Abstract
Pyrimidine nucleus is a significant pharmacophore that exhibited excellent pharmacological activities. A series of pyrimidine scaffolds was synthesized and its chemical structures were confirmed by physicochemical and spectral analysis. The synthesized compounds were evaluated for their antimicrobial potential towards Gram positive and negative bacteria as well as fungal species. They were also assessed for their anticancer activity toward a human colorectal carcinoma cell line (HCT116). Whilst results of antimicrobial potential revealed that compounds Ax2, Ax3, Ax8 and Ax14 exhibited better activity against tested microorganisms, the results of antiproliferative activity indicated that compounds Ax7 and Ax10 showed excellent activity against HCT116. Further, the molecular docking of pyrimidine derivatives Ax1, Ax9 and Ax10 with CDK8 (PDB id: 5FGK) protein indicated that moderate to better docking results within the binding pocket. Compounds Ax8 and Ax10 having significant antimicrobial and anticancer activities may be selected as lead compounds for the development of novel antimicrobial and anticancer agent, respectively.

Similar content being viewed by others
Introduction
Drug designing is a technique of searching and developing new molecules that exert specific action on a human kind [1]. The figure of multidrug resistant microbial infections is growing day by day which indicated that it is crucial to develop new class of antimicrobial drugs [2]. Tumor is a severe health issue and 2nd leading/most reason for mortality in the globe. It is caused by deregulation of the cell cycle which results in failure of cellular differentiation and unrestrained cellular growth [3, 4]. So, it is necessary to develop and synthesize new bioactive molecules whose chemical structure and mode of action are noticeably differing from the available agents [5].
Discovery of drug is a slow, lengthy costly and interdisciplinary procedure but the new developments have transformed the methods by which researchers generate new drug molecules e.g. CADD tool overcomes the cost of drug design up to 50% [1]. Molecular docking technique is used to understand the (i) drug-receptor interaction (ii) binding affinity (iii) orientation and approach of drug molecules to the target site. The main objectives of docking study are precise structural modeling, correct prediction of activity. It presents the most promising vision of drug–receptor interaction and generates a new rational approach to drug design [6]. RMSD is the average distance between the atoms of superimposed structures. This value is widely used parameter to rank the performance of docking methods. If the docked ligand shows < 2.0 Å RMSD value with the crystallographic ligand, it is considered as a successful docking. To calculate the relative free energy, an accurate MM-GBSA binding affinity computation can also be applied [7, 8].
Cyclin-dependent kinases play a significant role in the control of cell cycle. These holoenzymes have both catalytic (CDK) and regulatory (cyclin) subunits but present as higher order complexes that include additional proteins and are arbitrated by two classes of enzymes i.e. cyclin D- and E. The D-type cyclins (D1, D2 and D3) bind with two different catalytic sites (CDK4 and CDK6) to yield six possible holoenzymes that articulated in tissue-specific models [9].
CDKs are a class of enzymes that controls the cell cycle and are novel targets for prospective anticancer drugs [10]. A series of pyrimidines bearing 2-arylamino substituents was developed and screened for CDK1 and CDK2 inhibitory effect by Sayle et al. [11]. The SAR of 4-cyclohexylmethoxy-pyrimidines (inhibitors of CDK2) was explored [12]. The progression, transcription and other related functions of cell cycle are regulated by CDK8 that is a heterodimeric kinase protein. The carboxyterminal domain of RNA polymerase II is also phosphorylated by CDK-8. Hence, the inhibition of CDK-8 protein may be essential for regulating tumor [6, 13].
Pyrimidine is a heterocyclic nucleus containing nitrogen atom at 1 and 3 positions. It is the structural unit of DNA and RNA is an important molecule also plays a very significant role in the field of medicinal chemistry [14]. Pyrimidine is reported to have antimicrobial [15], anticancer [16, 17], anti-inflammatory [18], antioxidant [19], analgesic [20] and antiviral [21] and antimalarial [22] potentials. Number of marketed drugs contains pyrimidine ring such as proquazone (anti-inflammatory); idoxuridine (antiviral); trimethoprim (antibacterial); zidovudine (anti-HIV); pyrimethamine (antimalarial) and capecitabine (antiproliferative).
In the present study we have planned to synthesize heterocyclic pyrimidine analogues and evaluate their antimicrobial, antiproliferative and docking study.
Results and discussion
Chemistry
Synthesis of heterocyclic pyrimidine analogues followed the general procedure discussed in synthetic Scheme 1. The reaction of p-substituted acetophenone with substituted benzaldehyde resulted in the formation of Int- I. The resulted compound was treated with guanidine nitrate to yield pyrimidine ring (Int-II), which on reaction with corresponding substituted benzaldehyde in presence of glacial acetic acid yielded the final derivatives (Ax1–Ax19). The molecular scaffolds of the developed pyrimidine derivatives (Ax1–Ax19) were established by physicochemical properties (Table 1) and NMR, FTIR, MS spectra and elemental analysis (Table 2). The IR spectrum of synthesized compound showed bands around 2934–3093 cm−1 and 1462–1595 cm−1 which indicate the C–H and C=C group in aromatic nucleus, respectively. The Ar–Cl group in compounds Ax5, Ax12, Ax16 were displayed stretches in the scale of 712–757 cm−1. The IR str. vibrations at 512–628 cm−1 in the spectral data of compounds displayed the Ar–Br group at p-position of the aromatic nucleus. The existence of Ar-OCH3 in synthesized analogues is established by absorption band around 1177–1276 cm−1. The appearance of IR str. 1550–1685 cm−1 in the compounds (Ax1–Ax19) specified the existence of N=CH group. The Ar-NO2 group in compounds Ax1, Ax6 and Ax15–Ax19 were displayed by symmetric Ar-NO2 str. in the scale of 1345–1462 cm−1. The IR stretching 1270–1363 cm−1 of synthesized compounds specified the existence of C–N group. The impression of IR absorption band at 3231–3491 cm−1 in the spectral data of the molecules displayed the presence of Ar-OH group on the aromatic nucleus. The signals between 6.39 and 8.38 δ in NMR spectra are indicative of aromatic proton. The prepared derivatives exhibited singlet at 7.46–8.39 δ due to the presence of N=CH group in pyrimidine nucleus. Molecules displayed singlet at 7.56–7.91 δ due to the presence of –CH group in pyrimidine nucleus. The singlet at 3.71–3.87 δ indicated the presence Ar-OCH3. Compound Ax8 exhibited singlet at 2.67 δ due to presence of –N(CH3)2 at the p-position. The compound Ax14 exhibited quadrate at 3.38 δ and triplet at 1.14 δ due to presence of –N(C2H5)2 group at p-position. The 13C-NMR spectra of aromatic ring exhibited in the range of 102.0, 112.3, 117.3, 123.6, 124.4, 126.6, 126.3, 128.1, 129.3, 130.2, 133.2, 147.5, 153.2; pyrimidine nucleus exhibited around 111.5, 164.3, 168.2; N=CH group exhibited around 161.0; OCH3 group showed around 54.1, 60.8, 56.1. The elemental analysis (CHN) was found within ± 0.4% of the theoretical results of derivatives.
Antimicrobial screening results
The pyrimidine compounds (Ax1–Ax19) were examined for their antimicrobial potency towards Gram −ve and Gram +ve bacteria as well as fungal species by tube dilution technique. Table 3, Figs. 1 and 2 show the antimicrobial evaluation results. The compounds showed significant antimicrobial activity than standard drugs, norfloxacin (for antibacterial study) and fluconazole (for antifungal study). In Gram negative bacteria, compound Ax14 (MICec = 21.7 µM) exhibited better antibacterial potency toward E. coli. In the case of Gram positive bacteria, compound Ax8 (MICsa = 21.2 µM) and (MICbs = 10.6 µM) showed the significant potency towards S. aureus and B. subtilis, respectively. The antifungal screening results displayed that compounds, Ax2 (MICan = 9.40 µM) and Ax3 (MICca = 10.7 µM) showed the significant potency towards A. niger and C. albicans, respectively. The molecules may be used as the lead compounds for the development of new antimicrobial agents.
Antiproliferative screening results
Table 4 and Fig. 3 show the screening results of the developed pyrimidine compounds (Ax1–Ax19) towards human colorectal carcinoma cell line by SRB assay [23]. The synthesized compounds exhibited good anticancer activity, with some of the findings comparable or highly potent than 5-fluorouracil (standard drug). Compounds Ax2 (IC50 = 2.70 µM), Ax7 (IC50 = 1.90 µM), Ax8 (IC50 = 2.20 µM) and Ax10 (IC50 = 0.80 µM), in particular, were the four best compounds which elicited more potent anticancer activity when compared to the reference drug (IC50 = 6.20 µM). They may be used as lead molecules for the development of new anticancer agent.
Molecular docking results
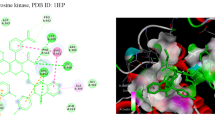
The CDKs is an enzyme family that plays an significant role in the regulation of the cell cycle and thus is an especially advantageous target for the development of small inhibitory molecules [13]. The crystal structure of cyclin dependent kinase 8 (PDB Id: 5FGK) which has a good resolution of about 2.36 Å was used for docking study. The binding site of the target was generated using co-crystallized ligand (5XG) as reference (X = − 0.138, Y = − 24.891, Z = 150.623). Root-mean square deviation (RMSD) value of docked pose of native co-crystallized ligand was calculated as 0.08 Å. The synthesized pyrimidine compounds were then docked to the active site of CDK8. The docking results were analysed based on the docking score obtained from GLIDE. Among the docked compounds, compounds Ax1, Ax9 and Ax10 displayed moderate to good docked score with anticancer potency against a HCT116 cancer cell line. Ligand interaction image and binding mode of compounds Ax1, Ax9 and Ax10 in the active site of CDK8 protein having co-crystallized ligand 5XG and 5-Fu is having a different binding mode to that of active compounds (Figs. 4, 5, 6 and 7). The molecular docking results depend on the statistical evaluation function according to which the interaction energy in numerical values as docking scores [24].
Molecular docking study of the selected compounds have good to better anticancer potency toward cancer cell line were displayed moderate to better docking score within binding pocket. Binding mode of active compounds Ax1, Ax9 and Ax10 within the binding region, compound Ax10 have moderate docked score (− 4.191) with better potency (0.80 μM) and formation of pi-cation interaction with amino acid residue Arg356; compound Ax1 have better docked score (− 5.668) with lowest potency (48.4 μM) and formation of H-bond with amino acid residues Val27 and Lys153, pi-cation interaction with Arg356 and salt bridge with Asp173, Lys52 and Glu66 within the binding pocket and compound Ax9 have moderate docked score (− 4.477) with moderate potency (16.7 μM) and formation of H-bond with amino acid residue Lys153 within the binding pocket and compared to 5-fluorouracil have better docked score (− 5.753) with good potency (6.20 μM) and formation of H-bond with amino acid residues Ala100 and Asp98 within binding pocket. The docking score results and interacting residues are showing in Table 5. Thus the docking analyses suggested that the pyrimidines can act as of great interest in successful chemotherapy. Cyclin dependent kinase-8 may be the target protein of pyrimidine derivatives for their antiproliferative activity.
SAR (structure activity relationship) study
The following SAR can be deduced from the antimicrobial and anticancer screening results of pyrimidine analogues (Fig. 8).
Antimicrobial activity
The presence of EWG (electron withdrawing group) (inductively)—Br at p-position of the substituted benzylidene aromatic nucleus of compound Ax2 improved the antifungal activity against A. niger and –N(CH3)2) (an electron donating group, by mesomeric affect) at p-position of the benzylidene nucleus of compound Ax8 enhanced the antibacterial activity towards S. aureus and B. subtilis.
On the other side, The presence of EWG (inductively)—Br at p-position of the substituted benzylidene aromatic nucleus of compound Ax3 improved the antifungal activity toward C. albicans and –N(C2H5)2) (an electron donating group, by mesomeric affect) at p-position the substituted benzylidene aromatic ring of compound Ax14 enhanced the antibacterial activity towards E. coli.
Anticancer activity
The presence of EWG (inductively)—Br at p-position of the substituted benzylidene aromatic nucleus of compounds Ax2 and –N(CH3)2) (an electron donating group, by mesomeric affect) at p-position of the substituted benzylidene aromatic ring of compound Ax8 enhanced the anticancer activity towards a human colorectal carcinoma cell line (HCT116), however, electron releasing groups like p-OCH3 and o-OH on substituted benzylidene aromatic ring of compounds Ax7 and Ax10, respectively showed significant role in improving the anticancer activity toward a HCT116 cell line. The SAR study is consistent the results of Kumar et al. [6, 15] and Xu et al. [25].
Experimental
Preparatory materials were obtained from commercial sources [CDH Pvt. Ltd, HiMedia Lab. Pvt. Ltd. and Loba Chemie, Pvt Ltd. Mumbai, India] for the research work. Reaction advancement was observed by TLC (silica gel plates) using chloroform: methanol as mobile phase. Melting point was determined in open capillary tube method. Elemental analysis of the derivatives was determined by Perkin–Elmer 2400 C, H and N instrument. FTIR spectrum was recorded on Bruker 12060280 spectrometer. The Mass spectrum of the molecules was recorded on Waters Micromass Q-ToF Micro instrument. 1H-NMR and 13C-NMR were recorded at 600 MHz and 150 MHz, respectively by Bruker Avance III 600. 1H-NMR data are given as multiplicity and number of protons.
Procedure for the synthesis of pyrimidine derivatives (Scheme 1, Ax1–Ax19)
(A): Synthesis of 1-(2-(3,4,5-trimethoxybenzylideneamino)-6-(4-nitrophenyl)pyrimidin-4-yl)-naphthalen-2-ol (Compound Ax1)
p-Nitroacetophenone (0.01 mol) and naphthaldehyde (0.01 mol) were added in 50 mL methanol after that 10 mL NaOH solution was added drop by drop to the reaction mixture and kept on vigorous stirring for 30 min. When the reaction mixture became turbid, it was maintained at 20–22 °C on magnetic stirrer for 4–5 h and then, the reaction mixture was neutralised by 0.1–0.2 N HCl to yield chalcone [Int-I]. The chalcone was filtered and recrystallised with methanol [26]. To the Int-I (0.01 mol), potassium hydroxide (0.01 mol) and guanidine nitrate (0.25 M) in methanol (30 mL) was added and refluxed for 5–6 h (RT). The reaction mixture was cooled and quenched with 20 mL of 0.5 M HCl solution in water to yield pyrimidine [Int-II] [27]. The Int-II (0.01 mol) was then refluxed with substituted benzaldehyde (0.01 mol) in methanol 50 mL in presence of glacial acetic acid for 2–3 h (RT). The precipitate generated by adding the reaction mixture to the ice cold water was filtered and recrystallised with methanol [28].
(B): Synthesis of 1-(2-substituted benzylideneamino)-6-(4-substituted benzylideneamino) phenyl)pyrimidin-4-yl) naphthalen-2-ol (Compounds Ax2, Ax7, Ax8 and Ax11)
p-Aminoacetophenone (0.01 mol) and naphthaldehyde (0.01 mol) were added in 50 mL methanol after that 10 mL NaOH solution was added drop by drop to the reaction mixture and kept on vigorous stirring for 30 min. When the reaction mixture became turbid, it was maintained at 20–22 °C on magnetic stirrer for 4–5 h. The reaction mixture was neutralised by 0.1–0.2 N HCl to yield chalcone [Int-I]. The chalcone was filtered and recrystallised with methanol [26]. To the Int-I (0.01 mol), potassium hydroxide (0.01 mol) and guanidine nitrate (0.25 M) in methanol (30 mL) was added and refluxed for 5–6 h (RT). The reaction mixture was cooled and quenched with 20 mL of 0.5 M HCl solution in water to yield pyrimidine [Int-II] [27]. The Int-II (0.01 mol) was then refluxed with substituted benzaldehyde (0.02 mol) in methanol 50 mL in presence of glacial acetic acid for 2–3 h (RT). The precipitate generated by adding the reaction mixture to the ice cold water was filtered and recrystallised with methanol [28].
(C): Synthesis of N-(2-substituted benzylidene)-4-(4-substituted phenyl)-6-(3,4,5-trimethoxy- phenyl)pyrimidin-2-amine (Compounds Ax3-Ax6, Ax9, Ax10, Ax12, Ax13, Ax14-Ax19)
p-Substituted acetophenone (0.01 mol) and 3,4,5-trimethoxybenzaldehyde (0.01 mol) were added in 50 mL methanol after that 10 mL NaOH solution was added drop by drop to the reaction mixture and kept on vigorous stirring for 30 min. When the reaction mixture became turbid it was maintained at 20–22 °C on magnetic stirrer for 4–5 h and then, the reaction mixture was neutralised by 0.1–0.2 N HCl to yield chalcone [Int-I]. The chalcone was filtered and recrystallised with methanol [26]. To the Int-I (0.01 mol), potassium hydroxide (0.01 mol) and guanidine nitrate (0.25 M) in methanol (30 mL) was added and refluxed for 5–6 h (RT). The reaction mixture was cooled and quenched with 20 mL of 0.5 M HCl solution in water to yield pyrimidine [Int-II] [27]. The Int-II (0.01 mol) was then refluxed with substituted benzaldehyde (0.01 mol) in methanol 50 mL and added few drops of glacial acetic acid for 2–3 h (RT). The precipitate generated by adding the reaction mixture to the ice cold water was filtered and recrystallised with methanol [28].
Biological evaluations (antimicrobial and anticancer)
The antimicrobial evaluation of developed derivatives (Ax1-Ax19) was carried out by tube dilution technique [29] towards Gram+ bacteria species (S. aureus MTCC3160; B. subtilis MTCC441) and Gram− ve bacterium species (E. coli MTCC443) and fungal species: C. albicans MTCC227; A. niger MTCC281. The stock solution of compounds and control drugs (norfloxacin and fluconazole) were prepared in DMSO to get a concentration of 100 µg/mL. Dilutions of test and reference compounds were prepared in Sabouraud dextrose broth I.P. (fungi) and double strength nutrient broth I.P. (bacteria) [30]. The test samples were incubated at 37 ± 1 °C for 48 h (C. albicans), at 25 ± 1 °C for 7 days (A. niger), 37 ± 1 °C for 24 h (bacteria) respectively and the screening results were recorded in terms of MIC. The antiproliferative potency of the developed derivatives was carried out by SRB assay [23] toward human colorectal carcinoma cancer cell line [HCT116 (ATCC CCL-247)]. Data was presented as mean IC50 of triplicates.
Molecular docking
The molecular docking study was performed of the synthesized pyrimidine derivatives by GLIDE docking program of maestro v11.5 (Schrodinger 2018-1). Among the docked compounds, compounds Ax1, Ax9 and Ax10 displayed moderate to good docked score within the binding pocket of the selected protein i.e. PDB Id: 5FGK with anticancer potency against a HCT116. The protein target for heterocyclic pyrimidine compounds was identified through the literature survey [6, 31]. Pyrimidine moiety has wide spectrum of biological potential in medicinal filed [32]. CDK8 (PDB Id: 5FGK) having native ligand 5XG (co-crystallized) with good resolution about 2.36 Å for docking study. Method: X-ray diffraction, R-value free: 0.237 [33]. The root-mean-square deviation is a measure of the average distance between the atoms of superimposed structures. RMSD value of the co-crystallized native ligand (5XG) was calculated. First, Grid is generated using ATP binding site, then docking scores are calculated (Schrodinger 2018-1, maestro v11.5) [34]. Ligand preparation is done using LigPrep module of maestro v11.5. To give the best results, the molecular structures that are docked must be good representations of the actual ligand structures as they would appear in a protein–ligand complex [35].
Conclusion
In the present study, a series of heterocyclic pyrimidine compounds was synthesized in considerable yield and confirmed by FTIR, NMR, MS, CHN analysis. The synthesized compounds showed appreciable antimicrobial and antiproliferative activities. Structure activity relationship study indicated that compounds (Ax2, Ax3, Ax8 and Ax14) having electron withdrawing and compounds (Ax7 and Ax10) have electron releasing groups at substituted benzylidene aromatic nucleus exhibited significant antimicrobial and antiproliferative activities. Further, molecular docking study demonstrated that compound Ax1 showed best docked score with lowest anticancer potency and compound Ax10 showed the moderate docked score with better anticancer potency and compared to the 5-fluorouracil having better docked score with good anticancer potency. Cyclin dependent kinase-8 may be the target protein of heterocyclic pyrimidine compound for their antiproliferative potency. Based on the docking results it is suggested that more structural modifications are required in derivatives Ax1 and Ax10 to make them more potent anticancer agents and these compounds may be used as leads for the development of novel antimicrobial and anticancer agents.
Availability of data and materials
We have presented all our main data in the form of tables and figures.
Abbreviations
- NMR:
-
nuclear magnetic resonance
- IR:
-
infrared
- MS:
-
mass spectrum
- CHN:
-
carbon hydrogen nitrogen
- Str:
-
starching
- CADD:
-
computer‐aided drug design
- MTCC:
-
microbial type culture collection
- E. coli :
-
Escherichia coli
- C. albicans :
-
Candida albicans
- S. aureus :
-
Staphylococcus aureus
- B. subtilis :
-
Bacillus subtilis
- A. niger :
-
Aspergillus niger
- MIC:
-
minimum inhibitory concentration
- ATCC:
-
American Type Culture Collection
- HCT116:
-
human colorectal carcinoma 116
- SRB:
-
sulforhodamine B
- SAR:
-
structure activity relationship
- μM:
-
micro mole
- CDK8:
-
cyclin dependent kinase 8
- PDB:
-
protein data bank
- RMSD:
-
root-mean-square deviation
- 2D:
-
2 dimensional
- 3D:
-
3 dimensional
- RNA:
-
ribonucleic acid
- DNA:
-
deoxyribonucleic acid
- CDH:
-
central drug house
- RT:
-
room temperature
- DMSO:
-
dimethyl sulfoxide
- 5-Fu:
-
5-fluorouracil
- O :
-
ortho
- p :
-
para
- EWG:
-
electron withdrawing group
References
Taft CA, da Silva VB, de Silva CHT (2008) Current topics in computer-aided drug design. J Pharm Sic 97(3):1089–1098. https://doi.org/10.1002/jps.21293
Kakkar S, Kumar S, Narasimhan B, Lim SM, Ramasamy K, Mani V, Shah SAA (2018) Design, synthesis and biological potential of heterocyclic benzoxazole scaffolds as promising antimicrobial and anticancer agents. Chem Cent J 12(96):1–11
Rani J, Saini M, Kumar S, Verma PK (2017) Design, synthesis and biological potentials of novel tetrahydroimidazo[1,2-a]pyrimidine derivatives. Chem Cent J 11(16):1–11
Hu Y, Fu L (2012) Targeting cancer stem cells: a new therapy to cure cancer patients. Am J Cancer Res 2(3):340–356
Kassab A, Gedawy E (2013) Synthesis and anticancer activity of novel 2-pyridyl hexahyrocyclooctathieno [2,3-d] pyrimidine derivatives. Eur J Med Chem 63:224–230
Kumar S, Lim SM, Ramasamy K, Vasudevan M, Shah SAA, Selvaraj M, Narasimhan B (2017) Synthesis, molecular docking and biological evaluation of bis-pyrimidine Schiff base derivatives. Chem Cent J 11(89):1–16
Plewczynski D, Lazniewski M, Augustyniak R, Ginalski K (2011) Can we trust docking results? Evaluation of seven commonly used programs on PDB bind database. J Comput Chem 32(4):742–755
Ece A (2019) Towards more effective acetylcholinesterase inhibitors: a comprehensive modelling study based on human acetylcholinesterase protein–drug complex. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2019.1583606
Sherr CJ (2000) The pezcoller lecture: cancer cell cycles revisited. Cancer Res 60:3689–3695
Ece A, Sevin F (2013) The discovery of potential cyclin A/CDK2 inhibitors: a combination of 3D QSAR pharmacophore modeling, virtual screening, and molecular docking studies. Med Chem Res 22(12):5832–5843
Sayle KL, Bentley JF, Boyle TA, Calvert H, Cheng YZ, Curtin NJ, Endicott JA, Golding BT, Hardcastle IR, Jewsbury P, Mesguiche V, Newell DR, Noble MEM, Parsons RJ, Pratt DJ, Wang LZ, Griffin RJ (2003) Structure-based design of 2-arylamino-4-cyclohexylmethyl-5-nitroso-6-aminopyrimidine inhibitors of cyclin-dependent kinases 1 and 2. Bioorg Med Chem Lett 13:3079–3082
Ece A, Sevin F (2010) Exploring QSAR on 4-cyclohexylmethoxypyrimidines as antitumor agents for their inhibitory activity of cdk2. Lett Drug Des Discov 7(9):625–631
Peyressatre M, Prével C, Pellerano M, Morris MC (2015) Targeting cyclin-dependent kinases in human cancers: from small molecules to peptide inhibitors. Cancer 7:179–237
Kumar S, Narasimhan B (2018) Therapeutic potential of heterocyclic pyrimidine scaffolds. Chem Cent J 12(38):1–29
Kumar S, Lim SM, Ramasamy K, Vasudevan M, Shah SAA, Narasimhan B (2017) Bis-pyrimidine acetamides: design, synthesis and biological evaluation. Chem Cent J 11(80):1–14
Kumar S, Lim SM, Ramasamy K, Mani V, Shah SAA, Narasimhan B (2018) Design, synthesis, antimicrobial and cytotoxicity study on human colorectal carcinoma cell line of new 4,4′-(1,4-phenylene)bis(pyrimidin-2-amine) derivatives. Chem Cent J 12(73):1–13
Guo Y, Li Jing, Ma J, Yu Z, Wang H, Zhua J, Liao X, Zhao Y (2015) Synthesis and antitumor activity of α-aminophosphonate derivatives containing thieno[2,3-d] pyrimidines. Chin Chem Lett 26:755–758
Yejella RP, Atla SR (2011) A study of anti-inflammatory and analgesic activity of new 2,4,6-trisubstituted pyrimidines. Chem Pharm Bull 59(9):1079–1082
Bhalgat CM, Ali MI, Ramesh B, Ramu G (2014) Novel pyrimidine and its triazole fused derivatives: synthesis and investigation of antioxidant and anti-inflammatory activity. Arab J Chem 7:986–993
Ashour HM, Shaaban OG, Rizk OH, El-Ashmawy IM (2013) Synthesis and biological evaluation of thieno[2′,3′:4,5]pyrimido[1,2-b][1,2,4]triazines and thieno[2,3-d] [1,2,4]triazolo[1,5-a]pyrimidines as anti-inflammatory and analgesic agents. Eur J Med Chem 62:341–351
Meneghesso S, Vanderlinden E, Stevaert A, McGuigan C, Balzarini J, Naesens L (2012) Synthesis and biological evaluation of pyrimidine nucleoside monophosphate prodrugs targeted against influenza virus. Antivir Res 94:35–43
Kumar D, Khan SI, Tekwani BL, Diwan PP, Rawat S (2015) 4-Aminoquinoline–pyrimidine hybrids: synthesis, antimalarial activity, heme binding and docking studies. Eur J Med Chem 89:490–502
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Bassyouni F, El Hefnawi M, El Rashed A, Rehim MA (2017) Molecular modeling and biological activities of new potent antimicrobial, anti-inflammatory and anti-nociceptive of 5-nitro indoline-2-one derivatives. Drug Des 6(2):1–6
Xu L, Zhang Y, Dai W, Wang Y, Jiang D, Wang L, Xiao J, Yang X, Li S (2014) Design, synthesis and SAR study of novel trisubstituted pyrimidine amide derivatives as CCR26 antagonists. Molecules 19:3539–3551
Kumar N, Jain JS, Sinha R, Garg VK, Bansal SK (2009) Evaluation of some novel chalcone derivatives for antimicrobial and anti-inflammatory activity. Der Pharmacia Lettre 1(1):169–176
Asiri AM, Khan SA (2011) Synthesis and antibacterial activities of a bis-chalcone derived from thiophene and its bis-cyclized products. Molecules 16:523–531
Sawarkar U, Narule M, Chaudhary M (2012) Synthesis of some new 3(4-hydroxyphenyl) prop-2-en-1-one 4-phenyl substituted Schiff’s bases and their antibacterial activity. Der Pharma Chemica 4(2):629–632
Cappuccino JC, Sherman N (1999) Microbiology—a laboratory manual. Addison Wesley, California, p 263
Pharmacopoeia of India, vol. Ӏ (2007) Controller of Publication, Ministry of Health Department, Govt. of India, New Delhi, pp 37
Kumar S, Singh J, Narasimhan B, Shah SAA, Lim SM, Ramasamy K, Mani V (2018) Reverse pharmacophore mapping and molecular docking studies for discovery of GTPase HRas as promising drug target for bis-pyrimidine derivatives. Chem Cent J 12(106):1–11
Kaur R, Kaur P, Sharma S, Singh G, Mehndiratta S, Bedi PM, Nepali K (2015) Anti-cancer pyrimidines in diverse scaffolds: a review of patent literature. Recent Pat Anti-Cancer 10(1):23–71
Amin KM, Awadalla FM, Eissa AAM, Abou- Seri AM, Hassan GS (2011) Design, synthesis and vasorelaxant evaluation of novel coumarin-pyrimidine hybrids. Bioorg Med Chem 19:6087–6097
Singh J, Kumar M, Mansuri R, Sahoo GC, Deep A (2016) Inhibitor designing, virtual screening and docking studies for methyltrans-ferase: a potential target against dengue virus. J Pharm Bioallied Sci 8(3):188–194
Driessche GVD, Fourches D (2017) Adverse drug reactions triggered by the common HLA-B*57:01 variant: a molecular docking study. J Cheminform 9(13):1–17
Acknowledgements
The authors are thankful to HOD, M.D. University, Rohtak, Haryana for providing necessary facilities to carry out this research work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Authors BN, AK and SK- performed synthesis, antimicrobial activity and molecular docking study of active anticancer compounds; SML, KR, VM and SAAS- performed characterization and antiproliferative study of synthesized pyrimidine compounds. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kumar, S., Kaushik, A., Narasimhan, B. et al. Molecular docking, synthesis and biological significance of pyrimidine analogues as prospective antimicrobial and antiproliferative agents. BMC Chemistry 13, 85 (2019). https://doi.org/10.1186/s13065-019-0601-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-019-0601-z