A, Mohebali M, Mahdian R, Abadi AR, Zarei Z, et al. Canine visceral leishmaniasis: a comparative study of real-time PCR, conventional PCR, and direct agglutination on sera for the detection of Leishmania infantum infection. Vet Parasitol. 2013;192:83–90.
Baneth G, Solano-Gallego L. Greene: infectious diseases of the dog and cat. 4th ed. Philadelphia: Elsevier Saunders; 2012.
Dos-Santos WLC, David J, Badaró R, De-Freitas LAR. Association between skin parasitism and a granulomatous inflammatory pattern in canine visceral leishmaniosis. Parasitol Res. 2004;92:89–94.
Solano-Gallego L, Fernández-Bellon H, Morell P, Fondevila D, Alberola J, Ramis A, et al. Histological and immunohistochemical study of clinically normal skin of Leishmania infantum-infected dogs. J Comp Pathol. 2004;130:7–12.
Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin diseases of the dog and cat—clinical and histopathologic diagnosis. Oxford: Blackwell; 2005.
de Amorim IFG, Da Silva SM, Figueiredo MM, Moura EP, de Castro RS, de Souza Lima TK, et al. Toll receptors type-2 and CR3 expression of canine monocytes and its correlation with immunohistochemistry and xenodiagnosis in visceral leishmaniasis. PLoS ONE. 2011. https://doi.org/10.1371/journal.pone.0027679.
Tafuri WL, Santos RDL, Arantes RME, Gonçalves R, De Melo MN, Michalick MSM, et al. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J Immunol Methods. 2004;292:17–23.
Di Mattia D, Fondevila D, Abramo F, Fondati A. A retrospective histopathological, immunohistochemical and molecular study of the presence of Leishmania spp. in the skin of cats with head and neck ulcerative dermatitis. Vet Dermatol. 2018;29:212-e76.
Xavier SC, De Andrade HM, Monte SJH, Chiarelli IM, Lima WG, Michalick MSM, et al. Comparison of paraffin-embedded skin biopsies from different anatomical regions as sampling methods for detection of Leishmania infection in dogs using histological, immunohistochemical and PCR methods. BMC Vet Res. 2006;2:1–7.
de Queiroz NM, da Silveira RC, de Noronha AC, Oliveira TM, Machado RZ, Starke-Buzetti WA. Detection of Leishmania (L.) chagasi in canine skin. Vet Parasitol. 2011;178:1–8.
Rolão N, Cortes S, Rodrigues OR, Campino L. Quantification of Leishmania infantum parasites in tissue biopsies by real-time polymerase chain reaction and polymerase chain reaction-enzyme-linked immunosorbent assay. J Parasitol. 2004;90:1150–4.
Gomes YM, Paiva Cavalcanti M, Lira RA, Abath FGC, Alves LC. Diagnosis of canine visceral leishmaniasis: biotechnological advances. Vet J. 2008;175:45–52.
Mohammadiha A, Mohebali M, Haghighi A, Mahdian R, Abadi AR, Zarei Z, et al. Comparison of real-time PCR and conventional PCR with two DNA targets for detection of Leishmania (Leishmania) infantum infection in human and dog blood samples. Exp Parasitol. 2013;133:89–94.
Di Muccio T, Veronesi F, Antognoni MT, Onofri A, Fioretti DP, Gramiccia M. Diagnostic value of conjunctival swab sampling associated with nested PCR for different categories of dogs naturally exposed to Leishmania infantum infection. J Clin Microbiol. 2012;50:2651–9.
Francino O, Altet L, Sánchez-Robert E, Rodriguez A, Solano-Gallego L, Alberola J, et al. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet Parasitol. 2006;137:214–21.
Solano-Gallego L, Rodriguez-Cortes A, Trotta M, Zampieron C, Razia L, Furlanello T, et al. Detection of Leishmania infantum DNA by fret-based real-time PCR in urine from dogs with natural clinical leishmaniosis. Vet Parasitol. 2007;147:315–9.
Michalsky ÉM, Rocha MF, da Rocha LACVM, França-Silva JC, Pires MQ, Oliveira FS, et al. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies. Vet Parasitol. 2007;147:67–76.
Giunchetti RC, Mayrink W, Genaro O, Carneiro CM, Corrêa-Oliveira R, Martins-Filho OA, et al. Relationship between canine visceral leishmaniosis and the Leishmania (Leishmania) chagasi burden in dermal inflammatory foci. J Comp Pathol. 2006;135:100–7.
Müller N, Hentrich B, Frey CF, Welle M. Quantitative PCR for the diagnosis of cutaneous leishmaniasis from formalin-fixed and paraffin-embedded skin sections. Mol Cell Probes. 2015;29:507–10.
de Almeida FS, Souza Leite R, Trindade Ituassu L, Almeida GG, Souza DM, Fujiwara RT, et al. Canine skin and conjunctival swab samples for the detection and quantification of Leishmania infantum DNA in an endemic urban area in Brazil. PLoS Negl Trop Dis. 2012;6: e1596.
Belinchón-Lorenzo S, Iniesta V, Parejo JC, Fernández-Cotrina J, Muñoz-Madrid R, Soto M, et al. Detection of Leishmania infantum kinetoplast minicircle DNA by Real Time PCR in hair of dogs with leishmaniosis. Vet Parasitol. 2013;192:43–50.
Rodríguez-Cortés A, Ojeda A, López-Fuertes L, Timón M, Altet L, Solano-Gallego L, et al. A long term experimental study of canine visceral leishmaniasis. Int J Parasitol. 2007;37:683–93.
Bourdoiseau G, Marchal T, Magnol JP. Immunohistochemical detection of Leishmania infantum in formalin-fixed, paraffin-embedded sections of canine skin and lymph nodes. J Vet Diagn Investig. 1997;9:439–40.
Roura X, Fondevila D, Sánchez A, Ferrer L. Detection of Leishmania infection in paraffin-embedded skin biopsies of dogs using polymerase chain reaction. J Vet Diagn Investig. 1999;11:385–7.
Noyes HA, Reyburn H, Bailey JW, Smith D. A nested-PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol. 1998;36:2877–81.
Lachaud L, Chabbert E, Dubessay P, Dereure J, Lamothe J, Dedet JP, et al. Value of two PCR methods for the diagnosis of canine visceral leishmaniasis and the detection of asymptomatic carriers. Parasitology. 2002;125:197–207.
Ordeix L. The spectrum of cutaneous manifestations in canine leishmaniosis: insights into diagnosis and immune responses. Doctoral thesis. Barcelona: Servei de Publicacions de la Universitat Autònoma de Barcelona; 2018.
Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D’Herde K, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–22.
Acknowledgements
The authors are grateful to Valeria Migni, Sara Leto, and Luca Stefanelli for their technical assistance.
Funding
This research was funded by the University of Perugia under the program “Ricerca di Base 2018”.
Author information
Authors and Affiliations
Contributions
IP, GM, FV and CB conceived and designed the study. IP, GM and MTA performed the sampling. IP, GM, KMW, CB, TF and SDA performed the formal analysis and visualization. FV and CBQ acquired and analyzed the data. FB and CB wrote the first draft and subsequent revisions. All authors edited the manuscript and commented on the text. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The Authors have declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Porcellato, I., Morganti, G., Antognoni, M.T. et al. Comparison of immunohistochemical and qPCR methods from granulomatous dermatitis lesions for detection of leishmania in dogs living in endemic areas: a preliminary study. Parasites Vectors 15, 104 (2022). https://doi.org/10.1186/s13071-022-05218-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05218-6