Abstract
Background
Acetic acid, generated from the pretreatment of lignocellulosic biomass, is a significant obstacle for lignocellulosic ethanol production. Reactive oxidative species (ROS)-mediated cell damage is one of important issues caused by acetic acid. It has been reported that decreasing ROS level can improve the acetic acid tolerance of Saccharomyces cerevisiae.
Results
Lycopene is known as an antioxidant. In the study, we investigated effects of endogenous lycopene on cell growth and ethanol production of S. cerevisiae in acetic acid media. By accumulating endogenous lycopene during the aerobic fermentation of the seed stage, the intracellular ROS level of strain decreased to 1.4% of that of the control strain during ethanol fermentation. In the ethanol fermentation system containing 100 g/L glucose and 5.5 g/L acetic acid, the lag phase of strain was 24 h shorter than that of control strain. Glucose consumption rate and ethanol titer of yPS002 got to 2.08 g/L/h and 44.25 g/L, respectively, which were 2.6- and 1.3-fold of the control strain. Transcriptional changes of INO1 gene and CTT1 gene confirmed that endogenous lycopene can decrease oxidative stress and improve intracellular environment.
Conclusions
Biosynthesis of endogenous lycopene is first associated with enhancing tolerance to acetic acid in S. cerevisiae. We demonstrate that endogenous lycopene can decrease intracellular ROS level caused by acetic acid, thus increasing cell growth and ethanol production. This work innovatively puts forward a new strategy for second generation bioethanol production during lignocellulosic fermentation.
Similar content being viewed by others
Background
Lignocellulosic ethanol is routinely recognized as one of the most promising renewable energy sources, and has received widespread attention due to its economic and environmental benefits [1, 2]. However, the inhibitory compounds generated during the pretreatment of lignocellulosic biomass, mainly including furan, weak acids, and phenolic mixtures, have a significant inhibitory effect on cell growth, metabolism, and ethanol production [3,4,5]. Acid-catalyzed hydrolysis of lignocelluloses usually generates acetic acid as the byproduct [6, 7], which is one of the main toxic inhibitors [8, 9]. Acetic acid results in a decrease in metabolic enzyme activity, cell growth, and ethanol production of Saccharomyces cerevisiae [10, 11]. Moreover, it can also induce the accumulation of reactive oxygen species (ROS) [12], resulting in damage to biological macromolecules such as DNA, lipids, and proteins, leading to the loss of protein function and even programmed cell death [13]. Therefore, strengthening the tolerance of fermented microorganisms to acetic acid is an important challenge in the lignocellulosic ethanol production process.
The development of acetic acid-tolerant yeast strains is mainly through reducing the absorption of acetic acid, enhancing the efflux of hydrogen ions and acetate ions, and enhancing the intracellular metabolism of acetic acid. Overexpressing of AZR1 [14], ACS2 [15], HAA1 [16,17,18], and HRK1 [18] could reduce the concentration of intracellular acetic acid, respectively. Knockouting of QDR3 [19] and overexpressing of WHI2 [20], SFP1, and ACE2 [21] could elicit endogenous acetic acid resistance in S. cerevisiae, respectively. Liu et al. [22] found changes in the gene expression in the acetic acid-resistant histone H3/H4 mutants were mainly related to energy production and antioxidative stress. Landolfo et al. [23] found that cellular ROS accumulation and scavenging status can significantly affect cell viability and ethanol production in S. cerevisiae. Acetic acid can provoke the oxidative stress of S. cerevisiae [12]. Antioxidants, due to their high ability to scavenge intracellular ROS species, have great potential to enhance the proliferation capacity of a broad range of cells. Therefore, adding of antioxidants has become a method of choice for construction of acetic acid-tolerant yeast strain. Qi et al. [24] increased the cell viability and ethanol titer of P. guilliermondii by adding biotin in seed cells. Wan et al. [25] proved that zinc addition decreased the release of ROS in the presence of chronic acetic acid stress. Previous study demonstrated that the addition of proline or overexpression of a proline synthesis-related gene (PRO1) led to an obvious increase in tolerance to acetic acid [26]; recently, overexpression of a peroxiredoxin in S. cerevisiae has showed an enhanced tolerance to lignocellulose-derived inhibitors including acetic acid [27]. Carotenoids are well known as antioxidants for protecting cells and organisms in nature [28, 29]. They are important biological compounds that can inactivate electronically excited molecules, a process termed quenching. However, it has not been reported that the utilization of carotenoids as antioxidant to protect of S. cerevisiae during ethanol fermentation from acetic acid stress.
In this study, the effects of lycopene on cell growth and ethanol production were investigated in S. cerevisiae under acetic acid stress. By accumulating lycopene during the aerobic fermentation of the seed stage, the ROS level of yeast was decreased and the ethanol production rate of yeast was increased under acetic acid stress during anaerobic fermentation. This work highlights that endogenous expression of lycopene in yeast can improve cell viability and ethanol production under acetic acid stress. The strategy proposed here may provide a new and alternative direction for the construction of tolerant inhibitor strains.
Results and discussion
Expressing endogenous lycopene to decrease intracellular ROS levels
To assay the intracellular ROS levels caused by acetic acid [30], CEN.PK2-1C incubated in YPD media with various concentrations of acetic acid was examined (Fig. 1a). The intracellular ROS levels of the cells incubated in YPD media with 0, 1, 2, 3, 4, 5, and 5.5 g/L of acetic acid were 0.0028, 0.0035, 0.0039, 0.0049, 0.0056, 0.0179, and 0.0196 fluorescence density per cell, respectively. It is demonstrated that the intracellular ROS level was strongly associated with extracellular acetic acid level.
Effects of lycopene on cell growth and ROS accumulation in acetic acid media. a Effects of acetic acid on ROS accumulation of CEN.PK2-1C. b Growth phenotypes of the yPS001 and yPS002 strains under conditions of acetic acid stress. c Effects of acetic acid on ROS levels of yPS001 and yPS002. d Fluorescence intensity of yPS001 and yPS002. HAc was abbreviated from acetic acid in figures. Data are averages from three independent experiments (error bars represent SD)
In this study, lycopene production yeast was generated by incorporating the lycopene pathway into CEN.PK2-1C (yPS002). Then, the CEN.PK2-1C with pRS415 was used as control (yPS001). To examine the effect of endogenous expression of lycopene on cell growth, yPS001 and yPS002 were cultured in YPD media until the late log phase, and serially diluted cells were spotted onto YPD agar plates containing 0–5.5 g/L acetic acid. As shown in Fig. 1b, the growth of yPS001 and yPS002 was almost the same in the absence of acetic acid, indicating that the expression of lycopene did not affect the cell growth. However, growth defects were observed in the control strain (yPS001) compared to the lycopene-expressing strain (yPS001) on plates containing 1.0, 2.0, 3.0, and 4 g/L acetic acid. There was a significantly better growth for the yPS002 over the yPS001 on media containing 5.0 and 5.5 g/L acetic acid. These results indicated that the incorporation of lycopene pathway could increase the cell growth in media with acetic acid. Moreover, to demonstrate the versatility of this method in different chassis strains, the lycopene pathway was transferred into BY4741 (yPS009) and BY4742 (yPS011), and the BY4741 and BY4742 with pRS415 were used as control (yPS008, yPS010), respectively. As shown in Additional file 1: Figure S1, significant growth advantages of yPS009 and yPS011 were observed in the presence of acetic acid. The experiment suggested that the lycopene production can be used to increase acetic acid tolerance of yeast in different yeast chassis.
To further explore the mechanism of the observed effects of endogenous lycopene on cell growth under acetic acid, we examined the intracellular ROS levels of yPS001 and yPS002. As shown in Fig. 1c, the intracellular ROS level of yPS002 was notably lower than that of yPS001 in groups of both acetic acid-free and acetic acid-treated. In the group of acetic acid-free, the ROS level of yPS001 was about 289-fold of that of yPS002. Under the condition of 5.5 g/L acetic acid, intracellular ROS levels of the two strains displayed a sharp increase in contrast to that of acetic acid-free group. Treated with 5.5 g/L acetic acid, yPS001 and yPS002 generated sevenfold and 27-fold ROS levels of that in the group of acetic acid-free, which further indicated that acetic acid could indeed cause ROS generating [12]. However, in the group of acetic acid-treated, the intracellular ROS level of yPS002 was just merely 1.4% of that of yPS001. Then, the fluorescence intensity of DCFH treatment was observed (Fig. 1d). The result of fluorescence intensity was consistent with the value of ROS, and the ROS level of yPS002 was indeed lower than that of yPS001 under the same condition.
Effects of various lycopene production on acetic acid tolerance
To study the effects of various lycopene production levels on acetic acid tolerance, lower lycopene production strains (yPS003, yPS004, and yPS005) were generated by adjusting the promoter strength of gene crtE. Nucleotide analog mutagenesis was used to generate a series of promoter mutants of varying strengths [31]. These promoter mutants were assembled with the crtE genes into a library of lycopene pathways with different expression patterns using the yeast assembly method [32]. In addition, higher lycopene production strains yPS006 and yPS007 were generated by incorporating another copy of lycopene pathway into yPS003 and yPS002, respectively. As shown in Fig. 2a, the lycopene yields of yPS007, yPS006, yPS002, yPS005, yPS003, yPS004, and yPS001 were 3.265, 2.311, 1.475, 1.322, 1.073, 0.759, and 0 mg/g DCW in the seed stage, respectively. Strains containing different lycopene produced during the aerobic fermentation of the seed stage were subjected to anaerobic fermentation at an initial optical density of 1.0 and acetic acid level of 5.5 g/L. As shown in Fig. 2b, the lag phases of yPS002, yPS005, yPS003, yPS004, yPS006, yPS007, and yPS001 were 12, 12, 24, 24, 24, 36, and 36 h, respectively. In addition, the highest cell density of yPS007 was 1.187-fold of yPS001. These results demonstrated that intracellular synthesis of lycopene in yeast could improve the tolerance to acetic acid. Moreover, it is indicated that yPS002, producing 1.475 mg/g DCW of lycopene, was the best strain in improving acetic acid tolerance in this study. Thus, it is important to regulate the endogenous concentration of lycopene to improve the tolerance of acetic acid to the yeast.
Effects of different lycopene levels on cell growth in yeast. a Lycopene production of different strains. b Growth curves of yeast containing different lycopene levels during ethanol fermentation (10% glucose, 1% yeast extract, 2% peptone, and 5.5 g/L acetic acid). Data are averages from three independent experiments. (error bars represent SD)
Endogenous lycopene-enhanced ethanol fermentation
To assay the performance of yeast containing the lycopene pathway during ethanol fermentation with acetic acid, yPS002, accumulating lycopene during the aerobic fermentation of the seed stage, was subjected to anaerobic fermentation at an initial optical density of 1.0 and acetic acid level of 5.5 g/L. The growth of cells was measured as optical density at specific intervals of time, within 60 h. As shown in Fig. 3 and Table 1, endogenous lycopene in yeast helped accelerate the process of ethanol fermentation under acetic acid and extremely reduce the lag phases in two groups. In the system of 40 g/L glucose, compared with yPS001, the highest cell density of yPS002 increased by 39%. Glucose consumption rate and ethanol production rate of yPS002 got to 1.49 and 0.67 g/L/h, respectively, which were 1.80- and 1.42-fold of the control strain. Evidently, there was a more strength in the group of fermentation with 100 g/L glucose. The lag phase was strongly reduced by intracellular synthesis of lycopene, which was 24 h shorter than that of control strain. The ethanol production was up to 44.25 g/L at 48 h; however, the control strain was just beginning to produce ethanol at that moment. The glucose consumption rate was 2.57-fold of that of yPS001. The above data demonstrated that intracellular synthesis of lycopene could increase acetic acid tolerance and ethanol production of yeast.
Effects of intracellular synthesis of lycopene on ethanol fermentation. a, b Fermentative profiles of two strains within 60 h (4% glucose, 1% yeast extract, 2% peptone and 5.5 g/L HAc) (pH = 4.05). c, d Fermentative profiles of two strains within 60 h (10% glucose, 1% yeast extract, 2% peptone and 5.5 g/L HAc) (pH = 4.05). Data are averages from three independent experiments (error bars represent SD)
Consequently, lycopene overexpressed in S. cerevisiae shorted the lag period and accelerated the ethanol fermentation process. A key feature of strains with an enhanced tolerance is shorter lag period relative to control. We conclude that intracellular synthesis of lycopene could increase acetic acid tolerance. Better fermentative performance was observed in strain yPS002 at higher concentrations of initial glucose medium, which was consistent with the literature [33].
In addition, to test whether the production of lycopene increases the metabolic burdens or hampers yeast fitness, we assayed growth profiles of the control and lycopene producing strains without acetic acid stress. As shown in Additional file 1: Figure S2, there was no significant difference in cell growth in lycopene-expressing strains compared to the control strains without acetic acid, which indicated that strains expressing lycopene in this study did not increase the metabolic burdens. It is possible that the yield of lycopene in the strains yPS002 was low and caused no growth defect during anaerobic fermentation.
Endogenous lycopene increasing oxidative stress resistance
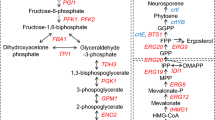
In general, acetic acid affects cell metabolism and stabilities of proteins by a drop in intracellular pH and potential, leading to negative effects on cell growth and proliferation [34]. Acetic acid diffusing across plasma membrane damages cells by accumulating ROS [3], which can inhibit cell viability and ethanol production in S. cerevisiae [23]. ROS is formed upon incomplete reduction of oxygen and includes the hydroxyl radical (HO−), superoxide anion (O2−), and hydrogen peroxide (H2O2) [35], which cause protein damage, DNA damage, and membrane damage to the normal cells (Fig. 4a). Moreover, high oxidative stress up-regulated some stress response genes [36]. Therefore, reducing intracellular ROS level has become an available method for construction of acetic acid-tolerant strains. Lycopene, synthesized from FPP through crtE, crtI, and crtB in yeast [37], is a highly unsaturated hydrocarbon open chain [38]. Hence, it can reduce ROS level by trapping chain-carrying peroxyl radicals to enhance the tolerance of the strain to acetic acid. The ability of suppressing singlet oxygen from lycopene is two times more effective than β-carotene and ten times more potent than α-tocopherol [39,40,41]. Our experiments proved that lycopene can reduce the intracellular ROS level caused by acetic acid stress.
Endogenous lycopene increasing oxidative stress resistance. a Hypothesis on the mechanism of an enhanced tolerance to acetic acid in lycopene-expressing strains. b Results of changes in the transcription level of CTT1 related to acetic acid stress in S. cerevisiae by RT-qPCR. c Results of changes in the transcription level of INO1 related to acetic acid stress in S. cerevisiae by RT-qPCR. HAc was abbreviated from acetic acid in figures. Data are averages from three independent experiments (error bars represent SD)
It was reported that lignocellulose-derived inhibitors can induce expression of oxidative stress-sensitive genes [36]. For example CTT1, encoding cytosolic catalase T, has a role in protection from oxidative damage by hydrogen peroxide [42]. Overexpression of CTT1 can reduce cellular oxidative stress. Previous work in our lab demonstrated INO1 is also an oxidative stress sensitive gene [28]. INO1 encodes inositol-3-phosphate synthase involved in synthesis of inositol phosphates and inositol-containing phospholipids [43]. Up-regulation of INO1 accelerated the membrane reconfiguration of cells to reduce oxidative damage. It is indicated that the expression of those genes is strongly associated with the intracellular oxidative stress. We, therefore, used the expression of the two genes as indicator for assaying the change of oxidative stress in lycopene production strains with acetic acid. As shown in Fig. 4b, c, the CTT1 gene and INO1 gene expressions of yPS001 with acetic acid were both significantly up-regulated than that of yPS001 without acetic acid, which were the same as other reports regarding yeast response to oxidative stress [19, 44]. However, the CTT1 gene and INO1 gene expressions of yPS002 with acetic acid decreased to 0.84- and 0.29-fold compared to that of yPS001 with acetic acid, respectively. It was indicated that intracellular lycopene reduced intracellular ROS stress and thus decreased the transcription levels of CTT1 and INO1. Our results confirmed that endogenous lycopene can decrease oxidative stress and, therefore, improve intracellular environment.
As the previous literature indicated both phenol and furfural can induce ROS accumulated [45, 46], we further evaluated the effects of endogenous lycopene on other lignocellulose-derived inhibitors, like phenol and furfural (Additional file 1: Figures S3, S4). Compared to the control, growth advantages were observed in the lycopene-expressing strains in the stress of phenol or furfural. These results indicated that endogenous lycopene has a positive role in increasing the yeast tolerance under appropriate concentrations of phenol and furfural in different yeast chassis. Above all, our study gives a new strategy for reducing the ROS burden by accumulating intracellular antioxidant in S. cerevisiae during lignocellulosic fermentation.
Conclusions
In this study, we illustrate that the intracellular ROS is associated with acetic acid stress in yeast. Incorporation of heterogeneous lycopene pathway in yeast can not only increase the cell viability via accumulating lycopene for antioxidant, but also improve the ethanol production level during anaerobic fermentation. Meanwhile, we developed a serial yeast strains producing different levels of lycopene and proved the strains with 1.475 mg/g DCW lycopene production have significant growth advantages. We demonstrate that endogenous lycopene can decrease intracellular ROS level caused by acetic acid, thus increasing cell growth and ethanol production. This work innovatively investigates a new strategy for second generation bioethanol production during lignocellulosic fermentation.
Methods
Strains, media, and plasmids
All the S. cerevisiae strains used in this study are listed in Table 2. S. cerevisiae strains were cultivated at 30 °C in YPD medium (1% (w/v) yeast extract, 2% (w/v) peptone, and 2% (w/v) glucose). The engineered yeast cells were selected and grown on synthetic dextrose (SD) medium containing 6.7 g/L yeast nitrogen base without amino acids, 2 g/L complete supplement mixture (without histidine, leucine, tryptophan, uracil), 20 g/L glucose, 20 mg/L histidine, 20 mg/L tryptophan, and 20 mg/L uracil (SD-Leu), or SD medium containing 6.7 g/L yeast nitrogen base without amino acids, 2 g/L complete supplement mixture (without histidine, leucine, tryptophan, uracil), 20 g/L glucose, 20 mg/L tryptophan, and 20 mg/L uracil (SD-Leu-His).
Plasmid cloning work and circuit construct characterization were both performed in Escherichia coli DH10B strains, which were cultured in LB (Luria–Bertani Broth) media (1% (w/v) peptone, 0.5% (w/v) NaCl, and 0.5% (w/v) yeast extract). The pCaro415 and pCaro413 were constructed using yeast assemble method. Specifically, The TEF1p, tTDH3p, tFBA1p, and TDH2t were PCR amplified from genome of S. cerevisiae. The crtE, crtI, and crtB were PCR amplified from the Registry of Standard Biological Parts (http://partsregistry.org). The library of mutagenized TEF1 promoter was generated via error-prone PCR with primers TEF1-F (5′-CTCACTATAGGGCGAATTGGGTACCGGGCCCCCCCTCGAG-3′) and TEF1-R (5′-ACTCGAGTGGAATTGCTGTGAGGATGTTCGCGTAATCCAT-3′) using pCaro415 as the template. About 0.8 mM Mn2+ was added to the Taq DNA polymerase buffer (Tiangen, China) to achieve the highest mutation rate and reduce the ratio of multiple mutants in one DNA fragment. The mutagenized TEF1 promoter and the PCR fragment of crtE-PDX1t-TDH3p-crtI-MPR1t- FBA1p-crtB-TDH2t and the pRS415 vector digested with BamHI and SalI were co-transformed to CEN.PK2-1C for yeast assemble. Colonies with lighter color were selected for fermentation analysis.
Reactive oxygen species analysis
The ROS levels were measured according to the reported method [47] with some modifications in which the two acetate groups of 2′7′-dichlorofluorescein diacetate (DCFH-DA) were cleaved by the intracellular esterase to yield 2′,7′-dichlorofluorescein (DCFH), and the DCFH was then oxidized by ROS resulting in forming the highly fluorescent 2′,7′-dichlorofluorescein (DCF). Briefly, the fermentation broth at 12 h containing 107 cells was centrifuged at 4 °C, washed twice with phosphate buffer (PBS, pH = 7.0), and resuspended in 1 mL PBS. Then, adding 10 μg of DCFH-DA (2.5 mg/mL of stock solution dissolved in DMSO) and incubated at 30 °C for 60 min. After the reaction, the broth was centrifuged for 5 min at 4 °C and 5000g. The cell pellets were washed twice with 1 mL PBS, and resuspended in 1 mL PBS. The fluorescence intensity was observed by Olympus CX41 fluorescence microscope, and the fluorescence was measured in a multimode plate reader (SpectraMax M2, Molecular Devices, USA) at excitation wavelength of 488 nm and emission wavelength of 525 nm. The whole process of analysis was carried out in dark. Diverse intensities of the fluorescence were transformed into electronic signals by the apparatus, and the data were given in relative fluorescence intensity. Colony-forming units (CFU) were measured with flat colony counting method. The fluorescence intensity per cell was used to represent the intracellular ROS levels. The DNA sequence of TEF1 promoter and the different mutants can be found in Additional File 2.
Acetic acid, phenol, and furfural tolerance assay
To examine the phenotype of endogenous expression of lycopene, yPS001 and yPS002 were cultured in SD-Leu media until the late log phase, and serially diluted cells were spotted onto YPD agar plates containing acetic acid. Approximately 106 cells and serial dilutions of 10−1–10−5 (from left to right) of strains were spotted on YPD plates with inhibitors. Plates were incubated at 30 °C for 48 h.
Anaerobic fermentation
Colonies on solid plates were picked up and cultured in 5 mL SD-Leu or SD-Leu-His medium and grown at 30 °C, 250 rpm for 24 h to exponential phase (OD600 = 8.0). Then, the preculture was transferred into 50 mL fresh SD-Leu or SD-Leu-His medium for further 24 h cultivation (to OD600 = 10.0). Then, the seed culture was transferred into a 250 mL shake-flask containing 50 mL fermentation medium (4% glucose, 1% yeast extract, and 2% peptone), or 100 mL fermentation medium (10% glucose, 1% yeast extract, and 2% peptone) with an initial OD600 of 1.0 and cultivated at 30 °C, 150 rpm for 60 h.
Analysis of cell growth, sugars, and ethanol
At designated time, the fermentation broth was taken using syringe for further analysis. To analyze the cell concentration, the optical densities were measured at 600 nm (TU-1810 UV spectrophotometer, Persee, China).
1 mL fermentation broth was taken by syringe and centrifuged immediately at 12,000 rpm for 5 min. Before analysis, the supernatant was filtered with a 0.22 μm filters to remove impurities and stored at − 80 °C. The concentration of glucose and ethanol was analyzed using HPLC (Waters Corp., USA) with a Aminex HP-87H column (Bio-Rad, Hercules, CA, USA) at 65 °C. The mobile phase and its flow rate were 5 mM H2SO4 and 0.6 mL/min, respectively [48].
Extraction and analysis of lycopene
Extraction of lycopene was as described by Chen et al. [49] with some modifications. Briefly, cells harvested from cultures were washed, resuspended in boiling 3 N HCl for 3 min, and cooled in an ice-bath for 3 min. Then, cells debris were washed twice with water, resuspended in acetone containing 0.1% BHT (w/v), vortexed until colorless, and followed by centrifugation. The acetone phase containing the extracted lycopene was filtered for HPLC analysis. An HPLC system (Waters e2695) equipped with a BDS Hypersil C18 column (5.0 × 2.1 mm, 2.7 µm) and a UV/VIS detector (Waters 2489) was used to analyze the produced lycopene. The signal of lycopene was detected 470 nm. The mobile phase consisted of methanol–acetonitrile–dichloromethane (9:40:1 v/v) with a flow rate of 0.3 mL/min at 22 °C.
Real-time reverse transcription-PCR
Real-time reverse transcription-PCR (RT-PCR) was as described by Wu et al. [50] with some modifications. The total RNA was extracted from yeast cells by Trizol. Then, complementary DNA (cDNA) was generated from isolated RNA using the TransScript First-Strand cDNA Synthesis Kit (Trans, China). Converted cDNA was added to Top/Tip Green qRCR SuperMix and specific Primer and subjected to RT-PCR analysis employing the CFX96 Cycler-Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA), in white-walled PCR plates (96 wells). The cycle conditions were set as follows: initial template denaturation at 94 °C for 3 min, followed by 45 cycles of denaturation at 94 °C for 5 s, and combined primer annealing/elongation at 60 °C for 30 s. The primer sequences used in the experiment are shown in Additional file 3: Table S1, and ALG9 was selected as the internal Ref. [51] .
Abbreviations
- S. cerevisiae :
-
Saccharomyces cerevisiae
- ROS:
-
reactive oxygen species
- HAc:
-
acetic acid
- LB:
-
Luria–Bertani Broth
- SD:
-
synthetic complete drop-out medium
- SD-Leu:
-
SD medium without histidine
- SD-Leu-His:
-
SD medium without leucine and histidine
- DCFH-DA:
-
2′,7′-dichlorofluorescein diacetate
- DCFH:
-
2′,7′-dichlorofluorescein
- DCF:
-
2′,7′-dichlorofluorescein
- PBS:
-
phosphate buffer
- CFU:
-
colony-forming units
- cDNA:
-
complementary DNA
- RT-PCR:
-
real-time reverse transcription-PCR
References
Jin M, Sarks C, Bals BD, Posawatz N, Gunawan C, Dale BE, Balan V. Toward high solids loading process for lignocellulosic biofuel production at a low cost. Biotechnol Bioeng. 2017;114:980–9.
Kwak S, Kim SR, Xu H, Zhang GC, Lane S, Kim H, Jin YS. Enhanced isoprenoid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol Bioeng. 2017;114:2581–91.
Martínez A, Rodríguez ME, Wells ML, York SW, Preston JF, Ingram LO. Detoxification of dilute acid hydrolyzates of lignocellulose with lime. Biotechnol Process. 2001;17:278–93.
Tao L, Aden A, Elander RT, Pallapolu VR, Lee YY, Garlock RJ, et al. Process and technoeconomic analysis of leading pretreatment technologies for lignocellulosic ethanol production using switchgrass. Bioresour Technol. 2011;102:11105–14.
Kim S, Dale BE, Ong RG. An alternative approach to indirect land use change: allocating greenhouse gas effects among different uses of land. Biomass Bioenerg. 2012;46:447–52.
Palmqvist E, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol. 2000;74:25–33.
Thomas KC, Hynes SH, Ingledew WM. Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl Environ Microbiol. 2002;68:1616–23.
Ding M-Z, Wang X, Yang Y, Yuan Y-J. Metabolomic study of interactive effects of phenol, furfural, and acetic acid on Saccharomyces cerevisiae. Omi A J Integr Biol. 2011;15:647–53.
Li BZ, Yuan YJ. Transcriptome shifts in response to furfural and acetic acid in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2010;86:1915–24.
Ding J, Bierma J, Smith MR, Poliner E, Wolfe C, Hadduck AN, Zara S, Jirikovic M, Zee K, Penner MH, Patton-Vogt J, Bakalinsky AT. Acetic acid inhibits nutrient uptake in Saccharomyces cerevisiae: auxotrophy confounds the use of yeast deletion libraries for strain improvement. Appl Microbiol Biotechnol. 2013;97:7405–16.
Narendranath NV, Thomas KC, Ingledew WM. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol. 2001;26:171–7.
Formigari A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:443–59.
Ludovico P, Sousa MJ, Silva MT, Leão C, Côrte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–15.
Tenreiro S, Rosa PC, Viegas CA, Sá-Correia I. Expression of the AZR1 gene (ORF YGR224w), encoding a plasma membrane transporter of the major facilitator superfamily, is required for adaptation to acetic acid and resistance to azoles in Saccharomyces cerevisiae. Yeast. 2000;16:1469–81.
Ding J, Holzwarth G, Penner MH, Patton-Vogt J, Bakalinsky AT. Overexpression of acetyl-CoA synthetase in Saccharomyces cerevisiae increases acetic acid tolerance. FEMS Microbiol Lett. 2015;362:1–7.
Keller G, Ray E, Brown PO, Winge DR. Haa1, a protein homologous to the copper-regulated transcription factor Ace1, is a novel transcriptional activator. J Biol Chem. 2001;276:38697–702.
Haitani Y, Tanaka K, Yamamoto M, Nakamura T, Ando A, Ogawa J, Shima J. Identification of an acetate-tolerant strain of Saccharomyces cerevisiae and characterization by gene expression analysis. J Biosci Bioeng. 2012;114:648–51.
Mira NP, Becker JD, Sá-Correia I. Genomic expression program involving the Haa1p-regulon in Saccharomyces cerevisiae response to acetic acid. Omi A J Integr Biol. 2010;14:587–601.
Ma C, Wei X, Sun C, Zhang F, Xu J, Zhao X, et al. Improvement of acetic acid tolerance of Saccharomyces cerevisiae using a zinc-finger-based artificial transcription factor and identification of novel genes involved in acetic acid tolerance. Appl Microbiol Biotechnol. 2015;99:2441–9.
Chen Y, Stabryla L, Wei N. Improved acetic acid resistance in Saccharomyces cerevisiae by overexpression of the WHI2 gene identified through inverse metabolic engineering. Appl Environ Microbiol. 2016;82:2156–66. https://doi.org/10.1128/aem.03718-15.
Chen Y, Sheng J, Jiang T, Stevens J, Feng X, Wei N. Transcriptional profiling reveals molecular basis and novel genetic targets for improved resistance to multiple fermentation inhibitors in Saccharomyces cerevisiae. Biotechnol Biofuels. BioMed Central. 2016;9:1–18.
Liu X, Zhang X, Zhang Z. Point mutation of H3/H4 histones affects acetic acid tolerance in Saccharomyces cerevisiae. J Biotechnol. 2014;187:116–23.
Landolfo S, Politi H, Angelozzi D, Mannazzu I. ROS accumulation and oxidative damage to cell structures in Saccharomyces cerevisiae wine strains during fermentation of high-sugar-containing medium. Biochim Bophys Acta Gen Subj. 2008;1780:892–8.
Qi K, Xia XX, Zhong JJ. Enhanced anti-oxidative activity and lignocellulosic ethanol production by biotin addition to medium in Pichia guilliermondii fermentation. Bioresour Technol. 2015;189:36–43.
Wan C, Zhang M, Fang Q, Xiong L, Zhao X. The impact of zinc sulfate addition on the dynamic metabolic profiling of Saccharomyces cerevisiae subjected to long term acetic acid stress treatment and identification of key metabolites involved in the antioxidant effect of zinc. Met R Soc Chem. 2015;7:322–32.
Wang X, Bai X, Chen DF, Chen FZ, Li BZ, Yuan YJ. Increasing proline and myo-inositol improves tolerance of Saccharomyces cerevisiae to the mixture of multiple lignocellulose-derived inhibitors. Biotechnol Biofuels BioMed Central. 2015;8:142.
Gao J, Feng H, Yuan W, Li Y, Hou S, Zhong S, Bai FW. Enhanced fermentative performance under stresses of multiple lignocellulose-derived inhibitors by overexpression of a typical 2-Cys peroxiredoxin from Kluyveromyces marxianus. Biotechnol Biofuels BioMed Central. 2017;10:1–13.
Martin H, Jäger C, Ruck C. Anti‐and prooxidant properties of carotenoids. J Prakt Chem. 1999;341:302–8.
Riccioni G. Marine carotenoids and oxidative stress. Mar Drugs. 2012;10:116–8.
Jensen TJ, Novak P, Eblin KE, Gandolfi JA, Futscher BW. Epigenetic remodeling during arsenical-induced malignant transformation. Carcinogenesis. 2008;29:1500–8.
Yuan Y, Du J, Zhao H. Customized optimization of metabolic pathways by combinatorial transcriptional engineering. Methods Mol Biol. 2013;985:177–209.
Lin Q, Jia B, Mitchell LA, Luo J, Yang K, Zeller KI, Zhang WQ, Xu ZW, Stracquadanio G, Bader JS, Boeke JD, Yuan YJ. RADOM, an efficient in vivo method for assembling designed DNA fragments up to 10 kb long in Saccharomyces cerevisiae. ACS Synth Biol. 2015;4:213–20.
Kim IS, Kim YS, Yoon HS. Expression of salt-induced 2-Cys peroxiredoxin from Oryza sativa increases stress tolerance and fermentation capacity in genetically engineered yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2013;97:3519–33.
Giannattasio S, Guaragnella N, Ždralević M, Marra E. Molecular mechanisms of Saccharomyces cerevisiae stress adaptation and programmed cell death in response to acetic acid. Front Microbiol. 2013;4:1–7.
D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–24.
Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–57.
Xie W, Lv X, Ye L, Zhou P, Yu H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab Eng. 2015;30:69–78.
Palozza P, Simone R, Catalano A, Russo M, Bohm V. Lycopene modulation of molecular targets affected by smoking exposure. Curr Cancer Drug Targets. 2012;12:640–57.
Campos KKD, Araújo GR, Martins TL, Bandeira ACB, de Paula Costa G, Talvani A, Garcia CCM, Oliveira LAM, Costa DC, Bezerra FS. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J Nutr Biochem. 2017;48:9–20.
Palozza P, Parrone N. Catalano a, Simone R. Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Curr Med Chem. 2010;17:2547–63.
Rao AV. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp Biol Med. 2002;227:908–13.
Lushchak VI, Gospodaryov DV. Catalases protect cellular proteins from oxidative modification in Saccharomyces cerevisiae. Cell Biol Int. 2005;29:187–92.
Majumder AL, Johnson MD, Henry SA. 1L-myo-Inositol-1-phosphate synthase. Biochim Biophys Acta Lipids Lipid Metab. 1997;1348:245–56.
Brickner JH, Walter P. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2004;2:e342.
Allen SA, Clark W, McCaffery JM, Cai Z, Lanctot A, Slininger PJ, Liu ZL, Gorsich SW. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels. 2010;3:2.
Nagata T, Ito S, Itoga K, Kanazawa H, Masaki H. The mechanism of melanocytes-specific cytotoxicity induced by phenol compounds having a prooxidant effect, relating to the appearance of leukoderma. Biomed Res Int. 2015;2015:12.
Liu J, Zhu Y, Du G, Zhou J, Chen J. Response of Saccharomyces cerevisiae to D-limonene-induced oxidative stress. Appl Microbiol Biotechnol. 2013;97:6467–75.
Zhu JQ, Qin L, Li WC, Zhang J, Bao J, Huang YD, Li BZ, Yuan YJ. Simultaneous saccharification and co-fermentation of dry diluted acid pretreated corn stover at high dry matter loading: overcoming the inhibitors by non-tolerant yeast. Bioresour Technol. 2015;198:39–46.
Chen Y, Xiao W, Wang Y, Liu H, Li X, Yuan Y. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact. 2016;15:113.
Wu X-L, Li B-Z, Zhang W-Z, Song K, Qi H, Dai J, Yuan YJ. Genome-wide landscape of position effects on heterogeneous gene expression in Saccharomyces cerevisiae. Biotechnol Biofuels. 2017;10:189.
Teste M-A, Duquenne M, François JM, Parrou J-L. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol. 2009;10:99.
Authors’ contributions
SP and BJ designed the experiments and wrote the manuscript. SP, BJ, MZC, and ZW performed the experiments. YJY, BZL, HL, MZD, XZ, XL, and CL analyzed the data and discussed the results. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (21750001, 21622605, 21621004, and 81502976).
Competing interests
The authors declare no competing financial interest.
Availability of supporting data
Data will be made available from the corresponding author on reasonable request.
Consent for publication
All authors read and approved the final manuscript.
Ethical approval and consent to participate
Not applicable.
Funding
The National Natural Science Foundation of China (21750001, 21622605, 21621004, and 81502976).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Figure S1.
Stress response of lycopene expression in BY4741 or BY4742 to the presence of acetic acid by serial dilution assay. Figure S2. Fermentative profiles of yPS001 and yPS002 within 60 h during anaerobic fermentation without acetic acid. Figure S3. Stress response of lycopene expression in CEN.PK2-1C to the presence of phenol or furfural by serial dilution assay. Figure S4. Stress response of lycopene expression in BY4741 or BY4742 to the presence of phenol or furfural by serial dilution assay.
Additional file 2:
Supporting Online Text: DNA sequences of TEF1 promoter and the different mutants obtained in this work.
Additional file 3.
Table S1. The primers for RT-qPCR analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pan, S., Jia, B., Liu, H. et al. Endogenous lycopene improves ethanol production under acetic acid stress in Saccharomyces cerevisiae. Biotechnol Biofuels 11, 107 (2018). https://doi.org/10.1186/s13068-018-1107-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-018-1107-y