Abstract
Four novel complexes [Co(H2O)4(sul)2] 1, [Co(2-ampy)2(sul)2] 2, [Co(H2O)2(1,10-phen) (sul)2] 3 and [Co(2,9-dimephen)(sul)2] 4 (sul = sulindac, 2-ampy = 2-amino pyridine, 1,10-phen = 1,10-phenanthroline and 2,9-dimeph = 2,9-dimethyl-1,10-phenanthroline) were prepared and characterized by IR, UV–Visible spectroscopy and magnetic properties. The crystal structures of complexes 1 and 4 were determined by single-crystal X-ray diffraction. In-vitro anti-bacterial activity for the prepared complexes against Gram-positive (Staphylococcus epidermidis, Staphylococcus aureus) and Gram-negative (Bordetella, Escherichia coli) bacteria and Yeast species (Saccharomyces and Candida) were performed using agar well-diffusion method. Only complex 4 showed reasonable activity against yeast. All compounds showed more anti-bacterial activity against Gram-positive bacteria than Gram-negative.

This work reports synthesis, crystallographic, spectroscopic studies and biological activity of new cobalt(II) complexes with bioactive mixed sulindac and nitrogen-donor ligands. The crystal structures of complexes 1 and 4 were determined using single-crystal X-ray diffraction. In-vitro anti-bacterial activity of the prepared complexes and their parent ligands were investigated against different Gram-positive and Gram-negative bacteria using agar diffusion method
Similar content being viewed by others
Background
Cobalt has a significant role in proteins; there are at least eight cobalt-dependent proteins. Moreover, cobalt is needed at the active center of certain coenzymes that are called cobalamins especially cyanocobalamins (Vitamin B12) which regulates indirectly the synthesis of DNA [1,2,3].
The first reported study about the biological activity of cobalt compounds was in 1952, where cobalt(III) compounds of bidentate mustard seemed to act as hypoxia-selective agents [4, 5]. Several compounds showed considerable activity against bacteria strains and against leukemia and lymphoma cell lines [6]. Furthermore, cobalt complexes possess in vivo insulin-like properties [7, 8], anti-fungal and anti-oxidant activities [9]. Several Co(III) complexes with anti-microbial activities have been reported [10,11,12,13,14]. For instance, a Co(III) complex of the known anti-ulcer drug famotidine turned out to have greater anti-microbial activity against M. lysodeikticus and Escherichia coli than the metal free drug [10,11,12,13,14].
Recently, metal(II) carboxylate compounds with nitrogen and/or oxygen-donor ligands have attracted an increasing interest because of their potential biological and chemical activities [15]. The interaction between heterocyclic compounds and metal ions is very important in biological systems such as drugs and vitamins [16]. In previous studies cobalt(II) compounds showed anti-fungal and anti-microbial activities; for example, imidazole-2-carbaldehyde semicarbazone was active against yeasts Candida tropicalis and Saccharomyces cerevisiae. Activity was most noticeable against phytopathogenic fungi such as Alternaria or Sclerotinia [17].
{(1Z)-5-fluoro-2-methyl-1-[4-(methylsulfinyl)benzylidene]-1H-indene-3-yl}acetic acid known as Sulindac, in the form of potassium salt has a wide spectrum of activity as non-steroidal anti-inflammatory drug (NSAIDs). The chemical classes of NSAIDs comprise phenylalkanoic acids, anthranilic acids, salicylate derivatives, oxicams, furanones and sulfonamides [18,19,20,21,22,23,24]. Sulindac belong to phenylalkanoic acids that are potent NSAIDs for the treatment of inflammatory conditions, such as pain, fever and inflammation. The transition metal coordination with NSAIDs caused many enhanced anti-inflammatory activity [25,26,27]. Some compounds of NSAIDs that can coordinate with transition metals have been synthesized and tested for their biological and pharmacological activity [28,29,30,31,32,33,34], to our best knowledge the synthesized cobalt complexes are the first reported structures, in addition to our previously reported zinc (Fig. 1) sulindac complexes [34].
Sulindac structure [37]
The synthesis, characterization and anti-bacterial activity of new cobalt(II) sulindac containing complexes with heterocyclic nitrogen based ligands (2-aminopyridine “2-ampy”, 1,10-phenanthroline “1,10-phen” and 2,9-dimethyl-1,10-phenanthroline “2,9-dimphen”) are described in the present work. The crystal structures of [Co(H2O)4(sul)2] (1) and [Co(2,9-dimephen)(sul)2] (4) are also reported.
Results and discussion
Synthesis of cobalt complexes
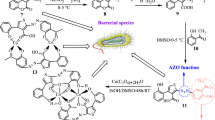
[Cobalt sulindac complex], 1 was prepared by mixing cobalt chloride and potassium sulindac in 1:2 molar ratios with methanol as a solvent. The desired product was obtained as a yellow solid (Scheme 1) and its structure was determined by single crystal X-ray diffraction. The novel mixed ligand cobalt(II) complexes were prepared by adding the appropriate N-donor ligand to complex 1 see (Scheme 2). The physical properties of 1–4 are summarized in Additional file 1: Table S1. Physical properties and yield of Cobalt(II) sulindac compounds.
Crystallographic study
Crystallographic study of complex 1
The atomic numbering scheme and atom connectivity for complex 1 are shown in Fig. 2. The asymmetric unit of the titled complex, contains a Co(II) cation, two monodentate sulindac groups and four water molecules.
Although the synthetic procedure and the recrystallization process of complex 1 were performed in methanol, a marked preference for coordination of water over methanol was observed and proved by single crystal X-ray determination. This phenomenon might be due to the stronger bond interaction between water and the metal center than methanol. In addition, the used methanol was not dry enough and wet, so it was possible to provide the four water molecules bonded to the metal center.
The two sulindaco groups are connected to the metal center in a monodentate coordination mode forming a symmetrical octahedral geometry with the additional four water molecules. The Co–O bond distances of 2.089(4), 2.100(5) and 2.141(4) Å are similar to previously reported values [38]. Selected bond angles and bond distances are listed in Table 1.
From the bonding angles in complex 1; O(1)#1–Co(1)–O(2W)#1 = 87.9(2)°, O(1)–Co(1)–O(2W) = 87.9(2)°, O(1)–Co(1)–O(1 W) = 92.09(17)°, O(1)#1–Co(1)–O(1W) = 87.91(17)° and O(2W)#1–Co(1)–O(1W) = 89.4(2)° a slight distortion from regular octahedral geometry was observed due to the expected Jahn–Teller effect which is also confirmed by the appearance of a shoulder in the d–d visible transition of this and other cobalt complexes.
Crystallographic study of complex 4
The atomic numbering scheme and atom connectivity for complex 4 are shown in Fig. 3. The asymmetric unit of the titled complex, contains a Co(II) cation, two sulindac groups and one 2,9-dimephen ligand. The Co–O bond distances of 2.117(8), 2.128(6), 2.220(10) and 2.220(10) Å are similar to reported values [39,40,41,42,43,44,45,46,47]. Co–N bond distances of 2.090(7) and 2.100(7) Å are also similar to reported values [39,40,41,42,43,44,45,46,47,48]. Selected angles and distances are listed in Table 1.
From bonding angles in complex 4, a slight deviation from octahedral geometry was observed, N(1)–Co(1)–O(1) = 108.2(3)°, N(2)–Co(1)–O(4) = 112.3(3)°, N(2)–Co(1)–O(5) = 102.5(4)°, N(2)–Co(1)–N(1) = 79.8(3)° and N(1)–Co(1)–O(2) = 104.3 (19)°.
Infrared spectra
Infrared spectral data of KBr pellet of cobalt sulindac complexes 1–4 in the 400–4000 cm−1 range are summarized in Additional files 2 and 3: Table S2. Comparison between some of principle peaks in IR for K(sul) and 1 (cm-1) and Table S3. Summary of principle peaks in IR for complexes 2, 3 and 4 (cm-1). In metal carboxylate complexes, the major characteristic of the IR spectra is the frequency of the υ asymmetric (υas) and υ symmetric (υs) of carbonyl (COO−) stretching vibrations and the difference between them Δυ(COO−). The frequency of these bands depends upon the coordination mode of the carboxylate ligand. Monodentate complexes exhibit Δυ(COO−) values that are much greater than the ionic complexes. Chelating (bidentate) complexes exhibit Δυ(COO−) values that are significantly less than the ionic values. Δυ(COO−) values for bridging complexes are greater than those of chelating complexes, and close to the ionic values [49]. In complex 1; υas(COO−) is at 1601 cm−1 and υs(COO−) at 1397 cm−1, Δυ(COO−) = 204 cm−1 which is close to that of potassium sulindac which supports a coordination mode for complex 1 as monodentate. The O–H vibration frequency at 3376 cm−1 indicates the presence of water molecules in the coordination geometry [Co(H2O)4(sul)2] as also supported by single crystal X-ray determination.
The assignments of IR frequencies for the asymmetric stretching υas(COO−), the symmetric stretching υs(COO−) and the difference between these two values of sulindac group in complexes 1–4 and those of potassium sulindac are shown in Additional file 1: Tables S2 and S3.
Complexes 2 and 3 have υas(COO−) at 1599, and 1600 cm−1, but υs(COO−) appear at 1390 and 1380 cm−1, so Δυ (COO−) are 219 and 220 cm−1, respectively which is larger than Δυ(COO−)K(sul) = 178 cm−1 and this supports monodentate coordination mode of the carboxylate groups. In addition, complex 3 has an absorption frequency at 3415 cm−1 which may indicate water molecules in the coordination geometry.
Moreover, in complex 2 two absorption frequencies υas(NH2) at 3374 cm−1 and υs(NH2) at 3268 cm−1 with Δυ(NH2) = 106 cm−1 were observed. These frequencies are assigned to the 1°-NH2 group indicating that the complexation with cobalt is through the pyridine nitrogen atom rather than the NH2 nitrogen atom [50, 51].
In complex 4 υas(COO−) was observed at 1599 cm−1, and υs(COO−) was at 1441 cm−1 giving a Δυ(COO−) of 158 cm−1 and this supports a bidentate coordination mode of the carboxylate groups. This result was also confirmed by X-ray structure determination of complex 4.
UV–Vis spectra
Generally, three types of electronic transitions have been observed for coordination compounds: Metal to ligand (MLCT) or ligand to metal (LMCT) charge-transfer absorption bands, d–d transition bands and intra-ligand (LC) transition bands [52, 53].
Co(II) metal ion with low spin d 7 electronic configuration showed two low intensity bands with small ε value (12–13 Lmol−1 cm−1) in the visible region. The source of these two bands is due to the d–d transition between 2E2→T1g and 2E→2T2g. LMCT was observed at (206–213 nm) with ε values between 1800 and 3000 Lmol−1 cm−1 [20, 21, 54,55,56,57,58,59,60,61,62,63,64,65,66,67]. All other bands are similar to nitrogen based ligand Π→Π* or n→Π* transitions with small blue or red shifts for cobalt coordination complexes [20, 21, 55,56,57,58,59,60,61,62,63,64,65,66,67]. The results are tabulated in Additional file 4: Table S4. UV-visible spectral data for compounds (1–4).
Complexes 3 and 4 adopted distorted octahedral geometries with different carboxylate coordination modes, e.g. monodentate, bidentate, in complex 3 the two water molecules were covalently coordinated to the central Co(II) cation which imposed monodentate coordination mode of the sulindaco groups. Whereas, the two sulindaco groups in complex 4 are both bidentately coordinated to the Co(II) center as a result of the increased steric hindrance effect by two methyl groups on the 1,10-phen ring. The electronic effect of the ligands in complexes 2–4 are almost identical.
Magnetic properties
The magnetic moment measurements of compounds 1–4 are given in Table 2. The value of magnetic moments for all complexes indicates that each compound has paramagnetic properties with one unpaired electron, which indicates that each Co(II) complex adopted a low spin, d 7 octhedral geometry. Low spin Co(II) octahedral complexes with nitrogen and/or oxygen-donor ligands are very rare [62]. Both structural, magnetic and spectral data are necessary to prove that a complex contains low spin Co(II) metal ion octahedral geometry with only few of these compounds have been structurally characterized by single crystal X-ray crystallography [68,69,70,71].
Anti-bacterial activity
Before measurement of their biological activity, the solution stability of the complexes were tested, as the complexes were crystallized by slow solvent evaporation at room temperature that took several days and the same physical properties of the compounds were obtained. Moreover, the relevant X-ray structure determination of some complexes showed that the structures were remained intact.
Two Gram positive bacteria (Staphylococcus epidermidis, Staphylococcus aureus), two Gram negative bacteria (Bordetella, E. coli) and yeast species (Saccharomyces and Candida) were used to test the compounds anti-bacterial activity. The results were obtained by the well-diffusion method using DMSO as a negative control to resist any tested microorganisms; Gentamycin as a positive control for Gram positive and Gram negative bacteria and Nystatin as a positive control for yeast. The parent ligand, potassium sulindac, did not show anti-bacterial activity against any of the tested microorganisms, but (CoCl2) showed anti-bacterial activity against all tested microorganisms (Table 3).
Complex 1 showed high activity against G− or G+ bacteria except against E. coli. Complexes 3 and 4 showed low activity against G− bacteria and high activity against G+ bacteria. Complex 2 showed high activity against S. epidermidis and low or zero activity against other bacteria. However, in yeast all complexes didn’t show any activity except complexes 4 showed high activity. Complexes 3 and 4 were chosen for further studies because of their higher IZD values. The complexes have been studied with their parent nitrogen donor ligands “1,10-phen and 2,9-dimephen” against all tested Gram-positive, Gram-negative bacteria and yeast to determine the effect of the complexation on anti-bacteria activity (Tables 4, 5).
Tables 4 and 5 show that the complexation process of cobalt-sulindac with 1,10-phen in complex 3 decreased the anti-bacterial activity considerably for both gram negative and gram positive bacteria, but complexation of cobalt-sulindac with 2,9-dimephen in complex 4 mostly showed similar behavior against S. epidermidis and yeast, but decreased the activity against S. aureus and increased the anti-bacterial activity against gram negative bacteria. The anti-bacterial activity of complexes 1–4 when compared with previously reported work would be considered as promising results [15, 28,29,30,31,32,33,34,35,36, 72,73,74,75,76,77,78].
Conclusion
Four new Co(II) complexes with sulindac in the presence of N-donor heterocyclic ligands (2-ampy, 1,10-phen and 2,9-dimephen) have been synthesized and characterized. Magnetic properties, infrared and UV–Vis spectrophotometric techniques were used to study the new complexes in addition to X-ray diffraction of complexes 1 and 4; which reveals distorted octahedral geometry of the Co(II) ion. In complex 1 the cobalt binds two monodentate sulindac groups and in complex 4 cobalt binds two bidentate sulindac groups and one 2,9-dimephen. The structures of the remaining complexes were proposed depending on IR, UV–Vis results and magnetic properties. Complexes 3 and 4 showed anti-bacterial activity against G+ and G− bacteria. Moreover, complex 4 have demonstrated the highest efficiency against yeast.
The results of this work was Submitted in Partial Fulfillment of the Requirements for the Degree of Masters in Applied Chemistry, Faculty of Graduate Studies, Birzeit University, Ramallah, Palestine. The thesis was published in 2015 on FADA Birzeit University Open Access Repository [79].
Experimental
Starting materials
Cobalt(II) chloride was purchased from Merck, sulindac, 2-aminopyridine, 1,10-phenanthroline and 2,9-dimethyl-1,10-phenanthroline were purchased from Sigma-Aldrich. All solvents used were of analytical reagent grade and purchased from commercial sources. E. coli, S. aureus, S. epidermidis, Bordetella and Yeast species (Saccharomyces and candida) were kindly obtained from the Drugs Department at Central Public Health Laboratory.
Synthesis
All Co(II) complexes were synthesized at room temperature in ambient conditions.
Synthesis of [Co(H 2 O) 4 (sul) 2 ] (1)
Sulindac (3.0 g, 8.4 mmol) was allowed to dissolve in a methanolic solution of potassium hydroxide (0.47 g, 4.2 mmol) (75 ml methanol). To this solution was added slowly CoCl2·7H2O (1.0 g, 4.2 mmol) in 15 ml of methanol. The mixture was allowed to stir for 24 h and the formed precipitate was collected, washed with cold water and air dried. Suitable crystals for X-ray structural analysis were obtained by recrystallization from hot methanol.
[Co(H 2 O) 4 (sul) 2 ] (1): 85% (3.81 g) yield; m.p. 201 °C; IR (cm−1, KBr): 3376, 3050, 2911, 2850, 1600, 1563, 1485, 1465, 1416, 1369, 1326,1268, 1217, 1203, 1171, 1133, 1086, 1024, 1008, 967, 918, 891, 891, 868, 805, 776, 717, 672, 659, 572, 473; UV–Vis [DMSO, λ (nm)(є/Lmol−1 cm−1)]: 211 (3283), 252 (828), 258 (872), 264 (850), 282 (771), 328 (514); μeff = 2.26 BM.
Synthesis of [Co(2-ampy) 2 (Sul) 2 ] (2)
Sulindac (3.0 g, 8.4 mmol) was allowed to dissolve in a methanolic solution of potassium hydroxide (0.47 g, 4.2 mmol) (40 ml methanol). To this solution was added slowly CoCl2·7H2O (1.0 g, 4.2 mmol) in 10 ml of methanol, then 2-ampy (0.79 g, 8.4 mmol) dissolved in 15 ml of methanol was added. The mixture was allowed to stir for 24 h, the solvent was evaporated then the residue was dissolved in dichloromethane which was then evaporated and the compound obtained was washed with petroleum ether and dried under vacuum.
[Co(2-ampy) 2 (Sul) 2 ] (2): 56% (2.50 g) yield; m.p. 180 °C (decomposed); IR (cm−1, KBr): 3374, 3268, 3015, 2914, 2860, 1599, 1515, 1494, 1464, 1424, 1380, 1267, 1195, 1164, 1137, 1086, 1031, 1010, 955, 915, 891, 846, 811, 727, 651, 593, 533, 474, 449; UV–Vis [DMSO, λ (nm); (є/Lmol−1 cm−1)]: 207 (1828), 286 (450), 329 (348), 655 (12.7); μeff = 2.41 BM.
Synthesis of [Co(H 2 O) 2 (1,10-phen)(sul) 2 ] (3)
Sulindac (3.0 g, 8.4 mmol) was allowed to dissolve in a methanolic solution of potassium hydroxide (0.47 g, 4.2 mmol) (40 ml methanol). To this solution was added slowly CoCl2·7H2O (1.0 g, 4.2 mmol) in 10 ml of methanol, then 1,10-phenanthroline (0.756 g, 4.2 mmol) dissolved in 15 ml of methanol was added. The mixture was allowed to stir for 24 h, the solvent was evaporated then the residue was dissolved in dichloromethane which was then evaporated and the compound obtained was washed with petroleum ether and dried under vacuum.
[Co(H 2 O) 2 (1,10-phen)(sul) 2 ] (3): 22% (1.0 g) yield; m.p. 140 °C; IR (cm−1, KBr): 3415, 3059, 2911, 2852, 1600, 1515, 1464, 1424, 1380, 1267, 1195, 1164, 1137, 1086, 1010, 956, 915, 891, 846, 811, 727, 651, 593, 533, 474, 441; UV–Vis [DMSO, λ (nm) (є/Lmol−1 cm−1)]: 208 (2152), 226 (700), 271 (535), 328 (224), 431 (16.3), 488 (13.2); μeff = 2.4 BM.
Synthesis of [Co(2,9-dimephen)(sul) 2 ] (4)
Sulindac (3.0 g, 8.4 mmol) was allowed to dissolve in a methanolic solution of potassium hydroxide (0.47 g, 4.2 mmol) (40 ml methanol). To this solution was added slowly CoCl2·7H2O (1.0 g, 4.2 mmol) in 10 ml of methanol, then 2,9-dimethyl-1,10-phenanthroline (0.875 g, 4.2 mmol) dissolved in 15 ml of methanol was added. The mixture was allowed to stir for 24 h, the solvent was evaporated then the residue was dissolved in dichloromethane which was then evaporated and the compound obtained was washed with petroleum ether and dried. Suitable crystals for X-ray structural analysis were obtained by recrystallization from 1:1 mixture of chloroform/acetonitrile.
[Co(2,9-dimephen)(sul) 2 ] (4): 34% (1.54 g) yield; m.p. 150 °C (decomposed); IR (cm−1, KBr): 3040, 2912, 2845, 1599, 1566, 1465, 1441, 1359, 1194, 1157, 1135, 1086, 1031, 954, 916, 891, 855, 812, 761, 728, 644, 533, 474; UV–Vis [DMSO, λ (nm) (є/Lmol−1 cm−1)]: 207 (2263), 229 (933), 274 (621), 328 (261), 432 (13.3); μeff = 2.4 BM.
Physical measurements
Infrared (IR) spectra were recorded in the 450–4000 cm−1 region (KBr) on a Perkin Elmer FT-IR spectrometer (2004). UV–Vis spectra were recorded using Hewlett Packard 8453 photo diode array spectrophotometer in the 200–800 nm region using DMSO as solvent. Melting points were determined in capillary tubes with B-545 melt apparatus without any correction. The magnetic susceptibility measurements were determined by Gouy method using mercury cobalt-thiocyanate complex, (HgCo(NSC)4) as standard. Calculation of the effective magnetic moment was obtained by using the following: μeff = 2.83 * (χmT)1/2 (Molar susceptibility, χm, and T is the temperature with K).
X-ray crystallography
X-ray intensity data of complexes 1 and 4 was carried out at room temperature on a Bruker SMART APEX CCD X-ray diffractometer system (graphite-monochromated Mo Kα radiation λ = 0.71073 Å) by using the SMART software package [80]. The data were reduced and integrated by the SAINT program package [81]. The structure was solved and refined by the SHELXTL software package [82]. H atoms were located geometrically and treated with a riding model. The R-factor above 10% reflects the low quality of crystals obtained in the process of recrystallization and better crystals could not been found. Crystal data and details of the data collection and refinement are summarized in Table 6 and in Additional file 5: Supplementary crystallographic data for complexes 1 and 4.
Anti-bacterial activity
Agar diffusion method [83] was used for screening the anti-bacterial activity measurements of the synthesized cobalt complexes. Different types of gram-negative bacteria (Bordetella, E. coli) and gram-positive (S. epidermidis, S. aureus) and Yeast species (Saccharomyces and Candida) were used in the present work.
In sterile saline single bacterial colonies were dissolved until the suspended cells reached the turbidity of McFarland 0.5 Standard. The bacterial inocula were spread on the surface of the Muller Hinton nutrient agar by means of a sterile cotton swab. Sterile glassy borer were used to make a 6 mm in diameter wells in the agar plate. Samples were dissolved in DMSO in concentration equal to (8 mg/ml), (4 mg/ml) and (2 mg/ml), then 50 μl of the test samples were introduced in the respective wells. DMSO was used as negative control while gentamycin used as positive control. Immediately the plate was incubated at 37 °C for 24 h. The anti-bacterial activity was determined by measuring the diameter inhibition zone of complete growth in millimeter (mm). The averages of two trials determined the results and are stated as average ± standard deviation.
References
Zhang KL, Lin JG, Wang YQ, Xu WL, Chen JT (2004) Aquabis (2-nitrobenzoato-κO)(1, 10-phenanthroline-κ2N, N′) zinc (II). Acta Crystallogr Sect C 60:m454–m456
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley, New York, pp 817–819
Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, Regtop HL, Davies NM (2002) Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord Chem Rev 232:95
Ott I, Kircher B, Gust R (2004) Investigations on the effects of cobalt-alkyne complexes on leukemia and lymphoma cells: cytotoxicity and cellular uptake. J Inorg Biochem 98:485–489
Yesilel OZ, Mutlu A, Darcan C, Buyukgungor O (2010) Syntheses, structural characterization and antimicrobial activities of novel cobalt-pyrazine-2, 3-dicarboxylate complexes with N-donor ligands. J Mol Struct 964:39–46
Lopez-Sandoval H, Londono-Lemos ME, Garza-Velasco R, Poblano-Melendez I, Granada-Macias P, Gracia-Mora I, Barba-Behrens N (2008) Synthesis, structure and biological activities of cobalt (II) and zinc (II) coordination compounds with 2-benzimidazole derivatives. J Inorg Biochem 102:1267–1276
Lv J, Liu T, Cai S, Wang X, Liu L, Wang Y (2006) Synthesis, structure and biological activity of cobalt (II) and copper (II) complexes of valine-derived schiff bases. J Inorg Biochem 100:1888–1896
Gust R, Ott I, Posselt D, Sommer K (2004) Development of cobalt (3, 4-diarylsalen) complexes as tumor therapeutics. J Med Chem 47:5837–5846
Dimiza F, Papadopoulos AN, Tangoulis V, Psycharis V, Raptopoulou CP, Kessissoglou DP, Psomas G (2010) Biological evaluation of non-steroidal anti-inflammatory drugs-cobalt (II) complexes. Dalton Trans 39:4517–4528
Miodragovic DU, Bogdanovic GA, Miodragovic ZM, Radulovic MD, Novakovic SB, Kaluderovic GN, Kozlowski H (2006) Interesting coordination abilities of antiulcer drug famotidine and antimicrobial activity of drug and its cobalt (III) complex. J Inorg Biochem 100:1568–1574
Nomiya K, Yoshizawa A, Tsukagoshi K, Kasuga NC, Hirakawa S, Watanabe J (2004) aluminium (III) and cobalt (II) complexes with 4-isopropyltropolone (hinokitiol) showing noteworthy biological activities. Action of silver (I)-oxygen bonding complexes on the antimicrobial activities. J Inorg Biochem 98:46–60
Rodriguez-Argüelles MC, Mosquera-Vazquez S, Sanmartin-Matalobos J, Garcia-Deibe AM, Pelizzi C, Zani F (2010) Polyhedron 29:864–866
Matsumoto K, Yamamoto S, Yoshikawa Y, Doe M, Kojima Y, Sakurai H, Hashimoto H, Kajiwara MN (2005) Antidiabetic activity of Zn (II) complexes with a derivative of L-glutamine. Bull Chem Soc Jpn 78:1077–1081
Dorkov P, Pantcheva IN, Sheldrick WS, Mayer-Figge H, Petrova R, Mitewa M (2008) Synthesis, structure and antimicrobial activity of manganese (II) and cobalt (II) complexes of the polyether ionophore antibiotic Sodium Monensin A. J Inorg Biochem 102:26–32
Abu Ali H, Darawsheh MD, Rappocciolo E (2013) Synthesis, crystal structure, spectroscopic and biological properties of mixed ligand complexes of zinc (II) valproate with 1, 10-phenanthroline and 2-aminomethylpyridine. Polyhedron 61:235–241
Szunyogova E, Gyoryova K, Hudecova D, Piknova L, Chomic J, Vargova Z, Zelenak V (2007) Thermal, spectral and biological properties of Zn (II) complex compounds with phenazone. J Therm Anal Calorim 88:219–223
Rodriguez-Arguelles MC, Mosquera-Vazquez S, Sanmartin-Matalobos J, Garcia-Deibe AM, Pelizzi C, Zani F (2010). Polyhedron: 867–870
Fountoulaki S, Perdih F, Turel I, Kessissoglou DP, Psomas G (2011) Non-steroidal anti-inflammatory drug diflunisal interacting with Cu (II). Structure and biological features. J Inorg Biochem. 105:1645–1655
Hwu JR, Tsay SC, Chuang KS, Kapoor M, Lin JY, Yeh CS, Su WC, Wu PC, Tsai TL, Wang PW, Shieh DB (2016) Syntheses of platinum–sulindac complexes and their nanoparticles as targeted anticancer drugs. Chem-A Eur J. 22:1926–1930
Dimiza F, Papadopoulos A, Tangoulis V, Raptopoulou C, Kessissglou D, Psomas G (2012) Biological evaluation of cobalt (II) complexes with non-steroidal anti-inflammatory drug naproxen. J Inorg Biochem 107:54–64
Tsiliou S, Kefala L, Perdih F, Turel I, Kessissoglou D, Psomas G (2012) Cobalt (II) complexes with non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological evaluation. Eur J Med Chem 48:132–142
Tsiliou S, Kefala Hatzidimitriou A, Kessissoglou D, Perdih F, Papadopoulos A, Turel I, Psomas G (2015) J Inorg Biochem: 1–15
Psomas G, Kessissoglou D (2013) Dalton Trans: 1–52
Patil A, Donde K, Raut S, Patil V, Lokhande R (2012) J Chem Pharm Res 4:1413–1425
Kovala-Demertzi D (2000) J Inorg Biochem 79:153–157
Krstic NS, Nikolic RS, Stankovic MN, Nikolic NG, Dordevic DM (2015) Coordination compounds of M (II) biometal Ions with acid-type anti-inflammatory drugs as ligands—a review. Trop J Pharm Res 14:337–349
Konstandinidou M, Kourounakis A, Yiangou M, Hadjipetrou L, Kovala-Demertzi D, Hadjikakou S, Demertzis M (1998) Anti-inflammatory properties of diclofenac transition metalloelement complexes. J Inorg Biochem 70:63–69
Abu Ali H, Fares H, Darawsheh M, Rappocciolo E, Akkawi M, Jaber S (2015) Synthesis, characterization and biological activity of new mixed ligand complexes of Zn (II) naproxen with nitrogen based ligands. Eur J Med Chem 89:67–76
Darawsheh M, Abu Ali H, Abuhijleh AL, Rappocciolo E, Akkawi M, Jaber S, Maloul S, Hussein Y (2014) New mixed ligand zinc (II) complexes based on the antiepileptic drug sodium valproate and bioactive nitrogen-donor ligands. Synthesis, structure and biological properties. Eur J Med Chem 82:152–163
Abu Ali H, Jabali B (2016) Polyhedron 107:97–106
Jabali B, Abu Ali H (2016) Non-steroidal Anti-Inflammatory Drug (indomethacin) and various nitrogen donor ligands. Synthesis, characterization and biological activity. Polyhedron 117:249–258
Abu Ali H, Omar S, Darawsheh M, Fares H (2016) Synthesis, characterization and antimicrobial activity of zinc (II) ibuprofen complexes with nitrogen-based ligands. J Coord Chem 69:1110–1122
Abu Ali H, Maloul S, Abu Ali I, Akkawi M, Jaber S (2016) Dichloro-bis-(pyridine-2-yl-undecyl-amine) zinc (II),[ZnCl2 (C16N2H26) 2]: Synthesis, characterization and antimalarial activity. J Coord Chem 69:2514–2522
Abu Ali H, Shalash A, Akawi M, Jaber S (2017) Synthesis, characterization and in vitro biological activity of new zinc (II) complexes of the nonsteroidal anti-inflammatory drug sulindac and nitrogen-donor ligands. Appl Organomet Chem. doi:10.1002/aoc.3772
Abu Ali H, Kamel S, Abu Shamma A (2017) Novel structures of Zn (II) biometal cation with the biologically active substituted acetic acid and nitrogen donor ligands: Synthesis, spectral, phosphate diester catalytic hydrolysis and anti-microbial studies. Appl Organomet Chem. doi:10.1002/aoc.3829
Abu Ali H, Abu Shamma A, Kamel S (2017) New mixed ligand cobalt (II/III) complexes based on the drug sodium valproate and bioactive nitrogen-donor ligands. Synthesis, structure and biological properties. J Mol Struct. doi:10.1016/j.molstruc.2017.04.048
http://en.wikipedia.org/wiki/Sulindac. Accessed 20 Jan 2015
www.wiley-vch.de/books/sample/3527331476_c01.pdf. Accessed 15 Mar 2015
Viossat V, Lemoine P, Dayan E, Dung N, Viossat B (2005) Synthesis, crystal structures and IR spectra of isotypic pseudopolymorphs complexes of Zn (II) by indole-2-carboxylic acid and 2, 9-dimethyl-1, 10-phenanthroline with different solvates (DMA, DMF or DMSO). J Mol Struct 741:45–52
Kadhiravan S, Sivajiganesan S (2015) J Appl Chem 8:73–84
Waizump K, Takuno M, Fukushima N, Masuda H (1998) Structures of pyridine carboxylate complexes of cobalt (II) and copper (II). J Coord Chem 44:269–279
Liu Z, Chen Y, Liu P, Wang J, Huang M (2005) Cadmium (II) and cobalt (II) complexes generated from benzimidazole-5-carboxylate: self-assembly by hydrogen bonding and π–π interactions. J Solid State Chem 178:2306–2312
Bu XH, Tong ML, Xie YB, Li JR, Chang HC, Kitagawa S, Ribas J (2005) Synthesis, structures, and magnetic properties of the copper (II), cobalt (II), and manganese (II) complexes with 9-acridinecarboxylate and 4-quinolinecarboxylate ligands. Inorg Chem 44:9837–9846
Rettig SJ, Thompson RC, Trotter J, Xia S (1999) rystal structure and magnetic properties of polybis (formamide) bis (μ-formato) cobalt (II): an extended two-dimensional square lattice material which exhibits spontaneous magnetization below 9 K. Inorg Chem 38:1360–1363
Greiner BA, Marshall NM, Narducci Sarjeant AA, McLauchlan CC (2007) Imidazole-based nickel (II) and cobalt (II) coordination complexes for potential use as models for histidine containing metalloproteins. Inorg Chim Acta 360:3132–3140
Singh UP, Aggarwal V, Sharma AK (2007) Mononuclear cobalt (II) carboxylate complexes Synthesis molecular structure and selective oxygenation study. Inorg Chim Acta 360:3226–3232
Khandar AA, Shaabani B, Belaj F, Bakhtiari A (2007) Synthesis, characterization, electrochemical and spectroscopic investigation of cobalt (III) Schiff base complexes with axial amine ligands: The layered crystal structure of [Co III (salophen)(4-picoline) 2] ClO 4· CH 2 Cl 2. Inorg Chim Acta 360:3255–3264
Lai CS, Tiekink ERT (2003) Appl Organomet Chem 17:255–256
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds, 6th edn. Wiley, Hoboken
Badshah KD (2011) Synthesis and characterization of zinc complexes with N- and O- donor ligands. AIOU, Islamabad
Zeleňák V, Vargová Z, Györyová K (2007) Correlation of infrared spectra of zinc (II) carboxylates with their structures. Spectrochim Acta, Part A 66:262–272
Zhang X, Yi ZH, Xue M, Xu Y, Yu JH, Yu XY, Xu JQ (2007) Chem Res Chin Univ 23:631–634
Yu HL, Yang J, Fu Q, Ma JC, Li WL (2008) Chem Res Chin Univ 24:123
Hasanvanda F, Hoseinzadeh A, Zolgharnein J, Amania S (2010) J Coord Chem 63:346–352
Ahmadia RA, Hasanvanda F, Brunob G, Rudbarib HA, Amania S (2013) Russ J Coord Chem 39:867–871
Komaei SA, Albada G, Reedijk AV (1999) J Trans Met Chem 24:104–107
Rodríguez L, Labisbal E, Sousa-Pedrares A, García-Vázquez JA, Romero J, Durán ML, Real A, Sousa A (2006) Coordination chemistry of amine bis (phenolate) cobalt (II), nickel (II), and copper (II) complexes. Inorg Chem 45:7903–7914
Shaker SA, Farina Y, Mahmmod S, Eskender M (2009) Co (II), Ni (II), Cu (II), Zn (II) and Cd (II) mixed ligand complexes of theophylline and cyanate: synthesis and spectroscopic characterization. Mod Appl Sci 3:88–93
Sunita Devi O, Manihar Singh AK (2011) J Chem Pharm Res 3:1055–1060
Al-Nahary TT (2009) Synthesis and characterization of metal complexes of Cr (III), Mn (II), Fe (III), Co (II), Ni (II), Cu (II), Ru (III), Rh (III) and Pd (II) with derivatives of 1, 3, 4-thiadiazole-2, 5-dithiol as new ligands. J Saudi Chem Soc 13:253–257
Al-Nahary TT (2007) ISESCO J Sci Technol Vis 3:16–22
Faus J, Julve M, Lloret F, Muiioz MC (1993) Bis (dimethylviolurato)(phenanthroline) cobalt (II), a low-spin octahedral cobalt (II) complex. Crystal structure of [Co (dmvi) 2phen]. 2CHCl3. Inorg Chem 32:2013–2017
Çukurovali A, Yilmaz I, Özmen H, Ahmedzade M (2002) Cobalt (II), copper (II), nickel (II) and zinc (II) complexes of two novel Schiff base ligands and their antimicrobial activity. Trans Met Chem 27:171–176
Pal S, Sengupta P, Ghosh S, Mukherjee G, Mostafa G (2002) Cobalt (III) and Low Spin Cobalt (II) Complexes of the Two Highly Flexible Hexadentate Ligands 1, 3-di (o-salicylaldiminophenylthio) propane and 1, 2-di (o-salicylaldiminophenylthio) xylene. J Coord Chem 55:271–280
Hitchman MA (1977) Electronic structure of low-spin cobalt (II) Schiff base complexes. Inorg Chem 16:1985–1993
Hartman JR, Hintsa EJ, Cooper SR (1986) J Am Chem Soc 108:1202–1208
Marques LF, Marinho MV, Speziali NL, Visentin LC, Machado FC (2011) Inorg Chim Acta 365:454–457
Bertrand JA, Carpenter DA, Kalyanaraman AR (1971) The structure of K2BaCo (NO2) 6 at 233° K.: a static Jahn-Teller distortion. Inorg Chim Acta 5:113–114
Hartman JR, Hintsa EJ, Cooper SR (1984) J Chem Soc Chem Commun: 287–386
Setzer WN, Ogle CA, Wilson GS, Glass RS (1983) Inorg Chem 22:266–271
Wilson GS, Swanson DD, Glass RS (1986) Inorg Chem 25:3827
Dimiza F, Perdih F, Tangoulis V, Turel I, Kessissoglou DP, Psomas G (2012) Eur J Med Chem 48:132–142
Tsiliou S, Kefala LA, Perdih F, Turel I, Kessissoglou DP, Psomas G (2013) Dalton Trans 42:6252–6276
Psomas G, Kessissoglou DP (2002) J Enzyme Inhib Med Chem 17:87–91
Chohan ZH, Iqbal MS, Iqbal HS, Scozzafava A, Supuran CT (2012) Eur J Med Chem 48:132–142
Tsiliou S, Kefala LA, Perdih F, Turel I, Kessissoglou DP, Psomas G (2012) J Inorg Biochem 107:54–64
Geraghtya M, Sheridana V, McCanna M, Devereuxb M, McKeec V (1999) Polyhedron 18:2931–2939
Podunavac-Kuzmanovic S, Vojinovic L, Cvetkovic D (2003) ISIRR
Shalash A, Abu Ali H (2015) Non-steroidal Zn(II) and Co(II) sulindac drugs and bioactive Nitrogen-donor ligands: synthesis, characterization, anti-bacterial effect, anti-malarial effect and the use as phosphate hydrolyzing enzymes, Master Thesis, Birzeit University
SMART-NT V5.6, B. A. G. (2002) Karlsruhe
SAINTL-NT V5.0, B. A. G. (2002) Karlsruhe
SHELXTL-NT V6.1, B. A. G. (2002) Karlsruhe
Rahman A, Choudhary MI, Thomsen WJ (2001) Bioassay techniques for drug development. Harwood Academic, Amsterdam
Authors’ contributions
Both authors read and approved the final manuscript.
Acknowledgements
The authors thank the office of Vice President for Academic Affairs at Birzeit University for their financial support.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
13065_2017_268_MOESM5_ESM.docx
Additional file 5: CCDC 1450310 and CCDC 1450311 contain the supplementary crystallographic data for complexes 1 and 4. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk. Supplementary data associated with this article can be found, in the online version.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shalash, A.M., Abu Ali, H.I. Synthesis, crystallographic, spectroscopic studies and biological activity of new cobalt(II) complexes with bioactive mixed sulindac and nitrogen-donor ligands. Chemistry Central Journal 11, 40 (2017). https://doi.org/10.1186/s13065-017-0268-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-017-0268-2