Abstract
Background
The secondary alcohol 2-butanol has many important applications, e.g., as a solvent. Industrially, it is usually made by sulfuric acid-catalyzed hydration of butenes. Microbial production of 2-butanol has also been attempted, however, with little success as witnessed by the low titers and yields reported. Two important reasons for this, are the growth-hampering effect of 2-butanol on microorganisms, and challenges associated with one of the key enzymes involved in its production, namely diol dehydratase.
Results
We attempt to link the metabolism of an engineered Lactococcus lactis strain, which possesses all enzyme activities required for fermentative production of 2-butanol from glucose, except for diol dehydratase, which acts on meso-2,3-butanediol (mBDO), with that of a Lactobacillus brevis strain which expresses a functional dehydratase natively. We demonstrate growth-coupled production of 2-butanol by the engineered L. lactis strain, when co-cultured with L. brevis. After fine-tuning the co-culture setup, a titer of 80 mM (5.9 g/L) 2-butanol, with a high yield of 0.58 mol/mol is achieved.
Conclusions
Here, we demonstrate that it is possible to link the metabolism of two bacteria to achieve redox-balanced production of 2-butanol. Using a simple co-cultivation setup, we achieved the highest titer and yield from glucose in a single fermentation step ever reported. The data highlight the potential that lies in harnessing microbial synergies for producing valuable compounds.
Similar content being viewed by others
Background
Fermentative production of bio-ethanol is a classic example of microbial solutions for bio-based fuel production [1]. Ethanol, however, compared to medium length alcohols, such as butanol, has less desirable fuel properties [2]. Atsumi et al. successfully demonstrated the feasibility of producing different butanol isomers by coupling branched chain amino acid synthesis with the Ehrlich pathway [3], however, this approach is not applicable for producing 2-butanol and despite several attempts at its bio-production, so far only limited success has been reported.
Production of 2-butanol therefore relies on chemical synthesis, and currently 811,000 tons are being produced annually [4]. Besides its potential to serve as a biofuel, 2-butanol has numerous applications, e.g., as solvent or in perfume manufacturing [4].
Microbial production of 2-butanol from sugar has been achieved in Klebsiella pneumonia [5] albeit with low titers. Very recently, it was reported that 13.4 g/L 2-butanol could be produced from mBDO. The mBDO was generated by Serratia marcescens and subsequently converted into 2-butanol by Lactobacillus diolivorans [6]. There are clear limitations to using this approach, e.g., a very low yield of only 0.24 mol/mol glucose, and formation of large amounts of by-products such as acetate, ethanol, and lactate (in total 815 mM, 4.5 mol per mol 2-butanol). Furthermore, the need for a 30-min heat treatment to inactivate S. marcescens, and the use of this opportunistic pathogen for producing mBDO, appear not to be compatible with large-scale production of 2-butanol. Cell-free multi-enzyme catalysis has also been utilized for synthesis of 2-butanol from ethanol through continued supply of coenzyme B12 and ATP [7]. Additionally, 1.3 g/L butanone was made from glycerol through 3-ketovaleryl-CoA and subsequent decarboxylation [8], however, significant amounts of acetone was generated as by-product. Thus, there is room for further improvements in microbial 2-butanol production.
Production of 2-butanol in one-step fermentation setups typically involves the conversion of pyruvate into α-acetolactate, a reaction catalyzed by the α-acetolactate synthase. The α-acetolactate then undergoes decarboxylation into acetoin and reduction into mBDO. mBDO is subsequently dehydrated to 2-butanone followed by reduction 2-butanol.
Notably, the dehydration of mBDO to 2-butanone is carried out by the coenzyme B12-dependent diol or glycerol dehydratases [9], which are typically found in microorganisms capable of producing 1,3-propanediol [10]. B12-independent dehydratases have been described in Clostridium butyricum, however, these require an S-adenosyl methionine co-factor [11]. The coenzyme B12-dependent dehydratase reaction is oxygen sensitive and susceptible to irreversible inactivation when substrates such as glycerol and mBDO are used [12, 13]. To maintain catalytic activity, the microorganisms rely on dehydratase re-activation systems, consisting of reactivases, that consume ATP to restore catalytic activity [14]. The intracellular activity of the dehydratase is known to be influenced by several factors such as carbon source, growth phase, and the availability of inducer molecules [15].
Interestingly, the obligate heterofermentative Lactobacillus brevis was found to be capable of producing 2-butanol from the mBDO produced by yeast during wine fermentation [16]. Later, the diol dehydratases from Lactobacillus brevis were found to be superior to dehydratases from Klebsiella oxytoca and Salmonella enterica [17]. Lactic acid bacteria (LAB), best known for their application in dairy fermentations and as human probiotics, have recently been demonstrated to have great potential for use in biotechnological applications [18]. The emergence of tools for genetic engineering of LAB [19], combined with their high metabolic rates and fast growth [20], make them interesting candidates for production of biofuels. One particular LAB, Lactococcus lactis, has received a lot of attention, and has been metabolically engineered into producing a broad variety of useful compounds [21].
In our previous work, we constructed an L. lactis strain that could be used as a platform for producing various pyruvate-derived compounds, with little by-product formation [22]. Recently, we expanded the metabolic repertoire of this strain by introducing genes needed for production of mBDO [23], the precursor for 2-butanol.
In the current study, we first investigate whether L. lactis is the right platform for producing 2-butanol and we do this by introducing a diol dehydratase from Klebsiella oxytoca and a 2-butanol dehydrogenase from Achromobacter xylosoxidans. Challenges in achieving a functional diol dehydratase prompt us to try out a different strategy, namely co-cultivation, where we explore whether the diol dehydratase of L. brevis can complement an incomplete 2-butanol biosynthetic pathway in an engineered L. lactis strain. We show that co-cultivation is an efficient approach for producing 2-butanol, and achieve the highest reported titer and yield from glucose in a one-step fermentation process.
Results and discussion
Assessing the potential of L. lactis for 2-butanol production
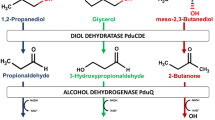
Lactococcus lactis is an established industrial workhorse within the dairy industry, where it is used to ferment in excess of 100 mio. tonnes of milk annually [24]. This lactic acid bacterium grows well, is easy to manipulate genetically [25,26,27] and there are many reports on its use as an efficient cell factory for producing useful compounds [21, 23, 28, 29]. Here we explore whether L. lactis can be transformed into a 2-butanol-producing cell factory. To assess the potential of L. lactis to become an efficient 2-butanol producer, we first introduced two genes necessary for 2-butanol formation from mBDO, namely a diol dehydratase for converting BDO into 2-butanone, and an alcohol dehydrogenase for reducing 2-butanone into 2-butanol. We used the L. lactis strain CS4363, which lacks lactate dehydrogenase, phosphotransacetylase, and alcohol dehydrogenase activities, and can only grow under aerated conditions where NADH oxidase regenerates NAD+ and its sole fermentation product is acetoin. By introducing the diol dehydratase and alcohol dehydrogenase enzyme activities into CS4363, redox-balanced production of 2-butanol from mBDO should in principle be possible (Fig. 1a). For the diol dehydratase, we decided to rely on the enzyme complex from K. oxytoca (PddABC), and the alcohol dehydrogenase was obtained from A. xylosoxidans (SadB). SadB has previously been found to be efficient at converting 2-butanone into 2-butanol [30] and the diol dehydratase from K. oxytoca has previously been demonstrated to be efficient at dehydrating mBDO [17]. One concern when using diol dehydratases for dehydrating mBDO, is substrate inactivation, and the enzyme needs to be re-activated by a dedicated reactivase. For this reason we additionally introduced the diol dehydratase reactivase from K. oxytoca (DdrAB), as the beneficial effect of this has been demonstrated previously [31].
Linking the metabolism of L. lactis and L. brevis to achieve 2-butanol production. a Metabolic pathway based on L. lactis CS4363 (mBDO added in the medium). The constructed strain encodes: PddABC, diol dehydratase, and DdrAB, reactivase from K. oxytoca; SadB, secondary alcohol dehydrogenase from A. xylosoxidans. Pathways in gray indicate activities that have been eliminated. Dashed lines indicate multiple enzymatic steps. b The combined metabolic pathway for L. lactis and L. brevis. Bdh, butanediol dehydrogenase from Enterobacter cloacae. DDH, diol dehydratase; DDHr, diol dehydratase reactivate; SAD, secondary alcohol dehydrogenase. Only heterologously expressed gene activities in L. lactis and activities related to 2-butanol synthesis in L. brevis are highlighted
After introducing the genes, we verified the respective enzyme activities. We found that the recombinant strain, in contrast to its parent lacking 2-butanol dehydrogenase activity (SadB), could grow anaerobically in the presence of 2-butanone with concurrent formation of 2-butanol, which confirmed the presence of SadB activity. The diol dehydratase activity was measured in crude cell extracts and was shown to be 0.32 ± 0.01 µmol min−1 mg protein−1. We subsequently examined if the engineered strain could grow and produce 2-butanol from mBDO in medium containing coenzyme B12, a co-factor needed for the function of the diol dehydratase. However, we did not observe restoration of anaerobic growth or formation of 2-butanol when mBDO was supplied. L. lactis lacks genes involved in coenzyme B12 biosynthesis, and our findings that coenzyme B12 is not taken up by the intact cells, is in accordance with the absence of an uptake system for vitamin B12 in L. lactis [32].
Lactobacillus brevis can serve as a whole-cell diol dehydratase catalyst
The observation above strongly indicates that the reason why our engineered L. lactis strain cannot produce 2-butanol is due to low or no diol dehydratase activity resulting from the lack of coenzyme B12 uptake. In principle, we could pursue heterologous introduction of genes involved in B12 synthesis from organisms possessing these, e.g., Lactobacillus reuteri [33], but the B12 synthesis pathway is encoded by 29 genes [34]. As an alternative, we decided to explore whether the diol dehydratase activity could be supplied, in-trans, from a second strain, used as a whole-cell catalyst. In the subsequent experiments we chose to express the Enterobacter cloacae meso-2,3-butanediol dehydrogenase (Bdh) and the 2-butanol dehydrogenase (SadB) in L. lactis, thus generating a strain which in principle only lacks a diol dehydratase in order to be able to generate 2-butanol (Fig. 1b). As a source of the diol dehydratase we chose L. brevis SE20, which previously has been shown to produce 2-butanol when supplied with mBDO and vitamin B12 [35].
Our hypothesis was that the mBDO formed in L. lactis would leave the cells and enter the L. brevis cells to be dehydrated into 2-butanone. 2-butanone would subsequently leave the L. brevis cells, reenter the L. lactis cells and be reduced into 2-butanol. In this way, the metabolism of L. lactis would be redox balanced, since the two NADH generated in glycolysis would be consumed by the 2,3-butanediol dehydrogenase and the 2-butanol dehydrogenase.
We found that glucose was a poor substrate for L. brevis, probably due to a low ATP yield on glucose of only one [36]. On xylose, however, the ATP yield is two (see Additional file 1: Figure S1). Using xylose as a fermentation substrate could be of interest, since this sugar is abundant in lignocellulose. However, since we intend to use L. brevis as an mBDO dehydratase cell catalyst, it is relevant to investigate on which substrate the highest in vivo enzyme activity is attained. We found a ninefold higher mBDO dehydratase activity for cells grown on glucose when compared to cells grown on xylose, and that mBDO acted as an inducer of activity (Table 1). For the following experiments, we therefore decided to rely on L. brevis cells grown on glucose.
The next step was to test if the diol dehydratase activity from L. brevis could complement the metabolism of the engineered L. lactis, and thereby enable production of 2-butanol by L. lactis. Indeed, 2-butanol synthesis was achieved in defined medium (SA) supplemented with 7.5 µM vitamin B12 and 5 mM 2-butanone. After 20 h, a titer of 14.2 ± 0.6 mM, with a yield of 0.5 ± 0.02 mol/mol was obtained. Production of 2-butanol was not observed when the cultures were incubated in medium without a small “catalytic” amount of 2-butanone added, which we speculate helped in the linking of the metabolisms of the two bacteria. We also demonstrated that 2-butanol production could be accomplished using a mix of lactose and xylose, although with a lower titer and yield (see Additional file 1: Table S1).
Co-cultivation of engineered L. lactis and L. brevis in M17 broth
After demonstrating proof-of-principle, we established a fermentation setup for co-culturing the two strains to enable more efficient 2-butanol production. The aim was to create an environment supporting a high metabolic flux in L. lactis, thus enabling efficient 2-butanol production, while concurrently preserving a high dehydratase activity in L. brevis. For the latter, an active L. brevis metabolism is needed, as re-activation of the diol dehydratase requires ATP. We decided to use rich M17 medium supplemented with 2% glucose, which supports optimal growth of L. lactis and to this medium 7.5 µM B12 was added. It has been shown previously that the ratio between the different strains present in a co-culture has a great impact on product formation [37]. For this reason, three different inoculation ratios of L. lactis to L. brevis were tested, 1:1, 1:4, and 4:1, using cell densities corresponding to an OD600 of either 0.06 or 0.24.
We found that 2-butanol was formed, when using M17 medium as well (Fig. 2 and Table 2). When using M17 medium, it was not necessary to add 2-butanone to facilitate 2-butanol generation. The best performance was observed when an excess of L. lactis was used (inoculation ratio 4:1), with a production of 80.0 ± 1.0 mM (5.9 ± 0.1 g/L) 2-butanol and a yield of 0.58 ± 0.01 mol/mol.
The 4:1 culture also resulted in the lowest production of the by-products acetate, ethanol, and lactate. None of the co-cultivations showed significant buildup of 2-butanol precursors, which suggests an effective transfer of intermediates between the two strains. 2-Butanol was not produced in any of the control cultivations with L. lactis or L. brevis alone, and only modest glucose consumption was observed in these cultures (data not shown). Additionally, growth of the L. lactis strain was dependent on the catalytic activity of the L. brevis strain.
Formation of the by-products acetate, ethanol, and lactate during co-cultivation was from 79 to 136 mM, as compared to L. brevis alone where 56 ± 1.5 mM was produced. The increase in by-product formation observed in the co-cultures suggests that L. brevis, in addition to catalyzing the conversion of mBDO to 2-butanone, reduce some of the 2-butanone to 2-butanol. This issue becomes more pronounced at higher initial culture ratios where the lack of 2-butanone in combination with the high acid production by L. brevis begins to inhibit L. lactis, which then reaches lower CFU/mL.
It therefore appears to be important to restrict the amount of L. brevis cells present to avoid excessive consumption of 2-butanone, while simultaneously ensuring that a sufficient diol dehydratase activity is available. We tested other inoculation ratios as well, however, this did not lead to higher yields of 2-butanol (Fig. 3). Previous research into co-culture fermentations highlights division of labor and functional enzyme expression to be the main burden of monocultures, whereas co-culturing is constrained by the need of population control and possible limitation by transfer of intermediates [38, 39].
We believe that there is potential for improving the titer and yield of 2-butanol by further engineering of the strains and by optimizing the fermentation setup. In the setup used here, we relied on a wild-type L. brevis strain to supply the important diol dehydratase activity. Protein engineering has been used to improve the diol dehydratase performance [40], and when the improved enzyme was introduced into K. pneumoniae, this resulted in improved 2-butanol production [5]. It has also been reported that over-expression of the transcription factor PocR in L. brevis can boost the diol dehydratase activity of L. brevis [41].
Conclusion
Our work highlights the possibility of linking the metabolisms of living microorganisms for producing useful compounds. Here, we have used an engineered L. lactis and a wild-type L. brevis strain for producing 2-butanol, where the L. lactis strain depends on the diol dehydratase activity of the L. brevis strain. We achieved the highest titer (5.9 g/L) and yield (0.58 mol/mol glucose) ever reported in a one-step production setup, and we believe that our work sets the stage for future studies where metabolisms of microorganisms are linked to enable superior production of a variety of useful compounds.
Methods
Strains and plasmids
Strain construction in L. lactis was based on MG1363, a plasmid-free derivative of L. lactis subsp. cremoris strain NCD0712 [42]. For optimized production of the precursor acetoin, CS4363 (MG1363 Δ3ldh Δpta ΔadhE) was used [22]. Expression of heterogeneous genes in L. lactis was done using plasmids pCI372 and pTD6. Derivatives of MG1363 and plasmids used in this study are described in Table 3.
Lactobacillus brevis SE20 [35], isolated from an ethanol pilot plant facility in Örnsköldsvik Sweden was kindly provided by Christer Larsson (Chalmers University of Technology, Sweden). Escherichia coli strain Top10 {F-mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacΧ74 recA1 araD139 Δ(ara-leu) 7697 galU galK rpsL (StrR) endA1 nupG λ-} was used for cloning purposes.
Growth conditions
Cultivation of L. lactis and L. brevis were carried out in 125-mL flasks with 100 mL medium and slow magnetic stirring at 30 °C.
For growth experiments, L. lactis was cultivated in M17 medium (Oxoid, England) or defined synthetic amino acid (SA) medium [45] with the following modification: 40 mM MOPS was replaced with 100 mM potassium phosphate buffer. Both media were supplemented with 1% glucose. For test of activity of the expressed diol dehydratase and alcohol dehydrogenase, cultivations were executed in M17 medium with 7.5 µM coenzyme B12 and 20 mM mBDO or 2-butanone. Strains unable to grow anaerobically were cultivated aerobically.
Lactobacillus brevis was grown in modified MRS medium [46] containing per liter: peptone, 10 g; meat extract, 10 g; yeast extract, 5 g; Tween 80, 1 mL; K2HPO4, 2 g; sodium acetate·3H2O, 5 g; triammonium citrate, 2 g; MgSO4·7H2O, 0.2 g; MnSO4·4H2O, 0.05 g; glucose or xylose, 20 g, 7.5 µmol vB12. When needed, 20 mM mBDO was added to stimulate expression of diol dehydratase.
Escherichia coli strains were grown aerobically at 37 °C in Luria–Bertani broth [47].
When required, antibiotics were added in the following concentrations: tetracycline, 8 µg/mL for E. coli and 5 µg/mL for L. lactis; chloramphenicol, 20 µg/mL for E. coli and 5 µg/mL for L. lactis.
DNA techniques
All manipulations were performed according to Sambrook and Russell [47]. E. coli was transformed using electroporation. L. lactis was made electrocompetent by growing in GM17 medium containing 1% glycine and transformed by electroporation as previously described by Holo and Nes, 1989 [48]. Chromosomal DNA from L. lactis was isolated using the method described for E. coli by Sambrook and Russel [47] with the modification that cells were treated with 20 μg of lysozyme per mL for 2 h prior to lysis.
Construction of strains
For construction of a 2-butanol-producing L. lactis, the diol dehydratase and reactivase from K. oxytoca ATCC 8724 [49] and 2-butanol dehydrogenase from A. xylosoxidans [30] were codon-optimized for L. lactis and synthesized by Genscript. pddABC and sadB and GapB promotor from L. lactis was amplified using the primers VP19 (SalI) and VP20 (PstI) (Table 4). The PCR products were further cloned into the XbaI/KpnI and PstI/SalI sites of pCI372, resulting in plasmid pButop. The plasmid was further transformed into strain CS4363 (MG1363 Δ3ldh Δpta ΔadhE), a plasmid-free derivative of L. lactis subsp. cremoris strain NCD0712 [42], resulting in strain MM10. ddrAB and GapB promotor from L. lactis was amplified using primers P001 and P002 and cloned at SalI/PstI of pTD6. The plasmid was transformed into MM10, resulting in strain MM01.
For application in co-cultivation, construction of a vector for high production of the precursor mBDO and expression of the 2-butanol dehydrogenase, sadB, was based on plasmid pJM001 [43]. pJM001 encode a codon-optimized butanediol dehydrogenase from E. cloacae, bdh. Plasmid pMM06 was constructed using Gibson assembly of sadB amplified using primers P038 and P039 and pJM001 amplified using primers P041 and P036. The plasmid was further transformed into L. lactis strain CS4363 to generate MM06.
Analytical methods
Cell growth was regularly monitored by measuring optical density at 600 nm (OD600) and the quantification of glucose, xylose, lactate, acetate, acetoin, ethanol, mBDO, 2-butanone, and 2-butanol was carried out using an Ultimate 3000 high-pressure liquid chromatography system (Dionex, Sunnyvale, USA) equipped with a Aminex HPX-87H column (Bio-Rad, Hercules, USA) and a Shodex RI-101 detector (Showa Denko K.K., Tokyo, Japan). The column oven temperature was set at 60 °C and the mobile phase consisted of 5 mM H2SO4, at a flow rate of 0.5 mL/min.
Assays
Diol dehydratase activity of MM10 towards 1,2-propanediol (PDO) was determined in cellular extracts using the 3-methyl-2-benzothiazolinone hydrazone (MBTH) method [50]. MBTH reacts with the produced propionaldehyde to form an azine derivate which can be determined by spectrophotometer [51]. Cells from a 100-mL culture were harvested, washed twice, and re-suspended in 10 mM potassium phosphate and 1 mM dithiothreitol buffer, pH 7.2. The cells were then disrupted by glass beads (106 µm, Sigma, Prod. No. G4649) using a FastPrep (MP Biomedicals, Santa Ana, USA). The reaction of 0.5 mL contained 50 mM potassium chloride, 35 mM potassium phosphate buffer pH 8, 0.015 mM coenzyme B12, 50 mM PDO, and appropriate amount of cellular extract. After incubation at 37 °C for 10 min, the reaction was terminated by addition of 0.5 mL potassium citrate buffer (0.1 M pH 3.6). 0.25 mL 0.5% MBTH hydrochloride was added, left to react at 37 °C after 15 min 0.5 mL water was added prior measurement at 305 nm using the Infinite M1000 PRO microplate reader. Absorbance values were converted to µmol propionaldehyde using standard curve. Protein concentration of cellular extracts was determined using the Bradford method, and bovine serum albumin served as the standard.
mBDO dehydratase activity was determined in vivo in cells of SE20 cultivated in modified MRS medium with 2% glucose or xylose, with or without addition of 20 mM mBDO. Cultures were harvested at late exponential phase, washed with 0.9% sodium chloride, and re-suspended to OD600 of 2.5 for conversion of 20 mM mBDO. Incubations were executed at 30 °C in SA medium added 7.5 µM vB12 and 1% glucose or xylose. Product formation was determined as the sum of 2-butanone and 2-butanol produced after 3 h of incubation.
Co-cultivation in SA medium
For co-cultivations, the strains were pre-cultivated separately to obtain biomass, harvested at late exponential phase by centrifugation (5000g, 10 min), and re-suspended in co-cultivation medium at the desired inoculum OD600. Pre-cultivation of L. lactis strains was done in SA medium with glucose, tetracycline and 20 mM 2-butanone to sustain anaerobic growth. L. brevis cultivations were done in modified MRS medium containing 2% glucose and 20 mM mBDO.
Co-cultivations were done in SA medium with 2% glucose, 7.5 µM vB12 and 5 mM 2-butanone. Inoculations were done in start OD600 of 1:1, 1:0, and 0:1, of L. lactis to L. brevis. Product formation was determined after 20 h of incubation. Cultures were prepared as biological triplicates.
Co-cultivation in M17 medium
For co-cultivations L. lactis MM06 and L. brevis SE20 were grown separately and harvested by centrifugation at late exponential phase (OD600 = 0.7 and OD600 = 0.4, respectively). L. lactis was cultivated in M17 supplemented with 1% glucose, tetracycline and 10 mM 2-butanone. L. brevis was cultivated in modified MRS with 1% glucose and supplemented with 20 mM mBDO.
Co-cultivations were executed in M17 with 2% glucose and 7.5 µM vB12. Minimal stirring was applied to the 50-mL tubes using 1 cm rod-shaped stirring magnets to keep the culture turbid while avoiding aeration. Cultures were inoculated to a final OD600 value of either 0.06 or 0.24 for each strain resulting in combinations of L. lactis:L. brevis of 1:1, 1:4, 4:1, 1:0, and 0:1. Cultures were incubated for 96 h and samples taken every 24 h for OD600, HPLC, and CFU analysis. Cultures were prepared as biological triplicates. To verify batch-to-batch replicability, an additional co-cultivation was executed using inoculation ratios of L. lactis:L. brevis of 1:4, 1:2, 1:1, 2:1, and 4:1. Product formation was evaluated after 72 h of cultivation.
Determination of colony forming units (CFU) during co-cultivation of L. lactis MM06 and L. brevis SE20 were done on agar plates consisting of a semi-defined medium [52] supplemented with 1.5% agar (w/v), 1% glucose (w/v) and 200 µM X-gluc (5-bromo-4-chloro-3-indolyl-beta-d-glucuronic acid) for colorimetric detection of β-glucuronidase activity. On these plates, the L. lactis appear as large blue colonies, whereas L. brevis appear as small white colonies.
Availability of data and materials
All data generated and analyzed during the current study are included in this published article and its supplementary information file.
Abbreviations
- L. lactis :
-
Lactococcus lactis
- L. brevis :
-
Lactobacillus brevis
- S. marcescens :
-
Serratia marcescens
- K. oxytoca :
-
Klebsiella oxytoca
- A. xylosoxidans :
-
Achromobacter xylosoxidans
- E. cloacae :
-
Enterobacter cloacae
- mBDO:
-
meso-2,3-Butanediol
- LAB:
-
Lactic acid bacteria
- NADH or NAD+ :
-
Reduced or oxidized form of nicotinamide adenine dinucleotide
- PddABC:
-
Diol dehydratase from K. oxytoca
- SadB:
-
Alcohol dehydrogenase from A. xylosoxidans
- DdrAB:
-
Diol dehydratase reactivase from K. oxytoca
- Bdh:
-
Butanediol dehydrogenase from E. cloacae
- OD600 :
-
Optical density at wavelength 600 nm
- SA:
-
Synthetic amino acid medium
References
Mussatto SI, Dragone G, Guimarães PMR, Silva JPA, Carneiro LM, Roberto IC, et al. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv. 2010;28(6):817–30.
Nanda S, Golemi-Kotra D, McDermott JC, Dalai AK, Gökalp I, Kozinski JA. Fermentative production of butanol: perspectives on synthetic biology. New Biotechnol. 2017;37(B):210–21.
Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451:86–9.
Hahn H-D, Dämbkes G, Rupprich N, Bahl H, Frey GD. Butanols. In: Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2013. https://doi.org/10.1002/14356007.a04_463.pub3.
Chen Z, Wu Y, Huang J, Liu D. Metabolic engineering of Klebsiella pneumoniae for the de novo production of 2-butanol as a potential biofuel. Bioresour Technol. 2015;197:260–5.
Russmayer H, Marx H, Sauer M. Microbial 2-butanol production with Lactobacillus diolivorans. Biotechnol Biofuels. 2019;12:262. https://doi.org/10.1186/s13068-019-1594-5.
Zhang L, Singh R, Sivakumar D, Guo Z, Li J, Chen F, et al. An artificial synthetic pathway for acetoin, 2,3-butanediol, and 2-butanol production from ethanol using cell free multi-enzyme catalysis. Green Chem. 2018;20(1):230–42.
Srirangan K, Liu X, Akawi L, Bruder M, Moo-Young M, Chou CP. Engineering Escherichia coli for microbial production of butanone. Appl Environ Microbiol. 2016;82(9):2574–84.
Jiang W, Wang S, Wang Y, Fang B. Key enzymes catalyzing glycerol to 1,3-propanediol. Biotechnol Biofuels. 2016;9:57. https://doi.org/10.1186/s13068-016-0473-6.
Yang M, Yun J, Zhang H, Magocha TA, Zabed H, Xue Y, et al. Genetically engineered strains: application and advances for 1,3-propanediol production from glycerol. Food Technol Biotechnol. 2018;56(1):3–15.
Raynaud C, Sarçabal P, Meynial-Salles I, Croux C, Soucaille P. Molecular characterization of the 1,3-propanediol (1,3-PD) operon of Clostridium butyricum. Proc Natl Acad Sci USA. 2003;100(9):5010–5.
Toraya T, Shirakashi T, Kosuga T, Fukui S. Substrate specificity of coenzyme B12-dependent diol dehydratase: glycerol as both a good substrate and a potent inactivator. Biochem Biophys Res Commun. 1976;69(2):475–80.
Moore KW, Richards JH. Stereospecificity and mechanism of adenosylcobalamin-dependent diol dehydratase. Catalysis and inactivation with meso- and dl-2,3-butanediols as substrates. Biochem Biophys Res Commun. 1979;87(4):1052–7.
Mori K, Hosokawa Y, Yoshinaga T, Toraya T. Diol dehydratase-reactivating factor is a reactivase—evidence for multiple turnovers and subunit swapping with diol dehydratase. FEBS J. 2010;277(23):4931–43.
Sauvageot N, Muller C, Hartke A, Auffray Y, Laplace J-M. Characterisation of the diol dehydratase pdu operon of Lactobacillus collinoides. FEMS Microbiol Lett. 2002;209(1):69–74.
Hieke E, Vollbrecht D. On the formation of butanol-2 by lactic acid bacteria and yeast. Arch Microbiol. 1974;99(1):345–51.
Chen Z, Sun H, Huang J, Wu Y, Liu D. Metabolic engineering of Klebsiella pneumoniae for the production of 2-butanone from glucose. PLoS ONE. 2015;10(10):e0140508. https://doi.org/10.1371/journal.pone.0140508.
Mazzoli R, Bosco F, Mizrahi I, Bayer EA, Pessione E. Towards lactic acid bacteria-based biorefineries. Biotechnol Adv. 2014;32(7):1216–36.
Börner RA, Kandasamy V, Axelsen AM, Nielsen AT, Bosma EF. Genome editing of lactic acid bacteria: opportunities for food, feed, pharma and biotech. FEMS Microbiol Lett. 2019;366(fny291).
Sauer M, Russmayer H, Grabherr R, Peterbauer CK, Marx H. The efficient clade: lactic acid bacteria for industrial chemical production. Trends Biotechnol. 2017;35(8):756–69.
Liu J, Wang Z, Kandasamy V, Lee SY, Solem C, Jensen PR. Harnessing the respiration machinery for high-yield production of chemicals in metabolically engineered Lactococcus lactis. Metab Eng. 2017;44:22–9.
Solem C, Dehli T, Jensen PR. Rewiring Lactococcus lactis for ethanol production. Appl Environ Microbiol. 2013;79(8):2512–8.
Kandasamy V, Liu J, Dantoft SH, Solem C, Jensen PR. Synthesis of (3R)-acetoin and 2,3-butanediol isomers by metabolically engineered Lactococcus lactis. Sci Rep. 2016;6:36769.
Teuber M. The genus Lactococcus. In: Wood BJB, Holzapfel WH, editors. The genera of lactic acid bacteria. Boston: Springer; 1995. p. 173–234.
Petersen KV, Martinussen J, Jensen PR, Solem C. Repetitive, marker-free, site-specific integration as a novel tool for multiple chromosomal integration of DNA. Appl Environ Microbiol. 2013;79(12):3563–9.
Solem C, Jensen PR. Modulation of gene expression made easy. Appl Environ Microbiol. 2002;68(5):2397–403.
Kleerebezem M, Beerthuyzen MM, Vaughan EE, De Vos WM, Kuipers OP. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl Environ Microbiol. 1997;63(11):4581–4.
Gaspar P, Neves AR, Ramos A, Gasson MJ, Shearman CA, Santos H. Engineering Lactococcus lactis for production of mannitol: high yields from food-grade strains deficient in lactate dehydrogenase and the mannitol transport system. Appl Environ Microbiol. 2004;70(3):1466–74.
Liu J, Kandasamy V, Würtz A, Jensen PR, Solem C. Stimulation of acetoin production in metabolically engineered Lactococcus lactis by increasing ATP demand. Appl Microbiol Biotechnol. 2016;100(22):1–9.
Bramucci MG, Eliot AC, Maggio-Hall LA, Nakamura CE. Butanol dehydrogenase enzyme from the bacterium Achromobacter xylosoxidans. United States patent; US 8,188,250 B2, 2012.
Yoneda H, Tantillo DJ, Atsumi S. Biological production of 2-butanone in Escherichia coli. Chemsuschem. 2014;7(1):92–5.
Wegmann U, O’Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, et al. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189(8):3256–70.
Morita H, Hidehiro T, Fukuda S, Horikawa H, Oshima K, Suzuki T, et al. Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res. 2008;15(3):151–61.
Santos F, Vera JL, van der Heijden R, Valdez G, de Vos WM, Sesma F, et al. The complete coenzyme B12 biosynthesis gene cluster of Lactobacillus reuteri CRL 1098. Microbiology. 2008;154(1):81–93.
Ghiaci P, Lameiras F, Norbeck J, Larsson C. Production of 2-butanol through meso-2,3-butanediol consumption in lactic acid bacteria. FEMS Microbiol Lett. 2014;360(1):70–5.
Zhang Y, Zeng F, Hohn K, Vadlani PV. Metabolic flux analysis of carbon balance in Lactobacillus strains. Biotechnol Prog. 2016;32(6):1397–403.
Jones JA, Vernacchio VR, Sinkoe AL, Collins SM, Ibrahim MHA, Lachance DM, et al. Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab Eng. 2016;35:55–63.
Zhang H, Wang X. Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng. 2016;37:114–21.
Jones JA, Wang X. Use of bacterial co-cultures for the efficient production of chemicals. Curr Opin Biotechnol. 2018;5333–8.
Maddock DJ, Gerth ML, Patrick WM. An engineered glycerol dehydratase with improved activity for the conversion of meso-2,3-butanediol to butanone. Biotechnol J. 2017;12:1700480.
Santos F, Spinler JK, Saulnier DM, Molenaar D, Teusink B, de Vos WM, et al. Functional identification in Lactobacillus reuteri of a PocR-like transcription factor regulating glycerol utilization and vitamin B12 synthesis. Microb Cell Fact. 2011;10:55.
Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154(1):1–9.
Liu J, Chan SHJ, Brock-Nannestad T, Chen J, Lee SY, Solem C, et al. Combining metabolic engineering and biocompatible chemistry for high-yield production of homo-diacetyl and homo-(S, S)-2,3-butanediol. Metab Eng. 2016;36:57–67.
Hayes F, Daly C, Fitzgerald GF. Identification of the minimal replicon of Lactococcus lactis subsp. lactis UC317 plasmid pCI305. Appl Environ Microbiol. 1990;56(1):202–9.
Jensen PR, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol. 1993;59(12):4363–6.
Elferink SJWHO, Krooneman J, Jan C, Spoelstra SF, Faber F. Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri. Appl Environ Microbiol. 2001;67(1):125–32.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2001.
Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55(12):3119–23.
Tobimatsu T, Hara T, Sakaguchi M, Kishimoto Y, Wada Y, Isoda M, et al. Molecular cloning, sequencing, and expression of the genes encoding adenosylcobalamin-dependent diol dehydrase of Klebsiella oxytoca. J Biol Chem. 1995;270(13):7142–8.
Toraya T, Ushio K, Fukui S, Hogenkamp HPC. Studies on the mechanism of the adenosylcobalamin-dependent diol dehydrase reaction by the use of analogs of the coenzyme. J Biol Chem. 1977;3:963–70.
Paz MA, Blumenfeld OO, Rojkind M, Henson E, Furfine C, Gallop PM. Determination of carbonyl compounds with N-methyl benzothiazolone hydrazone. Arch Biochem Biophys. 1965;109(3):548–59.
Kimmel SA, Roberts RF. Development of a growth medium suitable for exopolysaccharide production by Lactobacillus delbrueckii ssp. bulgaricus RR. Int J Food Microbiol. 1998;40(1–2):87–92.
Acknowledgements
We thank Christer Larsson from Chalmers University of Technology, Sweden, for providing L. brevis SE20. Additionally, we thank Shruti Harnal Dantoft from the Technical University of Denmark for her assistance with project management and revision of the manuscript.
Funding
The work is funded by the Danish Innovation Foundation (Grant ID: 4106-00037B). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Author information
Authors and Affiliations
Contributions
MJM carried out all the experimental work, analyzed the data, and wrote the manuscript. JMA analyzed the data and wrote the manuscript. VK was involved in strain construction and revised the manuscript. JL provided useful suggestions for experimental design and revised the manuscript critically. CS and PRJ participated in the design of the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Overview of glucose and xylose metabolism in L. brevis. On glucose, two NADH are formed in the oxidative pentose phosphate pathway, and these have to be oxidized through ethanol formation from acetyl-CoA. Thus, the Acetyl-P cannot give rise to ATP formation through the action of acetate kinase. On xylose, however, there is no such constraint, and the acetyl-P can be used for generating ATP. Table S1. Production of 2-butanol from lactose and xylose in defined SA medium using resting cells of L. lactis and L. brevis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mar, M.J., Andersen, J.M., Kandasamy, V. et al. Synergy at work: linking the metabolism of two lactic acid bacteria to achieve superior production of 2-butanol. Biotechnol Biofuels 13, 45 (2020). https://doi.org/10.1186/s13068-020-01689-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-020-01689-w