Abstract
Background
Microalgae are currently considered as a promising feedstock for the production of biofuels and high-value products. However, the efficient harvest of microalgal biomasses from their culture broth is a major challenge. The harvesting of algal biomass by flocculation combined with gravity sedimentation is more convenient and cost-effective than traditional methods such as centrifugation and filtration. Compared to inorganic and chemically synthetic flocculants, bioflocculants are a suitable choice for microalgal harvest due to their biodegradable and nontoxic properties. Nonetheless, the high production costs associated with expensive substrates hinder the commercial applications of bioflocculants. Previous studies have shown that the hydrolysates of lignocellulosic biomasses from dilute acid hydrolysis can be utilized as an inexpensive carbon source for the production of bioflocculants. However, the toxic by-products generated in the dilute acid hydrolysis step limit the efficiency of subsequent fermentation. The strains that produce bioflocculants by using untreated lignocellulosic materials can circumvent the pretreatment process, as well as promote the application of bioflocculants in microalgal harvest.
Results
Under alkaline fermentation conditions, the alkaliphilic strain Bacillus agaradhaerens C9 secreted 1.69 IU/mL of alkali-tolerant xylanase and 0.06 IU/mL of cellulase, indicating that this particular strain can efficiently convert untreated rice bran into bioflocculant (RBBF-C9), thereby circumventing rice bran pretreatment for downstream fermentation. The optimal fermentation conditions that result in the highest bioflocculant yield (12.94 g/L) were as follows: 20 g/L of untreated rice bran, 3 g/L of yeast extract, and 20 g/L of Na2CO3 at 37 °C for 24 h. RBBF-C9 contained 74.12% polysaccharides and 4.51% proteins, and was estimated to be 137 kDa. Furthermore, the bioflocculant RBBF-C9 exhibited good flocculating efficiency (91.05%) of oil alga Chlorella minutissima UTEX2341 when 60 mg/L of RBBF-C9 was added into the algal culture broth.
Conclusions
This study demonstrated that untreated rice bran is a suitable inexpensive substrate for the production of bioflocculants, and thus provides a novel approach in utilizing rice bran. The extracted bioflocculants may be potentially used in biomass harvesting of the oil algae C. minutissima UTEX2341 from the culture broth.
Similar content being viewed by others
Background
Microalgae have recently been considered as an attractive and renewable feedstock not only for biofuel production [1–3], but also for the production of many value-added products such as nutritional supplements, pigments, steroids, cosmetics, and pharmaceuticals [4–7] because they exhibit a higher growth rate and a shorter growth period, and requires less water and land area compared to traditional crops [8, 9]. However, harvesting and dewatering of microalgal biomass from the culture broth remains a major challenge due to their small cell size (3–20 μm), low biomass concentration, and colloidal stability of microalgal cells [9, 10]. It has been estimated that the cost of biomass harvesting generally accounts for more than 30% of the total production cost of microalgal biofuels. Various harvesting strategies have been developed to separate the microalgae cells from their culture broth, including centrifugation, air flotation, ultrasound, filtration, flocculation, electrolytic method, magnetic coagulant, gravity sedimentation [1, 5, 11–14], and bio-flocculation based on algal/bacterial, algal/fungal, or algal/algal interactions [15, 16]. Centrifugation, air flotation, and ultrasound are not economical for large-scale harvest of microalgae due to the requirement of high energy inputs [17]. Filtration is only suitable for the large multicellular microalgae, and the frequent filter changes increase its operational complexity [18]. Magnetic coagulant and electrolytic method are reported to be able to enhance the harvest efficiency of certain microalgal species; however, they are also expensive and will contaminate the microalgae biomass [19]. Although fungal-assisted algae harvest is found to be effective for some microalgae, it is a challenge for this method to separate the fungi and microalgal cells. And the harvest method based on mixing self-flocculating and non-flocculating microalgae is only suitable for specific microalgae [15]. Flocculation is known as one of the inexpensive strategies for microalgae harvesting [20], which can increase the aggregation size of microalgae, and thus enhance the efficiency of gravity sedimentation or flotation [19].
Flocculating agents are generally classified into three main groups: inorganic flocculants such as poly-aluminum chloride and aluminum sulfate, organic polymeric flocculants such as polyethyleneimine, and bioflocculants that are a complex mixture of biopolymers secreted by microorganisms during their growth and cell lysis, including polysaccharides, proteins, and lipids [21–24]. The use of organic/inorganic flocculants caused serious health and environmental problems [25, 26]. Thus, bioflocculants have received increasing attention as nontoxic and biodegradable substitutes for conventional inorganic and chemically synthetic flocculants [24, 27] in various industrial fields such as wastewater treatment and microalgae harvest [28–34]. However, the commercial applications of bioflocculants are often limited by their high production cost due to the use of expensive substrates [35, 36]. To reduce production costs, activated sludges or various wastewaters such as potato starch wastewater and phenol-containing wastewater are used as inexpensive culture media for the production of bioflocculants [37–41]. Various lignocellulosic biomasses such as peanut hull, rice hull, and corn stover whose hydrolyzates from dilute acid hydrolysis have been used as inexpensive carbon sources for the production of bioflocculants [42–44]. However, toxic by-products, including furan derivatives, organic acids, phenolic compounds, and lignin derivatives, are generated during the dilute-acid hydrolysis process [45, 46]. These toxins not only severely inhibit fermentation efficiency, but also influence the safety of the bioflocculant products. The pH neutralization and detoxification steps are often part of the process of generating dilute acid hydrolysates prior to downstream fermentation [47]. Therefore, bioflocculant production using a strain that can secrete lignocellulolytic enzymes and thereby produce bioflocculants by directly degrading untreated lignocellulosic materials is a promising strategy because it integrates enzyme production, enzymatic saccharification of lignocellulosic biomass, and fermentation into a single process [48–50]. In a previous study, Cellulosimicrobium cellulans L804 has been reported as a lignocellulose-degrading strain that could convert untreated corn stover into bioflocculant [51]. However, the optimal pH for activities of lignocellulolytic enzymes secreted by C. cellulans L804 is around 5.2–6.0, which largely varies from its optimal fermentation pH (pH 8.2) for bioflocculant production, thereby decreasing the conversion efficiency of corn stover into bioflocculant due to low enzyme activity at an unsuitable pH.
Rice bran is a by-product of the rice milling industry with world production of 50–60 million each year [52]. However, its industrial applications are limited to its use as animal feed, pollutant adsorbent, or for the production of rice bran oil [53]. The amount of rice bran available is far in excess of its local applications, thereby frequently leading to disposal problems [54]. Rice bran mainly contains carbohydrates, proteins, fats, and minerals, and the presence of a high amount of carbohydrates (cellulose and hemicellulose) makes it an alternative inexpensive feedstock for the conversion into value-added microbial products [55].
The present study has shown that under alkaline conditions, B. agaradhaerens C9 secretes alkali-tolerant lignocellulolytic enzymes and thereby directly converts untreated rice bran into bioflocculant (named as RBBF-C9). High conversion efficiency was achieved due to the similarity between the optimal pH range for bioflocculant fermentation and enzyme activities. The highest bioflocculant yield (12.94 g/L) was obtained within 24 h. Moreover, the obtained bioflocculant RBBF-C9 was applied in harvesting the oil algae C. minutissima UTEX2341.
Results and discussion
Selection of lignocellulose biomass for bioflocculant production
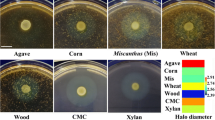
Agricultural wastes, whose hydrolyzates generally contain glucose and xylose, are abundantly available in China. Therefore, the strain that can secrete lignocellulolytic enzymes and thereby produce bioflocculants by directly degrading untreated agricultural wastes may be an effective approach in reducing the production cost of bioflocculants. A previous study described B. agaradhaerens C9 as a highly active polysaccharide bioflocculant-producing strain under alkaline conditions [56]. To test whether B. agaradhaerens C9 can utilize untreated lignocellulosic biomasses as carbon source, the lignocellulolytic enzymes of B. agaradhaerens C9 were analyzed in this study. Figure 1a, b shows the clearing zones surrounding the colonies that were grown on the agar plates that contained xylan and cellulose, indicating that B. agaradhaerens C9 secretes xylanase and cellulase. Therefore, B. agaradhaerens C9 may be potentially used in the production of bioflocculants by directly degrading and utilizing untreated lignocellulosic biomasses.
Xylanase (a) and cellulase (b) of B. agaradhaerens C9 were evaluated using agar plates containing xylan or CMC. Images were captured at 48 h of culture. c Production of RBBF-C9 when different agricultural wastes were used as carbon source. B. agaradhaerens C9 was grown in the media containing 3 g/L yeast extract and different biomasses, and in the control media without inoculation of B. agaradhaerens C9. The medium added with 3 g/L yeast extract but without biomasses was used as blank. 100-μL fermentation broth was collected at 24 h of culture and used in the flocculating activity assay. The residues of lignocellulosic biomasses in the broths with (d) and without (e) inoculation of B. agaradhaerens C9. Images were captured at 24 h of culture
Five kinds of untreated lignocellulosic biomasses, including rice bran, corn stover, corn cob, wheat straw, and peanut hull were directly used as the sole carbon source of fermentation media. Figure 1c shows that >87% flocculating activity was achieved when rice bran and corn stover were used as carbon sources, which is significantly higher than that using corn cob, wheat straw, and peanut hull. On the other hand, the control broth (without inoculation of B. agaradhaerens C9) showed relatively low flocculating activity, which might be attributable to the release of macromolecular substances such as pectin from the lignocellulosic biomasses under alkaline conditions. The flocculating activities of all fermentation broths with five kinds of lignocellulosic biomasses were significantly higher than that of the control broth without inoculation, and fewer residues of lignocellulosic biomasses were observed after fermentation by B. agaradhaerens C9 compared to that of the control broths (Fig. 1d, e), thereby suggesting that the bioflocculant products were mainly produced from the conversion of lignocellulosic biomasses by B. agaradhaerens C9. The flocculating activity of the blank broth (without adding lignocellulosic biomasses) was much lower than that of the broths with lignocellulosic biomasses, and no bioflocculant product was obtained from the blank broth, further indicating that the bioflocculant products were mainly from the conversion of lignocellulosic biomasses by B. agaradhaerens C9. Moreover, the highest flocculating activity of 88.42% and a yield of 3.67 g/L were observed when rice bran was used as carbon source, which is slightly higher than the flocculating activity of 87.24% and the yield of 3.65 g/L that was achieved using corn stover as carbon source. Based on these findings, untreated rice bran was selected for the subsequent studies.
Effects of various rice bran dosages on the flocculating activity and yield of RBBF-C9
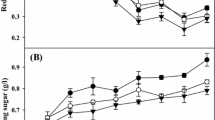
The ratio of the carbon source to nitrogen source significantly influences cell growth and bioflocculant production. The optimal carbon/nitrogen ratio leads to the highest flocculating activity within the shortest culture time [24]. Figure 2 shows the effects of various rice bran dosages on RBBF-C9 production when 3 g/L of yeast extract was added as nitrogen source. An enhancement of flocculating activity and yield was observed with increasing rice bran dosages from 0 to 20 g/L, and a flocculating activity of 87% and the highest bioflocculant yield of 3.83 g/L were achieved when B. agaradhaerens C9 was cultured in the medium containing 20 g/L of rice bran. A further increase in the rice bran dosage inhibited RBBF-C9 production, which may be due to limitations in oxygen transfer in the fermentation medium with excessive amounts of solid rice bran, thereby influencing cell growth and enzymatic secretion of B. agaradhaerens C9.
Effects of Na2CO3 concentrations on the flocculating activity and yield of RBBF-C9
The initial pH of the fermentation medium influences the charge of the molecules on the cell surface, oxidation–reduction potential, microbial nutriment assimilation, and enzyme reactions [24, 57]. A previous study showed that under the alkaline conditions using Na2CO3 to adjust pH, B. agaradhaerens C9 produces the bioflocculant because it could buffer changes in pH levels [56]. Thus, the effects of various Na2CO3 concentrations on the flocculating activity and yield of RBBF-C9 were analyzed. Figure 3 shows that the flocculating activity and yield of RBBF-C9 improved with increasing Na2CO3 concentrations from 0 to 20 g/L. However, a further increase in the Na2CO3 concentration eventually resulted in a decrease in flocculating activity. Thus, the optimal Na2CO3 concentration of 20 g/L was selected to promote the enzymatic hydrolysis of rice bran, and simultaneously provide a buffer for the production of the acidic polysaccharide bioflocculant RBBF-C9. Previous studies have shown that alkaline pretreatment could cause spelling of lignocellulosic substances, thereby resulting in an increase in internal surface area, and the separation of structural linkages between lignin and carbohydrates [47]. Thus, alkaline fermentation conditions could increase the conversion efficiency of lignocellulosic biomasses into valuable products [51].
The optimal pH range for the activities of xylanase and cellulase
A previous study has shown that under weak alkaline conditions (pH 8.2), C. cellulans L804 produces bioflocculants by using untreated corn stover. However, the optimal pH for the activities of both xylanase and cellulase from C. cellulans L804 is around 5.2–6.0 [51], which is significantly different from its optimal fermentation pH (pH 8.2) for bioflocculant production, thereby decreasing the conversion efficiency of corn stover into bioflocculant due to the low activity of these two hydrolytic enzymes at an unsuitable pH. In the present study, the effects of pH on the activities of xylanase and cellulase were analyzed. Figure 4 shows that the optimal pH range for xylanase activity was highly alkaline pH 9.1–10.3, which was similar to another alkaliphilic xylanase (optimal pH 11) that is produced by an alkaliphilic strain Bacillus halodurans PPKS-2 [58]. The optimal pH for activity of cellulase from B. agaradhaerens C9 was 5.9. Although alkaline conditions were not optimal for cellulase activity, 60–70% of the cellulase activity relative to its highest activity remained within the alkaline pH range of 9.1–10.8. The optimal fermentation pH range for B. agaradhaerens C9 was 9.3–10.4 (Fig. 5), which was similar to the optimal pH range for the activity of xylanase that is secreted by B. agaradhaerens C9. Thus, compared to the reported strain C. cellulans L804, B. agaradhaerens C9 shows higher efficiency in converting the lignocellulosic biomasses into bioflocculants.
Time profiles for cell growth, xylanase, cellulase, pH, and RBBF-C9 production
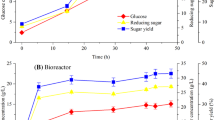
The time curves for cell growth, cellulase, xylanase, pH, and RBBF-C9 production were analyzed when B. agaradhaerens C9 was cultured in a medium that was supplemented with rice bran as carbon source. Figure 5 shows that the flocculating activity and yield of bioflocculant RBBF-C9 sharply increased from 6 to 24 h, which is almost parallel to its cell growth, thus indicating that RBBF-C9 is mainly produced by B. agaradhaerens C9 and does not result from the release of macromolecular substances from rice bran under alkaline conditions. The highest flocculating activity (92.67%) of kaolin clay solution was achieved after 24 h of culture. After 36 h of culture, the cells entered the death phase. The drop in flocculating activity and yield of RBBF-C9 during this period may be due to deflocculation enzyme activity that is caused by cell lysis [36, 59]. The pH significantly decreased from 10.4 to 9.3 in the first 24 h, which might have been caused by the production of acidic polysaccharides during cell growth [51, 56, 60]. After 24 h of culture, the increase in pH might have been due to the release of intracellular substances during cell lysis. A similar change in pH was reported in a previous study [60]. Figure 5 also shows sharp increases in xylanase and cellulase activities in the first 24 h of incubation. A positive correlation between the enzymatic activities and RBBF-C9 production was observed, thereby suggesting that the production of RBBF-C9 from rice bran was dependent on the activities of xylanase and cellulose secreted by B. agaradhaerens C9. The highest levels of xylanase (1.69 IU/mL) and cellulase (0.06 IU/mL) were observed within the fermentation pH range of 9.3–10.4 at 24 h of culture, which is significantly higher than the activity of xylanase (0.6 IU/mL) and cellulase (0.046 IU/mL) produced by C. cellulans L804 after 36 h of culture in a medium with untreated corn stover as carbon source [51], thereby indicating that B. agaradhaerens C9 more efficiently converts untreated lignocellulosic biomasses into bioflocculants than C. cellulans L804. The highest bioflocculant yield (12.94 g/L) was achieved at 24 h, which is significantly higher than that of C. cellulans L804 (4.75 g/L) after 48 h of culture [51].
Properties of the bioflocculant RBBF-C9
The composition analysis indicated the presence of 74.12% polysaccharides and 4.51% proteins in RBBF-C9, suggesting that polysaccharides are the main functional components. According to the result of gel permeation chromatography (GPC), the molecular weight of RBBF-C9 was estimated to be 137 kDa. Elemental analysis showed that the mass proportion of C, H, N, and S was 35.06, 5.72, 7.21, and 3.94%, respectively. FTIR spectrum of RBBF-C9 (Additional file 1: Fig. S1) exhibited a strong broad peak around 3323 cm−1, which is identified as hydroxyl groups, and a weak C–H stretching peak at 2929 cm−1. The spectrum also displayed bands at 1655 and 1440 cm−1, which are contributed to carboxyl groups. The absorption peak ranged from 1000 to 1200 cm−1 suggests the presence of sugar derivatives. This result is consistent with the FTIR spectrum of bioflocculant produced by B. agaradhaerens C9 using glucose as carbon source [56], and confirms that polysaccharides are the main components of RBBF-C9.
Application of RBBF-C9 in harvesting C. minutissima UTEX2341 cells
Chlorella minutissima is currently considered as a promising feedstock for biofuel production due to its capability for rapid growth and its high lipid content. Significant environmental factors that influence the growth of C. minutissima UTEX2341 have been previously investigated [61, 62]. In addition, the culture conditions of C. minutissima UTEX2341 were optimized to enhance its cell growth and lipid production [63, 64]. Flocculation is a suitable strategy for harvesting C. minutissima UTEX2341 biomass. Inorganic flocculants such as aluminum, ferric, and zinc salts have been used to flocculate the C. minutissima biomass, and the optimum concentration was 0.75 g/L for their sulfate salts. However, inorganic flocculants are harmful to microalgal cells. For example, aluminum salts induce cell lysis, and ferric salts resulted in changes in the color of microalgal cells [65].
In the present study, the feasibility of harvesting C. minutissima UTEX2341 cells using bioflocculant RBBF-C9 was evaluated. Figure 6 shows the flocculating rates of C. minutissima UTEX2341 when different concentrations of RBBF-C9 were added into the algal broth. A positive correlation between the flocculating activity and RBBF-C9 dosage was observed when RBBF-C9 dosage varied from 5 to 60 mg/L. The highest flocculating efficiency of 91.05% was achieved at the optimal dosage of 60 mg/L. A further increase in RBBF-C9 dosage caused a slight decrease in the harvest efficiency of microalgal biomass. Similar trends were observed in previous studies [51, 56]. This slight decrease could be explained by the excessive addition of negatively charged polysaccharide bioflocculants that induced the repulsion between similarly charged particles.
The flocculating efficiency of microalgae by bioflocculant is dependent on their species. The cell sizes and cell surface properties of microalgae can significantly influence their harvest efficiency. For example, the flocculating rate of bioflocculant produced by strain C. cellulans L804 to microalga Chlamydomonas reinhardtii was 99%, while only 93% to C. minutissima [51]. A potent bioflocculant γ-PGA produced by Bacillus licheniformis CGMCC2876 exhibited a highest flocculating rate of 99% to Desmodesmus sp. F51 [66]. The cell size of Chlorella sp. is smaller compared to most microalgae (only 3–8 μm). The flocculating rates of γ-PGA produced by Bacillus subtilis to C. vulgaris and C. protothecoides were 91 and 98%, respectively [67]. In addition, most bioflocculants used in the harvest of microalgal biomass are generally produced from expensive substrates [56]. The bioflocculant γ-PGA is produced from trisodium citrate, glycerol, and sodium glutamate [66]. In the present study, the bioflocculant RBBF-C9 was produced through directly degrading untreated agricultural by-product (rice bran), which could reduce the production cost of bioflocculant, achieve the resourceful utilization of abundantly available agricultural wastes and promote the efficient harvesting of microalga C. minutissima UTEX2341 cells.
Conclusions
This study has shown that under alkaline conditions, B. agaradhaerens C9 secretes alkali-tolerant xylanase and cellulase, and simultaneously produces bioflocculant RBBF-C9 by directly degrading untreated rice bran. The highest yield of RBBF-C9 (12.94 g/L) was achieved at the following optimized conditions: 20 g/L of untreated rice bran as carbon source, 3 g/L of yeast extract as nitrogen source, and supplemented with 20 g/L of Na2CO3. Furthermore, RBBF-C9 exhibited the highest flocculating efficiency (91.05%) for microalga C. minutissima UTEX2341 cells, which thereby promotes the application of bioflocculant in the low-cost harvesting of microalgae for biofuel production.
Methods
Strain and culture conditions
The bioflocculant-producing strain B. agaradhaerens C9 was isolated [56] and deposited to China General Microbiological Culture Collection Center (CGMCC, Beijing, China) under the accession number CGMCC13057. The strain was inoculated into 25 mL of seed medium and cultured on a reciprocal shaker at 180 rpm and 37 °C. After 12 h of incubation, the obtained seed culture was used for further experiments. The composition of the seed medium was as follows: 10 g/L glucose, 10 g/L yeast extract, 1.3 g/L K2HPO4, and 0.2 g/L MgSO4·7H2O. After sterilization at 115 °C for 30 min, the medium was supplemented with 10 g/L Na2CO3. To evaluate the activity of cellulase and xylanase that were secreted by B. agaradhaerens C9, the seed culture was dropped onto an agar plate containing 10 g/L of CMC-Na (for cellulase assay) or oat spelt xylan (sigma) (for xylanase assay), 10 g/L of yeast extract, 1.3 g/L K2HPO4, 0.2 g/L MgSO4·7H2O, 15 g/L agar, and supplemented with 10 g/L Na2CO3. After 48 h of incubation at 37 °C, the plates were stained using a 0.3% (w/w) congo red solution for 30 min, then destained using 1 M NaCl for 30 min to observe the hydrolytic zones.
Flocculating activity assay
The flocculating activity assays were performed according to a previous report, with minor modifications [56]. Briefly, the mixture containing 60 mL of 5 g/L kaolin clay suspension, 100 μL of bioflocculant solution, and 1 mL of 1% CaCl2 solution was added into a 100-mL beaker, and then rapidly stirred for 2 min, followed by slow mixing for 1 min. After standing for 1 min, the turbidity (OD550) of the supernatant was determined using an ultraviolet spectrophotometer (Unic-7200, Shanghai, China). A control experiment using the same procedure but was instead mixed with the same volume of distilled water was performed. The flocculating activity was calculated by measuring the decrease in turbidity of the supernatant according to the equation: \({\text{Flocculating activity}} = [A-B]/A \times 100\%\), where A is the absorbance of the control at a wavelength of 550 nm, and B is the absorbance of the sample at a wavelength of 550 nm.
Selection of the optimal lignocellulosic biomass for bioflocculant production
Agricultural wastes were collected from the Xuzhou suburb of China, including rice bran, corn stover, corn cob, wheat straw, and peanut hull. After crushing into powder, these lignocellulosic biomasses were added into fermentation media, and the flocculating activities were compared to select the suitable agricultural waste for the production of bioflocculant. The composition of the fermentation medium was as follows: 20 g/L of dry power of different agricultural wastes, 3 g/L of yeast extract, 1.3 g/L of K2HPO4, 0.2 g/L of MgSO4·7H2O, and supplemented with 10 g/L Na2CO3. The medium without B. agaradhaerens C9 inoculation was used as control to rule out that RBBF-C9 was mainly released from the natural lignocellulosic biomasses. In addition, the medium added with 3 g/L of yeast extract but without biomass was used as blank to rule out that the bioflocculant was mainly converted from yeast extract.
Effects of rice bran dosages and Na2CO3 concentrations on RBBF-C9 production
To optimize the fermentation conditions of converting rice bran into RBBF-C9, the effects of various rice bran dosages and Na2CO3 concentrations on RBBF-C9 production were analyzed. Rice bran at concentrations of 0, 4, 8, 12, 16, 20, 25, and 30 g/L was added into the fermentation medium with 3 g/L of yeast extract as nitrogen source. The Na2CO3 concentration was adjusted to 0, 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 25, and 30 g/L in the fermentation medium with 20 g/L of rice bran and 3 g/L of yeast extract. The flocculating activities and yields achieved at different fermentation conditions were compared.
Enzyme activity assay
The substrates were dissolved in 0.2 M phosphate buffer (pH 5.6–8.9) and 0.2 M NaHCO3–Na2CO3 buffer (pH 8.5–11.3). The fermentation broth was collected after 24 h culture and centrifuged at 10,000 rpm for 10 min. The enzyme activities in the supernatant under different pH levels were assessed to determine the optimal pH range for the activities of xylanase and cellulase.
To determine the time profiles of xylanase and cellulase, the supernatants of fermentation broth obtained at different time points were used as enzyme solution. The cellulase activity was determined according to a previous study with slight modifications [60]. Carboxymethyl cellulose (1%, w/v) was dissolved in 0.2 M NaHCO3–Na2CO3 buffer (pH 10) using as substrate. A reaction mixture containing 150 μL of carboxymethyl cellulose solution and 50 μL of the enzyme solution was incubated at 50 °C for 30 min, followed by the addition of 100 μL of 1 M NaOH and 150 μL dinitrosalicylic acid (DNS) to stop the reaction. After boiling for 5 min, 550 μL of H2O was added to the mixture, and the OD540 was measured using a Unic-7200 spectrophotometer. The enzyme solution inactivated by boiling was used as control. One unit of cellulase activity was defined as the amount of enzyme liberating 1 μM of glucose per minute.
The amount of reducing sugars produced from 1% (w/v) oat spelts xylan (sigma) dissolved in 0.2 M NaHCO3–Na2CO3 buffer (pH 10.0) was determined in the xylanase activity assay. 50 μL of the enzyme solution and 150 μL of the oat spelts xylan solution were mixed and incubated at 50 °C for 30 min. The DNS method was performed to determine the amount of reducing sugars that was released, using xylose as a standard sample. The inactivated enzyme solution was used as control. One unit of xylanase was defined as the enzyme amount that released 1 μM of xylose per minute.
Production and extraction of the bioflocculant product
Bacillus agaradhaerens C9 cells were cultured in 25 mL of the seed medium at 37 °C for 12 h. 1 mL of seed culture was inoculated into 500-mL Erlenmeyer flasks containing 100 mL of optimized fermentation medium. The optimal fermentation medium contained 20 g/L of untreated dry rice bran, 3 g/L of yeast extract, 1.3 g/L of K2HPO4, 0.2 g/L of MgSO4·7H2O, and supplemented with 20 g/L of Na2CO3. After 24 h of incubation with 180-rpm shaking at 37 °C, the cells and rice bran residues in fermentation broth were removed by centrifugation at 12,000 rpm at 4 °C for 10 min. The bioflocculant RBBF-C9 was precipitated from the supernatant by adding two volumes of cold absolute ethanol. The resulting RBBF-C9 product was centrifuged at 10,000 rpm for 5 min, washed with 75% ethanol twice, and lyophilized to dryness using a BETA 1-8 LD plus Freeze Dryer (Christ, Germany).
Characteristics of bioflocculant RBBF-C9
The general properties of RBBF-C9 were determined according to a previous study [16]. The content of polysaccharides in RBBF-C9 was analyzed by using the phenol–sulfuric acid method using glucose as the standard [68]. The Bradford method was performed to determine the protein content with bovine serum albumin as the standard [69]. Gel permeation chromatography was carried out to identify the molecular weight of RBBF-C9 using a Hitachi L-6200 system controller [42]. Elemental analysis was performed using an elemental analyzer (Elementar vario EL, Germany). The functional groups of RBBF-C9 were analyzed by using a fourier transform infrared (FTIR) spectroscopy (Bruker Tensor 27, Germany) in a wavelength range from 600 to 4000 cm−1.
Culture and harvest of C. minutissima UTEX2341 cells
The oil algae C. minutissima UTEX2341 was stored in our laboratory and cultured in IM medium as described in a previous study [51, 61]. C. minutissima UTEX2341 was cultured in 500-mL Erlenmeyer flasks containing 200 mL of liquid IM medium at 24 °C, with a 14-/10-h light/dark cycle for 2 weeks.
The extracted RBBF-C9 was resolved in deionized water to prepare a 5 g/L RBBF-C9 solution, and the pH of this RBBF-C9 solution was adjusted to 7.0 using 1 M NaOH or HCl solution. To evaluate the flocculating efficiency of RBBF-C9 to C. minutissima UTEX2341 cells, 60 mL of the algal culture broth was poured into 100-mL beakers, added with RBBF-C9 solution to different concentrations, and mixed with 0.8 mL 10% CaCl2. The mixtures were rapidly stirred for 5 min, followed by slow mixing for 1 min. After standing for 10 min, the supernatant was collected to determine the OD675 by using a Unic-7200 spectrophotometer (Shanghai, China). Algal culture broth supplied with same volume of deionized water and subjected to the same procedure was used as control. The flocculating efficiency of the C. minutissima UTEX2341 cells was calculated according to the following equation: \({\text{Flocculating efficiency}} = (X-Y)/X \times 100\%\), where X is the OD675 value of the control, and Y is the OD675 value of the supernatant of the flocculated sample.
Abbreviations
- RBBF-C9:
-
the bioflocculant converted from untreated rice bran by Bacillus agaradhaerens C9
- CMC:
-
carboxymethyl cellulose
- DNS:
-
3, 5-dinitrosalicylic acid
- CGMCC:
-
China General Microbiological Culture Collection Center
- GPC:
-
gel permeation chromatography
- FTIR:
-
fourier transform infrared spectroscopy
References
Brennan L, Owende P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev. 2010;14:557–77.
Man KL, Lee KT. Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv. 2012;30:673–90.
Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels. Science. 2010;329:796–9.
Skjånes K, Rebours C, Lindblad P. Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit Rev Biotechnol. 2013;33:172–215.
Christenson L, Sims R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv. 2011;29:686–702.
Guo SL, Zhao XQ, Wan C, Huang ZY, Yang YL, Alam MA, Ho SH, Bai FW, Chang JS. Characterization of flocculating agent from the self-flocculating microalga Scenedesmus obliquus AS-6-1 for efficient biomass harvest. Bioresour Technol. 2013;145:285–9.
Barros AI, Gonçalves AL, Simões M, Pires JCM. Harvesting techniques applied to microalgae: a review. Renew Sustain Energy Rev. 2015;41:1489–500.
Stephens E, Ross I, King Z, Mussgnug JH, Kruse O, Posten C, Borowitzka MA, Hankamer B. An economic and technical evaluation of microalgal biofuels. Nat Biotechnol. 2010;28:126–8.
Liu J, Zhu Y, Tao Y, Zhang Y, Li A, Li T, Sang M, Zhang C. Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol Biofuels. 2013;6:1–11.
Talukder MMR, Das P, Wu JC. Immobilization of microalgae on exogenous fungal mycelium: a promising separation method to harvest both marine and freshwater microalgae. Biochem Eng J. 2014;91:53–7.
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol. 2011;102:71–81.
Muradov N, Taha M, Miranda AF, Wrede D, Kadali K, Gujar A, Stevenson T, Ball AS, Mouradov A. Fungal-assisted algal flocculation: application in wastewater treatment and biofuel production. Biotechnol Biofuels. 2015;8:1–23.
Zhou W, Cheng Y, Yun L, Wan Y. Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatment. Appl Biochem Biotechnol. 2012;167:214–28.
Xie S, Sun S, Dai SY, Yuan JS. Efficient coagulation of microalgae in cultures with filamentous fungi. Algal Res. 2013;2:28–33.
Alam MA, Vandamme D, Wan C, Zhao X, Foubert I, Wang Z, Muylaert K, Yuan Z. Bioflocculation as an innovative harvesting strategy for microalgae. Rev Environ Sci Biotechnol. 2016;15(4):573–83.
Alam MA, Wan C, Guo SL, Zhao XQ, Huang ZY, Yang YL, Chang JS, Bai FW. Characterization of the flocculating agent from the spontaneously flocculating microalga Chlorella vulgaris JSC-7. J Biosci Bioeng. 2014;118(1):29–33.
Garg S, Wang LG, Schenk PM. Effective harvesting of low surface-hydrophobicity microalgae by froth flotation. Bioresour Technol. 2014;159(2):437–41.
Molina GE, Belarbi EH, Acién Fernández FG, Robles MA, Chisti Y. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv. 2003;20(7):491–515.
Ummalyma SB, Gnansounou E, Sukumaran RK, Sindhu R, Pandey A, Sahoo D. Bioflocculation: an alternative strategy for harvesting of microalgae—an overview. Bioresour Technol. 2017. doi:10.1016/j.biortech.2017.02.097.
Banerjee C, Ghosh S, Sen G, Mishra S, Shukla P, Bandopadhyay R. Study of algal biomass harvesting using cationic guar gum from the natural plant source as flocculant. Carbohydr Polym. 2013;92:675–81.
Salehizadeh H, Shojaosadati S. Extracellular biopolymeric flocculants: recent trends and biotechnological importance. Biotechnol Adv. 2001;19:371–85.
Liu W, Wang K, Li B, Yuan H, Yang J. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour Technol. 2010;101:1044–8.
Zheng Y, Ye ZL, Fang XL, Li YH, Cai WM. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour Technol. 2008;99:7686–91.
Salehizadeh H, Yan N. Recent advances in extracellular biopolymer flocculants. Biotechnol Adv. 2014;32:1506–22.
Rudén C. Acrylamide and cancer risk–expert risk assessments and the public debate. Food Chem Toxicol. 2004;42:335–49.
Campbell A. The potential role of aluminium in Alzheimer’s disease. Nephrol Dial Transpl. 2002;17:17–20.
Liu W, Liu C, Yuan H, Yang J. The mechanism of kaolin clay flocculation by a cation-independent bioflocculant produced by Chryseobacterium daeguense W6. AIMS Environ Sci. 2015;2:169–79.
Lei X, Chen Y, Shao Z, Chen Z, Li Y, Zhu H, Zhang J, Zheng W, Zheng T. Effective harvesting of the microalgae Chlorella vulgaris via flocculation-flotation with bioflocculant. Bioresour Technol. 2015;198:922–5.
Pathak M, Devi A, Bhattacharyya KG, Sarma HK, Subudhi S, Lal B. Production of a non-cytotoxic bioflocculant by a bacterium utilizing a petroleum hydrocarbon source and its application in heavy metal removal. RSC Adv. 2015;5:143–9.
Sun PF, Lin H, Wang G, Lu LL, Zhao YH. Preparation of a new-style composite containing a key bioflocculant produced by Pseudomonas aeruginosa ZJU1 and its flocculating effect on harmful algal blooms. J Hazard Mater. 2015;284:215–21.
Yi L, Xu Y, Lei L, Jiang X, Zhang K, Zheng T, Wang H. First evidence of bioflocculant from Shinella albus with flocculation activity on harvesting of Chlorella vulgaris biomass. Bioresour Technol. 2016;218:807–15.
Gang C, Zhang Y, Li C, Jie L, Mao K, Li K, Zhou J. Production of a bioflocculant from methanol wastewater and its application in arsenite removal. Chemosphere. 2015;141:274–81.
Ndikubwimana T, Zeng X, Murwanashyaka T, Manirafasha E, He N, Shao W, Lu Y. Harvesting of freshwater microalgae with microbial bioflocculant: a pilot-scale study. Biotechnol Biofuels. 2016;9:1–11.
Wan C, Zhao XQ, Guo SL, Alam MA, Bai FW. Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresour Technol. 2013;135:207–12.
Guo J, Jing M. Bioflocculant from pre-treated sludge and its applications in sludge dewatering and swine wastewater pretreatment. Bioresour Technol. 2015;196:736–40.
Aljuboori AHR, Uemura Y, Osman NB, Yusup S. Production of a bioflocculant from Aspergillus niger using palm oil mill effluent as carbon source. Bioresour Technol. 2014;171:66–70.
Guo J, Lau AK, Zhang Y, Zhao J. Characterization and flocculation mechanism of a bioflocculant from potato starch wastewater. Appl Microbiol Biotechnol. 2015;99:5855–61.
Guo J, Yang C, Zeng G. Treatment of swine wastewater using chemically modified zeolite and bioflocculant from activated sludge. Bioresour Technol. 2013;143:289–97.
Zhong C, Xu A, Chen L, Yang X, Yang B, Hong W, Mao K, Wang B, Zhou J. Production of a bioflocculant from chromotropic acid waste water and its application in steroid estrogen removal. Colloids Surf B Biointerfaces. 2014;122:729–37.
Pu SY, Qin LL, Che JP, Zhang BR, Xu M. Preparation and application of a novel bioflocculant by two strains of Rhizopus sp. using potato starch wastewater as nutrilite. Bioresour Technol. 2014;162:184–91.
Chen H, Zhong C, Berkhouse H, Zhang Y, Lv Y, Lu W, Yang Y, Zhou J. Removal of cadmium by bioflocculant produced by Stenotrophomonas maltophilia using phenol-containing wastewater. Chemosphere. 2016;155:163–9.
Guo J, Yu J, Xin X, Zou C, Cheng Q, Yang H, Nengzi L. Characterization and flocculation mechanism of a bioflocculant from hydrolyzate of rice stover. Bioresour Technol. 2015;177:393–7.
Wang L, Ma F, Lee DJ, Wang A, Ren N. Bioflocculants from hydrolysates of corn stover using isolated strain Ochrobactrum ciceri W2. Bioresour Technol. 2013;145:259–63.
Liu W, Hao Y, Jiang J, Zhu A, Zhu J, Dong Z. Production of a bioflocculant from Pseudomonas veronii L918 using the hydrolyzate of peanut hull and its application in the treatment of ash-flushing wastewater generated from coal fired power plant. Bioresour Technol. 2016;218:318–25.
Jian Z, Zhu Z, Wang X, Nan W, Wei W, Jie B. Biodetoxification of toxins generated from lignocellulose pretreatment using a newly isolated fungus, Amorphotheca resinae ZN1, and the consequent ethanol fermentation. Biotechnol Biofuels. 2010;3:26.
Mussatto SI, Roberto IC. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol. 2004;93:1–10.
Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11.
Hasunuma T, Kondo A, Xu JH, Zhao XQ. Development of yeast cell factories for consolidated bioprocessing of lignocellulose to bioethanol through cell surface engineering. Biotechnol Adv. 2012;30:1207–18.
Olson DG, Mcbride JE, Shaw AJ, Lynd LR. Recent progress in consolidated bioprocessing. Curr Opin Biotechnol. 2012;23:396–405.
Daas MJA, Van dWAHP, De VWM, John VDO, Richard VK. Isolation of a genetically accessible thermophilic xylan degrading bacterium from compost. Biotechnol Biofuels. 2016;9(1):210.
Liu W, Zhao C, Jiang J, Lu Q, Hao Y, Wang L, Liu C. Bioflocculant production from untreated corn stover using Cellulosimicrobium cellulans L804 isolate and its application to harvesting microalgae. Biotechnol Biofuels. 2015;8:1.
Pourali O, Asghari FS, Yoshida H. Sub-critical water treatment of rice bran to produce valuable materials. Food Chem. 2009;115:1–7.
Zullaikah S, Lai CC, Vali SR, Ju YH. A two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresour Technol. 2005;96:1889–96.
Montanher SF, Oliveira EA, Rollemberg MC. Removal of metal ions from aqueous solutions by sorption onto rice bran. J Hazard Mater. 2005;117:207–11.
Najeeb Kaid NAS, Kalil MS, Mohtar WYW. Biobutanol production from rice bran and de-oiled rice bran by Clostridium saccharoperbutylacetonicum N1-4. Bioprocess Biosyst Eng. 2012;35:817–26.
Liu C, Wang K, Jiang JH, Liu WJ, Wang JY. A novel bioflocculant produced by a salt-tolerant, alkaliphilic and biofilm-forming strain Bacillus agaradhaerens C9 and its application in harvesting Chlorella minutissima UTEX2341. Biochem Eng J. 2015;93:166–72.
Xia S, Zhang Z, Wang X, Yang A, Chen L, Zhao J, Leonard D, Jaffrezicrenault N. Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Bioresour Technol. 2008;99:6520–7.
Prakash P, Jayalakshmi SK, Prakash B, Rubul M, Sreeramulu K. Production of alkaliphilic, halotolerent, thermostable cellulase free xylanase by Bacillus halodurans PPKS-2 using agro waste: single step purification and characterization. World J Microbiol Biotechnol. 2012;28:183–92.
Xiong Y, Wang Y, Yu Y, Li Q, Wang H, Chen R, He N. Production and characterization of a novel bioflocculant from Bacillus licheniformis. Appl Environ Microbiol. 2010;76:2778–82.
Song JM, Wei DZ. Production and characterization of cellulases and xylanases of Cellulosimicrobium cellulans grown in pretreated and extracted bagasse and minimal nutrient medium M9. Biomass Bioenergy. 2010;34:1930–4.
Cao J, Yuan H, Li B, Yang J. Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour Technol. 2014;152:177–84.
Yang JS, Cao J, Xing GL, Yuan HL. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour Technol. 2015;175:537–44.
Yang JS, Rasa E, Tantayotai P, Scow KM, Yuan HL, Hristova KR. Mathematical model of Chlorella minutissima UTEX2341 growth and lipid production under photoheterotrophic fermentation conditions. Bioresour Technol. 2011;102:3077–82.
Li ZS, Yuan HL, Yang JS, Li BZ. Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341. Bioresour Technol. 2011;102:9128–34.
Papazi A, Makridis P, Divanach P. Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol. 2010;22:349–55.
Ndikubwimana T, Zeng X, Liu Y, Chang JS, Lu Y. Harvesting of microalgae Desmodesmus sp. F51 by bioflocculation with bacterial bioflocculant. Algal Res. 2014;6:186–93.
Zheng H, Gao Z, Yin J, Tang X, Ji X, Huang H. Harvesting of microalgae by flocculation with by poly (γ-glutamic acid). Bioresour Technol. 2012;112:212–20.
Chaplin MF, Kennedy JF. Carbohydrate analysis: a practical approach. 2nd ed. Oxford: IRL Press Ltd.; 1994.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–54.
Authors’ contributions
CL produced and analyzed the bioflocculant product and drafted the manuscript. YH participated in the optimization of fermentation conditions, the analysis of enzyme activity, and drafted the manuscript. JJ contributed in the study design and helped in revising the manuscript. WL conducted the culture and harvest of C. minutissima UTEX2341 cells, performed the statistical analysis, and drafted the manuscript. All authors have approved the submission of the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Prof. Jinshui Yang for his gift of Chlorella minutissima UTEX2341.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All the data related to the present manuscript will be available from the corresponding author on reasonable request.
Funding
This work was supported by National Natural Science Foundation of China (31300054), Youth Fund of the Natural Science Foundation of Jiangsu Province of China (BK20130228), Grants from Natural Science Foundation of Xuzhou city (KC15N0014), Postgraduate Research and Innovation Plan Project of Jiangsu Normal University (2016YZD019) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, C., Hao, Y., Jiang, J. et al. Valorization of untreated rice bran towards bioflocculant using a lignocellulose-degrading strain and its use in microalgal biomass harvest. Biotechnol Biofuels 10, 90 (2017). https://doi.org/10.1186/s13068-017-0780-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-017-0780-6