Abstract
Glomerular filtration rate (GFR) is usually determined by estimation of iothalamate (IOT) clearance. We have developed and validated an accurate and robust method for the analysis of IOT in human plasma and urine. The mobile phase consisted of methanol and 50 mM sodium phosphate (10:90; v/v). Flow rate was 1.2 mL/min on a C18 reverse phase column, Synergi-hydro (250 × 4.6 mm) 4 µm 80 Å, with an ultraviolet detector set to 254 nm. Acetonitrile was used for the deproteination and extraction of IOT from human plasma and urine. Precision and accuracy were within 15% for IOT in both plasma and urine. The recoveries of IOT in urine and plasma ranged between 93.14% and 114.74 and 96.04–118.38%, respectively. The linear range for urine and plasma assays were 25–1500 and 1–150 µg/mL respectively. The lower limits of detection were 0.5 µg/mL for both urine and plasma, with no interference from plasma and urine matices. This method has been fully validated according to FDA guidelines and the new HPLC assay has been applied to a new formulation of IOT (Conray™ 43), to calculate GFR in healthy volunteers. The new method is simple, less expensive and it would be instrumental in future clinical and pharmacokinetic studies of iothalamate in kidney patients.
Similar content being viewed by others
Background
Renal function is best studied with accurate measurement of glomerular filtration rate (GFR) in pre-clinical studies, clinical practice and clinical trials [1–3]. Inulin [4, 5] and creatinine [6, 7] clearances are accepted reference standards for determining GFR but are expensive and laborious. Colorimetric assays are used to detect Inulin, which is very expensive and also in very short supply and many problems with the assay [8]. 24-h creatinine clearance overestimates GFR in patients with poor renal function and a variety of other glomerular diseases and drugs can make creatinine clearance and serum creatinine a poor markers of GFR estimation [9, 10]. GFR estimated from serum creatinine using prediction equations may result in unpredictable error when the diagnosis of chronic kidney disease is unknown [1–3, 6, 7, 11–23]. Cystatin C, a low molecular weight protein that is produced by nucleated cells at a constant rate and is then filtered by glomeruli and reabsorbed and catabolized by tubular epithelial cells, has been suggested as an alternative for creatinine [24–26]. It seems to be affected by factors other than renal function alone [27–33]. Usually, iothalamate (IOT) clearance [2, 3, 15, 20, 22, 34, 35] are used to measure GFR. Mostly, radioactive compounds are also used to perform IOT clearances [36–38], which have radioactive health and safety issues. That’s why we have used non-radioactive IOT for analysis.
Recently, several high-performance liquid chromatography (HPLC) techniques have been published for the analysis of IOT in serum and urine, but are not without problems [8, 39–46]. Initial methods for sample preparation consisted of several time-consuming extraction and evaporation steps. Also, the sensitivity of some of the previously reported assays is not great, for example, an HPLC–UV method published by Farthing et al. had a linear range for the detection of IOT in plasma from 10 to 50 µg/mL [40]. Later developed procedures reduced sample preparation to a single precipitation step or an ultrafiltration step prior to analysis. LC–MS methods are also used [36, 47] along with capillary electrophoresis [34], both of which are more expensive and require specialized training and expertise to operate.
Herein we report on a method using standard C18 columns, which allow estimation of IOT in a single chromatographic run along with a simple and inexpensive sample preparation method using acetonitrile precipitation as compared to ultrafiltration as done in the past. We don’t require a very expensive instruments to carry out such analysis. Our extraction procedure does not require extensive sample extraction procedures, shows no peak deterioration with high injection volume. The sample preparation method is a much improved version of existing methods [8, 41, 43, 48], notably because the present work describes a more sensitive and simple assay for IOT in a relatively large number of patients, who are given a new formulation of IOT (Conray 43™). Furthermore, IOT concentrations were determined in both, plasma as well as urine samples to calculate GFR, and lastly, we report a fully validated assay according to FDA guidelines.
Our aim was to develop an inexpensive, robust and reliable HPLC method for analysis of IOT in human plasma and urine along with comparing two sample preparation methods (ACN precipitation method compared to ultrafiltration) using two internal standards allopurinol and BHET.
Experimental
Materials
Iothalamic acid (P/N USP34500-2) was bought from Promochem, Welwyn Garden City, Herts, UK. Internal Standards Allopurinol (P/N H-9006), Hydroxyethyl-Theophylline (BHET) (P/N H-9006) were purchased from Sigma, Chemical Co, Poole, Dorset, UK, as was Sodium phosphate (Monobasic-ACS reagent grade). HPLC grade acetonitrile, methanol and water were purchased from Hichrom Ltd, Berkshire, UK. Drug free control serum was purchased from Charter House Clinical Research Unit, Slough, UK.
Micro-centrifuge tubes 1.5 mL, Kimble Amber auto-sampler vials, glass inserts, caps and 12 × 75 mm test tubes were all bought from Fisher Scientific Loughborough, UK. Other instruments used were heater block with nitrogen cannula to evaporate samples, platform mixer or vortex to mix samples.
Apparatus and chromatographic conditions
Hewlett Packard model 1100 high performance liquid chromatography (HPLC) system consisted of HP 1100 series degasser, quaternary pump, auto-injector, column heater and a UV detector. The system was controlled through Chemstation software, version B.04.02 loaded on a HP (Vectra Vm) series computer with a printer. Mobile phase consisted of Methanol: 50 mM Sodium Phosphate (10:90) (unadjusted about PH 4.67). Flow rate was 1.2 mL/min, column used was Synergi-4µ-hydro-RP-80A (250 × 4.6 mm) with a guard column Symmetry Shield RP-18 (15 mm × 4.5 mm, 5 µm) with an ultraviolet Detector set at 254 nm, column temperature was set to 30 °C, injection volume was 10 µL, run time for the run was 15 min. All analyses were performed at room temperature.
Solution preparation
Stock solutions
Preparation of stock iothalamic acid solutions 10 mg/mL. Solutions were prepared in duplicate one for calibration standards and one for quality control. 100 mg of iothalamic acid was weighed out and placed in a 10 mL volumetric flask. Then dissolved in 10 mL methanol and stored in the refrigerator (0–5 °C) (Final conc. = 10 mg/mL). 10 mg of Allopurinol was weighed out and placed in a 10 mL volumetric flask. It was dissolved in 10 mL of methanol and stored in the refrigerator (0–5 °C) (Final conc. = 1.0 mg/mL). β-Hydroxyethyl-Theophylline (BHET), stock internal standard (IS) solution was prepared having a concentration of 1.0 mg/mL. 10 mg of BHET was weighed out and placed in a 10 mL volumetric flask. It was dissolved in a 10 mL of methanol. The solutions were stored in the refrigerator at 0–5 °C (Final conc. = 1.0 mg/mL) and these solution was marked as IS-Stock.
Working solutions, calibrants and quality controls
Working internal standard solution was prepared by diluting 1.0 mL of IS Stock solution in a 10 mL volumetric flask and diluted to 10 mL with acetonitrile (final conc. 0.1 mg/mL Allopurinol). Working iothalamic standard solutions were prepared by dissolving 1 mL of stock iothalamic (IOT) solution (conc. = 10 mg/mL) in a 10 mL volumetric and diluted with 9 mL of methanol. Iothalamic acid stock solutions were stored in 15 mL polypropylene screw cap test-tube and stored at 0–5 °C (refrigerated). Calibrants were prepared by serial dilutions and then the volumes were pipetted into clean, labelled 1.5 mL micro-centrifuge tubes, and evaporated to dryness and reconstituted with 100 µL of control plasma or urine to obtain the desired concentrations. Quality control solutions were prepared in the concentration of 1, 2, 20 and 120 µg/mL for plasma and 25, 40, 200 and 1200 for urine. Calibration curve were made in plasma in the range 1–150 µg/mL and in urine in the range 25–1500 µg/mL.
Phosphate buffer
50 mM monobasic sodium phosphate was prepared by weighing out 6 g of sodium phosphate (Monobasic) and dissolving in 1000 mL of HPLC water, and stored at room temperature.
Sample preparation and extraction
For plasma samples, working standards and controls were evaporated under a stream of nitrogen and 100 µL of control plasma was added for calibrants and quality control samples preparation, the samples were mixed vigorously for 5–10 s using a platform (vortex) mixer. 50 μL of working internal standard solution of allopurinol was added to each sample, standard and quality control. Next 300 μL of acetonitrile was added to each sample, standard and quality control. All samples were mixed vigorously and centrifuged at 1200×g for 10 min at room temperature (18–25 °C). The supernatant were transferred into clean, labelled 12 × 75 mm tubes and evaporated to dryness under a gentle stream of nitrogen. The dried residue was reconstituted with 100 µL of mobile phase and transferred to a glass insert inside a 2 mL standard HPLC auto injector vial. These were vortex mixed and centrifuged for 5 min at 1200×g and 10 µL of the supernatant injected onto the HPLC system.
For urine samples, working standards and quality controls were evaporated under a stream of nitrogen and reconstituted in 100 μL of tenfold diluted blank urine. These were mixed vigorously for 5–10 s using a platform (vortex) mixer. This urine solution was then placed in a 1.5 mL micro-centrifuge tube and 50 μL mobile phase containing the internal standard (100 μg/mL Allopurinol) was added to each sample, standard and quality controls. These are mixed vigorously and transferred to a glass insert inside a Kimble vial and 10 μL was injected onto the HPLC system.
Calculations
Peak areas/heights for each peak were obtained from the computer data capture system. The standard curves were generated by weighted (1/y2) linear regression of peak area/height ratios of IOT to allopurinol versus supplemented urine concentrations. Quantitation of unknown samples were estimated by applying the linear regression equation of the standard curve to the unknown sample’s peak area/height ratio.
Validation
All frozen samples (control and test serum samples) were thawed for approximately half an hour at room temperature, vortexed before processing and transferred to appropriately labelled tubes. The usual analytical run consisted of two control blank matrix samples, calibrators S1-S8, quality control samples (minimum two sets of quality control low -QCL, quality control medium—QCM and quality control high–QCH per run), one set run after blanks and calibrators and then after approximately every 15 test samples. Mobile phase was injected between the analytical control samples and the blanks and also after the calibrators to minimise carryover. Each analytical run was prepared and assayed within a 28-h period.
A quality control was rejected on the standard curve estimations if deviated 15% or more, or in case of limit of quantitation (LOQ), quality controls deviating 20% or more was rejected. Any sample was discarded if a peak of interest had obvious chromatographic interference. A sample, standard, or quality control sample was rejected if the internal standard (peak height/area/injected volume) was one standard deviation from the normal.
Precision/accuracy and LOQ
The accuracy and precision of the method was determined by assaying 1 mL aliquots of blank human plasma and urine was fortified with four quality control (QC) samples of concentrations 1, 2, 20 and 120 µg/mL for plasma and 25, 40, 200 and 1200 µg/mL for urine. To assess the inter-assay precision and accuracy, samples were analysed on five separate days. To assess the intra-assay precision, these same QC concentrations were analysed during 1 day. Precision and accuracy are reported as percent coefficient of variation and percent accuracy [(observed − expected) × 100/expected concentration], respectively.
The results of QC sample analysis provide the basis for accepting or rejection the run. Variability of the quality control samples was assessed from the validation procedure. For each individual QC, the acceptance criterion was not more than a 15% deviation from the nominal value for accuracy. LOQ was defined as the lowest concentration of an analyte in a sample that was determined with acceptable precision and accuracy under the stated operational conditions of the method. An acceptable range for LOQ was nominal value ±20%.
Specificity
The responses of interfering peaks at the retention time of the analyte were less than 20% of the response of an LOQ standard. Responses of interfering peaks at the retention time of the internal standard were ≤5% of the response of the concentration of the internal standard to be used in studies which demonstrated the specificity of the validated analytical procedure.
Linearity
The ratio of UV area response for the peak corresponding to iothalamic acid in the HPLC chromatogram to the UV area response of the internal standard against the nominal concentrations of iothalamic acid represents the linearity. Applying linear regression to the calibration data obtained from seven calibration standards gives the equation of the linear line. A calibration point was rejected as an outlier if the back-calculated concentration for a calibrator (on the basis of the corresponding calibration curve) deviated more than 15% at all concentrations covered by the calibration range except at LOQ where the deviation of 20% was acceptable. A calibration curve was accepted with a minimum of 6 out 8 acceptable calibration levels.
Recovery
The recovery of IOT from urine and plasma was determined by calculating the ratio of slopes of IOT standard curves against the slope of the same standards prepared in distilled deionized water.
Stability
Human plasma at concentration of QCL-2, QCM-20 and QCH-120 µg/mL and urine samples at concentrations QCL-40, QCM-200 and QCH-1200 were subjected to 3 freeze and thaw cycles. The results obtained after each freeze and thaw cycle was expressed as a percentage change from the results for QCL-40, QCM-200 and QCH-1200 in the intra-assay run (validation run-1, these samples were prepared fresh and had not experienced any freezing conditions). The test compound was considered to be stable if the percentage change from freshly prepared samples was within ±15% of the nominally spiked level.
GFR calculations
An effective way of assessing how well the kidneys are working is to calculate the glomerular filtration rate (GFR). GFR is a measurement of how many millilitres (mL) of waste fluid the kidneys can filter from the blood in a minute (measured in mL/min). A healthy pair of kidneys should be able to filter more than 90 mL/min. The result is called the estimated GFR or eGFR.
The IOT clearance was calculated by the formula
Here glomerular filtration rate was calculated by multiplying urine flow rate with concentration of IOT in urine divided by IOT concentration in plasma. The GFR was recorded in units of volume per time i.e.; mL/min. The following table shows the different stages of renal function [49]. The urine flow was measured using uroflowmetry device, Flow-Med™ 1100 and measuring flow of urine over time.
Subjects
Twenty healthy young volunteers were recruited for the study. Each volunteer received a 1.5 mL bolus injection of Conray™ 43 containing 645 mg of iothalamate meglumine (Malinkrodt Plc, Ireland) and a 5-mL sterile normal saline flush. To estimate GFR over 150 min period blood and urine samples were collected. Blood and urine samples were first collected after 60 min and then samples were collected every 30 min for three consecutive time interval. All samples were stored at −20 °C until analysed (within 1 month). Kingston University research ethics committee approved the study protocol and the volunteers provided informed written consent to participate in the study. This study was conducted according to the principles of the Declaration of Helsinki [50].
Results and discussion
Chromatography
A representative chromatogram of IOT and internal standard, are shown in Fig. 1. A representative chromatogram, showing an overlay of blank human serum and urine along with a representative chromatogram of urine and plasma, after injecting IOT in a human subject, is shown in Fig. 1. The chromatographic conditions were adapted from a previously published report [26]. The mobile phase elution of 10% methanol and 90% 50 mM sodium phosphate in water gave maximum separation. Both IOT and the internal standard showed sharp, well-defined peaks at retention times of 6.9 and 10.4 min, respectively.
Intra-day and inter-day precision and accuracy
The accuracy and precision of the method was determined by assaying 100 μL aliquots of human plasma fortified with 1, 2, 20 and 120 μg/mL of iothalamic acid (representing quality controls: LOQ, QCL, QCM and QCH respectively) and 25, 40, 200 and 1200 concentrations for human urine. These fortified samples were assayed by HPLC–UV. Intra-assay accuracy and precision for iothalamic acid in urine were calculated from results obtained from 6 replicate analyses of quality controls each at 4 concentrations (1, 2, 20 and 120 μg/mL of iothalamic acid in human plasma, and 25, 40, 200 and 1200 concentrations for human urine). Different stanges of renal function are given in Table 1 below.
The intra and inter assay results show 1 μg/mL to be the limit of quantitation level for iothalamic acid in human plasma and 25 μg/mL for urine, using developed analytical method. These are summarised below in Table 2.
Specificity
Chromatogram showing plasma and urine samples from six subjects without any interference from endogenous compounds in the retention time regions of the internal standard and iothalamic acid.
Linearity
The plots were linear over the concentration range 1–150 μg/mL IOT in human plasma and the fit statistically tested by slope, intercept and correlation coefficient (r2) and linear regression with 1/y*y fit was found to be suitable. For urine the plots were linear over the concentration range 25–1500 μg/mL. None of the intercepts were significantly different from zero.
Recovery of calibration standards
Determination of recovery of iothalamic acid from human plasma and urine was determined by taking the UV area ratio response of iothalamic acid to internal standard in the extracted sample divided by the area ratio response determined in an un-extracted sample and multiplied by 100 gave the percent recovery.
Stability of iothalamic acid in plasma to repeated freezing and thawing cycles
The QCL-2 µg/mL samples gave a mean result of 1.97, 1.87 and 2.37 μg/mL (n = 6) with the corresponding percentage change from freshly prepared samples of 12.44, 12.59 and −5.19% for freezing and thawing cycles 1,2 and 3 respectively. The QCM-20 samples gave a mean result of 21.37, 20.73 and 20.20 μg/mL (n = 6) with the corresponding percentage change from freshly prepared samples of −6.08, −2.87 and −0.26% for freezing and thawing cycles 1, 2 and 3 respectively. The QCH-120 samples gave a mean result of 122.12, 121.99 and 121.71 μg/mL (n = 6) with the corresponding percentage change from freshly prepared samples from time t = 0 to −1.89, −1.49 and −1.54% for freezing and thawing cycles 1, 2 and 3 respectively. The data indicates that iothalamic acid was stable in plasma to at least 3 freezing and thawing cycles.
Stability of iothalamic acid in urine to repeated freeze/thaw cycles
The QCL-40 samples gave a mean result of 37.63, 40.19 and 36.07 μg/mL with the corresponding percentage change from freshly prepared samples of +6.31, −13.54 and +1.88% for freeze and thaw cycles 1, 2 and 3 respectively. The QCM-200 samples gave a mean result of 208.34, 211.68 and 213.08 μg/mL with the corresponding percentage change from freshly prepared samples of −9.38, +7.93 and −7.90% for freeze and thaw cycles 1, 2 and 3 respectively. The QCH-1200 samples gave a mean result of 1144.84, 1192.05 and 1227.22 μg/mL with the corresponding percentage change from freshly prepared samples of −13.11, +9.53 and −7.37% for freeze and thaw cycles 1, 2 and 3 respectively. The data indicates that iothalamic acid was stable in urine to at least 3 freeze and thaw cycles.
Applications in humans
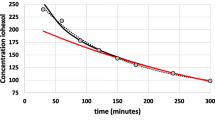
Plasma IOT concentration was plotted against time after a single injection (1.5-mL Conray™ 43) as shown in Fig. 2. 0 point on the graph shows the time before the bolus injection was administered. Since the first sample collection and measurement was carried out at 60 min, it is quite likely that the peak concentration might have spiked at a much earlier time point than 60 min. However, the main aim of this part of the study was to measure GFR rates. Figure 2 shows that on the average the IOT reaches its peak plasma concentration in an hours’ time and then it starts to decline. The concentration of IOT in plasma ranged between 4.32 and 16.36 µg/mL in 20 healthy volunteers.
Figure 3 shows how the urine IOT concentration changed over time after a single injection (1.5-mL Conray™ 43). The figure also shows how the average concentration of IOT clearance reaches its maximum in an hour time and then it starts to decline. The concentration of IOT in urine ranged between 76.92 and 311.32 µg/mL in 20 healthy volunteers.
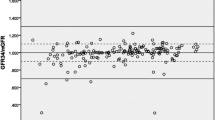
Figure 4 shows the GFR value of 20 volunteers in mL/min against time. GFR was calculated by dividing (concentration of urine excreted × flow of urine) by concentration of plasma for each subject. The 5 columns under each subject represent the five time points of sample collection (0, 60, 90, 120 and 150). Subjects 1, 2, 3, 4, 5, 6, 7, 9, 15, 16, 18, 19 and 20 shows that the glomerular filtration rate was optimum at around 90 min, and subjects 10, 11, 12, 13 and 14 show a high renal clearance at 60 min, while 17 shows maximum GFR from 90 to 120 min. Moreover, subject 8 shows maximum GFR before IOT injection at 0 min.
Figure 5 shows a comparative concentration–time profile of 20 volunteers, where blood and urine was collected at 60, 90, 120 and 150 min from subjects after a single intravenous bolus injection of 1.5-mL Conray™ 43. The figure describe how the GFR varies between subjects at different time intervals.
In conclusion, we report on a new, simple and sensitive method for the measurement of IOT, which was then validated and used to estimate GFR. The current method employed a new internal standard, allopurinol (after trying BHET as well) and had the added advantage of only a single step extraction from plasma and urine. The method was further validated in healthy volunteers and showed that GFR was rapidly estimated with minimal blood and urine sampling. GFR determination by IOT clearance is safe in diabetic patients with mild to moderate renal impairment [51]. Furthermore, single plasma sampling 4 h after injection of IOT was safely and accurately undertaken to determine GFR. This enables the technique to be used more frequently in clinical practice as it reduces the cost of GFR determination, and allows the procedure to be undertaken easily in an outpatient setting.
References
Lamb EJ, Brettell EA, Cockwell P, Dalton N, Deeks JJ, Harris K, Higgins T, Kalra PA, Khunti K, Loud F (2014) The eGFR-C study: accuracy of glomerular filtration rate (GFR) estimation using creatinine and cystatin C and albuminuria for monitoring disease progression in patients with stage 3 chronic kidney disease-prospective longitudinal study in a multiethnic population. BMC Nephrol 15:13
Gaspari F, Ruggenenti P, Porrini E, Motterlini N, Cannata A, Carrara F, Sosa AJ, Cella C, Ferrari S, Stucchi N (2013) The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 84:164–173
Francoz C, Nadim MK, Baron A, Prié D, Antoine C, Belghiti J, Valla D, Moreau R, Durand F (2014) Glomerular filtration rate equations for liver-kidney transplantation in cirrhotic patients: validation of current recommendations. Hepatology 59:1514–1521
Baccard N, Hoizey G, Frances C, Lamiable D, Trenque T, Millart H (1999) Simultaneous determination of inulin and p-aminohippuric acid (PAH) in human plasma and urine by high-performance liquid chromatography. Analyst 124:833–836
Maher F, Nolan, N, Elveback L (1971) Comparison of simultaneous clearances of 125-I-labeled sodium lothalamate (Glofil) and of inulin. In: Mayo Clinic proceedings
Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST (2013) Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 83:1169–1176
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254
Agarwal R, Vasavada N, Chase SD (2003) Liquid chromatography for iothalamate in biological samples. J Chromatogr B 785:345–352
Branten AJ, Vervoort G, Wetzels JF (2005) Serum creatinine is a poor marker of GFR in nephrotic syndrome. Nephrol Dial Transplant 20:707–711
Mussap M, Dalla Vestra M, Fioretto P, Saller A, Varagnolo M, Nosadini R, Plebani M (2002) Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int 61:1453–1461
Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G (2004) Clinical practice guidelines for chronic kidney disease in adults: part II. Glomerular filtration rate, proteinuria, and other markers. Am Fam Phys 70:1091–1097
Al K, YT A, JC J (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67:2089–2100
Lin J, Knight EL, Hogan ML, Singh AK (2003) A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. JASN 14:2573–2580
Tsai C-W, Grams ME, Inker LA, Coresh J, Selvin E (2013) Cystatin C-and creatinine-based estimated glomerular filtration rate, vascular disease, and mortality in persons with diabetes in the United States. Diabetes Care 37:1002–1008
Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, Larson TS (2004) Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 43:112–119
Fontseré N, Salinas I, Bonal J, Bayés B, Riba J, Torres F, Rios J, Sanmartí A, Romero R (2006) Are prediction equations for glomerular filtration rate useful for the long-term monitoring of type 2 diabetic patients? Nephrol Dial Transplant 21:2152–2158
Padmasree D, Kumar MA, Ukey Ujwala U (2013) Glomerular filtration rate in healthy indian adults by the modification of diet in renal disease equation. IJRTSAT 8:143–145
Valente MA, Hillege HL, Navis G, Voors AA, Dunselman PH, van Veldhuisen DJ, Damman K (2013) The chronic kidney disease epidemiology collaboration equation outperforms the modification of diet in renal disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail 16:86–94
Kilbride HS, Stevens PE, Eaglestone G, Knight S, Carter JL, Delaney MP, Farmer CK, Irving J, O’Riordan SE, Dalton RN (2013) Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 61:57–66
Blake GM, Sibley-Allen C, Hilton R, Burnapp L, Moghul MR, Goldsmith D (2013) Glomerular filtration rate in prospective living kidney donors. Int Urol Nephrol 45:1445–1452
Chin P, Florkowski C, Begg E (2013) The performances of the Cockcroft-Gault, modification of diet in renal disease study and chronic kidney disease epidemiology collaboration equations in predicting gentamicin clearance. Ann Clin Biochem 50:546–557
Bragadottir G, Redfors B, Ricksten S-E (2013) Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury-true GFR versus urinary creatinine clearance and estimating equations. Crit Care 17:R108
Willems JM, Vlasveld T, den Elzen WP, Westendorp RG, Rabelink TJ, de Craen AJ, Blauw GJ (2013) Performance of Cockcroft-Gault, MDRD, and CKD-EPI in estimating prevalence of renal function and predicting survival in the oldest old. BMC Geriatr 13:113
Whelly S, Serobian G, Borchardt C, Powell J, Johnson S, Hakansson K, Lindstrom V, Abrahamson M, Grubb A, Cornwall GA (2014) Fertility defects in mice expressing the L68Q variant of human cystatin C: a role for amyloid in male infertility. J Biol Chem 289:515–759
Abrahamson M, Olafsson I, Palsdottir A, Ulvsback M, Lundwall A, Jensson O, Grubb A (1990) Structure and expression of the human cystatin C gene. Biochem J 268:287–294
Dönmez O, Korkmaz HA, Yildiz N, Ediz B (2015) Comparison of serum cystatin C and creatinine levels in determining glomerular filtration rate in children with stage I to III chronic renal disease. Ren Fail 37:784–790
Vega A, de Vinuesa SG, Goicoechea M, Verdalles Ú, Martínez-Pueyo ML, Chacón A, Quiroga B, Luño J (2014) Evaluation of methods based on creatinine and cystatin C to estimate glomerular filtration rate in chronic kidney disease. Int Urol Nephrol 46:1161–1167
Nasseri-Moghaddam S, Tofangchiha S, Ganji M (2013) Serum cystatin-C is not superior to serum creatinine in predicting glomerular filtration rate in cirrhotic patients. MEJDD 5:209–216
Shima T, Khatun A, Yeasmin F, Ferdousi S, Kirtania K, Sultana N (2013) Cystatin C: a better predictor of kidney function in diabetic patients. BSMB 4:16–20
Nehus EJ, Laskin BL, Kathman TI, Bissler JJ (2013) Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol 28:453–461
Ristiniemi N, Savage C, Bruun L, Pettersson K, Lilja H, Christensson A (2012) Evaluation of a new immunoassay for cystatin C, based on a double monoclonal principle, in men with normal and impaired renal function. Nephrol Dial Transplant 27:682–687
Bansal N, Vittinghoff E, Peralta CA, Shlipak MG, Grubbs V, Jacobs DR, Siscovick D, Steffes M, Carr JJ, Bibbins-Domingo K (2013) Estimated kidney function based on serum cystatin C and risk of subsequent coronary artery calcium in young and middle-aged adults with preserved kidney function: results from the CARDIA study. Am J Epidemiol 178:410–417
Isakova T, Craven TE, Scialla JJ, Nickolas TL, Schnall A, Barzilay J, Schwartz AV (2015) Change in estimated glomerular filtration rate and fracture risk in the action to control cardiovascular risk in diabetes trial. Bone 78:23–27
Wilson DM, Bergert JH, Larson TS, Liedtke RR (1997) GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis 30:646–652
Barbour GL, Crumb CK, Boyd CM, Reeves RD, Rastogi SP, Patterson RM (1976) Comparison of inulin, iothalamate, and 99mTc-DTPA for measurement of glomerular filtration rate. J Nucl Med 17:317–320
Rhea JM, Ritchie JC, Molinaro RJ (2013) Development of a liquid chromatography tandem mass spectrometry method for iothalamate measurement to assess renal function for potential kidney donation. Clin Chim Act 420:104–108
Smith T, Veall N, Altman D (1981) Dosimetry of renal radiopharmaceuticals: the importance of bladder radioactivity and a simple aid for its estimation. Br J Radiol 54:961–965
Israelit A, Long D, White M, Hull A (1973) Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate. Kidney Int 4:346–349
Hagan AS, Jones DR, Agarwal R (2013) Use of dried plasma spots for the quantification of iothalamate in clinical studies. Clin J Am Soc Nephrol 8:909–914
Farthing D, Sica DA, Fakhry I, Larus T, Ghosh S, Farthing C, Vranian M, Gehr T (2005) Simple HPLC–UV method for determination of iohexol, iothalamate, p-aminohippuric acid and n-acetyl-p-aminohippuric acid in human plasma and urine with ERPF, GFR and ERPF/GFR ratio determination using colorimetric analysis. J Chromatogr B 826:267–272
Kos T, Moser P, Yilmatz N, Mayer G, Pacher R (2000) High-performance liquid chromatographic determination of p-aminohippuric acid and iothalamate in human serum and urine: comparison of two sample preparation methods. J Chromatogr B Biomed Sci Appl 740:81–85
Agarwal R (1998) Chromatographic estimation of iothalamate and p-aminohippuric acid to measure glomerular filtration rate and effective renal plasma flow in humans. J Chromatogr B Biomed Sci Appl 705:3–9
Bell RR, Bombardt PA, DuCharme DW, Kolaja GJ, Packwood WH, Bothwell BE, Satoh PS (1994) A non-radioactive iothalamate and p-aminohippuric acid high-performance liquid chromatographic method for simultaneously measuring glomerular filtration rate and renal blood flow in the rat. Biomed Chromatogr 8:224–229
Seneviratne AK, Jayewardene AL, Gambertoglio JG (1994) Paired ion reversed-phase HPLC assay for the determination of iothalamic acid and para aminohippuric acid in urine. J Pharm Biomed Anal 12:1311–1316
Gaspari F, Mainardi L, Ruggenenti P, Remuzzi G (1991) High-performance liquid chromatographic determination of iothalamic acid in human plasma and urine. J Chromatogr B Biomed Sci Appl 570:435–440
Reidenberg M, Lorenzo B, Drayer D, Kluger J, Nestor T, Regnier J, Kowal B, Bekersky I (1987) A nonradioactive iothalamate method for measuring glomerular filtration rate and its use to study the renal handling of cibenzoline. Ther Drug Monit 10:434–437
Seegmiller JC, Burns BE, Fauq AH, Mukhtar N, Lieske JC, Larson TS (2010) Iothalamate quantification by tandem mass spectrometry to measure glomerular filtration rate. Clin Chem 56:568–574
Prueksaritanont T, Chen M-L, Chiou WL (1984) Simple and micro high-performance liquid chromatographic method for simultaneous determination of p-aminohippuric acid and iothalamate in biological fluids. J Chromatogr B Biomed Sci Appl 306:89–97
NHS. Kidney disease, chronic—diagnosis; NHS choices your health, your choices 2012. http://www.nhs.uk/Conditions/Kidney-disease-chronic/Pages/Diagnosis.aspx
World MAGA (2004) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Int Bioethique 15:124
Group, DER (2011) Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011:2366–2376
Authors’ contributions
IS, JB, SJB and DPN initiated the study. All authors contributed to the study design, interpretation of the results and preparation of the manuscript. The method development, validation and sample analyses were conducted by IS with contributions from JB, SSA. SJB and DPN also contributed by recruitment and in the blood sample collection. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shah, I., Barker, J., Naughton, D.P. et al. HPLC estimation of iothalamate to measure glomerular filtration rate in humans. Chemistry Central Journal 10, 80 (2016). https://doi.org/10.1186/s13065-016-0227-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-016-0227-3