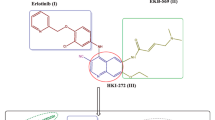
Abstract
Background
Quinoline derivatives have diverse biological activities including anticancer activity. On the other hand, many sulfonamide derivatives exhibited good cytotoxic activity. Hybrids of both moieties may present novel anticancer agents.
Results
Chloroquinoline incorporating a biologically active benzene-sulfonamide moieties 5–21 and diarylsulfone derivatives 22 and 23 were prepared using (E)-1-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-(dimethyl-amino)prop-2-en-1-one 4 as strategic starting material. The structure of the newly synthesized compounds were confirmed by elemental analyses and spectral data. Compound 4 was confirmed by X-ray crystallographic analysis. The prepared compounds were evaluated for their anticancer activity against Lung, HeLa, Colorectal and breast cancer cell lines. Compounds 2, 4, 7, 11, 14 and 17 showed better or comparable activity to 2′, 7′-dichlorofluorescein (DCF) as reference drug. Molecular docking of the active compounds on the active site of PI3K enzyme was performed in order to explore the binding mode of the newly synthesized compounds.
Conclusion
Compounds 2, 4, 7, 11, 14 and 17 are novel quinoline derivatives that may represent good candidates for further evaluations as anticancer agents. The mechanism of action of these compounds could be through inhibition of PI3K enzyme.

Compound 17 on the active site of PI3K
Similar content being viewed by others
Background
Quinoline scaffold has been broadly distributed in sundry natural and synthetic compounds with multipurpose biological activities [1–3]. The antitumor activity of the quinoline derivatives for instance camptothecin [4], luotonin [5], ascididemin [6], TAS-103 A that displayed IC50 value of: 0.0030–0.23 microM hostile to various cell lines [7], CIL-102 B that unveiled IC50 value of: 0.31–2.69 microM hostile to countless cell lines [8], cryptolepin [9] and indolo[2,3-b]quinolines [10] has been described. Numerous mechanisms of action were optional for such action among them was the strong suppression of E2F1 that inhibits growth by thwarting cell cycle progression and fasters differentiation by creating a permissive environment for cell distinction [11]. Chloroquinolines were valuable in sundry cancer sorts remarkably, breast cancer with high aptitude to induce apoptosis [12]. Heterocyclic sulfonamides have publicized good anticancer bustle with diversity of mechanisms embracing cell cycle perturbation at G1 phase, disruption of microtubules assembly and the eminent carbonic anhydrase inhibition activity with selectivity to the tumor allied isoforms hCA IX and hCA XII [13–17]. Merging quinoline scaffold with the biologically active benzene-sulfonamide moiety has received immense attention as PI3K inhibitor which is an vital enzyme regulatory signal transduction [16, 18–20]. Freshly, diaryl sulfones that were prepared from Dapson have shown respectable cytotoxic activity on breast cancer cell line [21]. Based on the aforementioned and as a continuation for our effort to synthesize a novel anticancer agents [18–25], we have prepared novel quinolone-sulfonamide and diarylsulfone derivatives. Prepared compounds were subjected to cytotoxic assay on lung, hela, colorectal and breast cancer cell lines. Likewise, “the highest active compounds were docked on the active site of PI3K enzyme” to recommend their binding mode in a trial to explore their mechanism of action expecting to reach innovative anticancer agents. 

Results and discussion
Chemistry
The ambition of this effort was to prepare a new series of chloroquinolines carrying biologically active benzene-sulfonamide moieties and to assess their anticancer activity. Thus, interaction of 2 [26] with dimethylformamide-dimethylacetal (DMF-DMA) in dry xylene yielded the unexpected 4 instead of expected 3. “The structural assignments to synthesized compounds were based on their physico-chemical characteristics and spectroscopic (FT-IR, 1H-NMR, 13C-NMR, and mass spectroscopy) investigations”. Structure of 4 was confirmed by X-ray crystallographic analysis [27] (Figs. 1, 2). IR of 4 revealed the disappearance of NH band and presence of absorption bands for (aromatic), (aliphatic), (CO), (CN), (CCl). 1H-NMR showed the presence of a singlet at 2.4 ppm attributed to N-(CH3)2, singlet at 3.4 ppm assigned to N-CH3, two doublet at 5.4, 6.5 ppm for CH = CH of quinolone ring, two doublet at 6.1,7.4 ppm assigned to CH = CH group. Enaminones are highly reactive intermediates extensively used for the preparation of heterocyclic derivatives. Thus, treatment of 4-(7-chloro-1-methylquinolin-4-(1H)-ylideneamino) phenyl-3-(dimethyl-amino)-prop-2-en-1-one 4 with sulfonamide derivatives in refluxing ethanol/acetic acid mixture (2:1) afforded the sulfonamide derivatives 5–21 (Scheme 1). “Structures of the latter products were assigned on the basis of their analytical and spectral data”. 1H NMR of 5–21 support the assumption that these structures were in E-form and not in Z form, while the coupling constant of doublet signals for olefinic protons was equal to 6.1–7.7 Hz. IR of the reaction products showed in each case three absorption bands for 2NH functions in the 3446–3143 cm−1 region, in addition to carbonyl functions 1654–1635 cm−1 region and CCl functions 883–763 cm−1 (Scheme 1). 1H-NMR of 5 showed singlet at 12.0 ppm assigned to NH group, while 13C NMR revealed singlet at 189.3 ppm for CO group. 1H-NMR of 6 exhibited singlet at 2.0 ppm according to COCH3 group.1H-NMR of 7 revealed singlet at 9.4 ppm for NH group. 1H-NMR of 8 showed singlet at 2.3 ppm for CH3 group, while 1H NMR of 9 exhibited two signals at 1.9, 2.6 assigned to 2CH3 groups. 1H NMR of 10 revealed two signals at 10.2, 12.0 ppm assigned to NH, SO2NH groups. 1H-NMR of 11 exhibited two signals at 6.6, 6.8 ppm for CH = CH of thiazole ring. 1H-NMR of 12 exhibited singlet at 2.4 ppm for CH3 of thiadiazole ring. 13C NMR of 13 showed signal at 186.6 ppm due to CO group. 1H-NMR of 15 exhibited singlet at 2.3 ppm for CH3 of pyrimidine ring. 1H-NMR of 16 revealed singlet at 2.2 ppm for 2CH3 of pyrimidine ring. 1H-NMR of compound 17 exhibited singlet at 3.9 ppm for OCH3 group. 1H-NMR of 18 showed singlet at 3.7 ppm assigned to 2OCH3 groups, while 1H NMR of 19 exhibited two signals at 3.6, 3.8 ppm attributed to 2OCH3 groups. 1H NMR of 20 revealed singlet at 12.0 according to NH group of indazole ring. 13C-NMR of 21 showed singlet at 186.7 ppm for CO group. Interaction of 4 with Dapson in molar ratio (1:1 mol) afforded the mono compound 22, while the bis-compound 23 was achieved in the same condition but in molar ratio (2:1 mol). Compounds 22 and 23 were confirmed by microanalyses, IR, 1H-NMR, 13C-NMR and mass spectral data. IR of 22 revealed the characteristic bands at 3446, 3348, 3213 cm−1 (NH2, NH), 1635 cm−1 (CO), 1591 cm−1 (CN), 1369, 1180 cm−1 (SO2), 821 cm−1 (CCl). 1H-NMR of 22 exhibited signals at 3.4 ppm corresponding to N-CH3 group, 5.9 ppm due to NH2 group, two doublet at 6.1, 7.4 ppm for 2 CH quinoline, two doublet at 6.5, 6.6 ppm assigned to CH = CH groups, singlet at 12.0 NH. 13C-NMR of 22 showed singlet at 186.6 ppm attributed to (CO) group. Mass of 22 revealed a molecular ion peak m/z at 569 [M+] (19.87) with a base peak appeared at 90 (100). IR of 23 showed a characteristic bands at 3143 cm−1 (2NH), 1635 cm−1 (2CO), 1570 cm−1 (2CN), 1375, 1180 cm−1 (SO2), 819 cm−1 (2CCl). 1H-NMR of 23 revealed signals at 3.4 ppm for N-CH3, two doublets at 6.2, 7.3 ppm due to 4CH quinoline, two doublets at 6.6, 7.2 assigned to 2CH = CH, two singlet’s at 9.3, 12.0 for 2NH groups. 13C-NMR of 23 revealed singlet at 186.7 ppm for (2CO) groups. Mass of 23 showed a molecular ion peak m/z at 889 [M+] (6.48) with a base peak appeared at 272 (100) (Scheme 2).
In vitro cytotoxic screening
The newly synthesized compounds were evaluated for their in vitro cytotoxic activity against human lung (A549-Raw), hela, colorectal (lovo) and breast (MDA-MB231) cancer cell lines and 2′,7′-dichlorofluorescein (DCF) was used as the reference drug in this study. The relationship between surviving fraction and drug concentration was plotted to obtain the survival curve of cancer cell lines. The response parameter calculated was the IC50 value, which corresponds to the concentration required for 50 % inhibition of cell viability. Table 1 shows the in vitro cytotoxic activity of the newly synthesized compounds. In a closer look to Table 1, we can see that compounds 1, 2, 4, 7, 11, 14 and 17 were active towards all the tested cell line while the rest of compounds were inactive. Regarding the activity towards lung cancer cell line (A549-Raw), all the aforementioned compounds were more active than DCF as reference drug. Compound 2 was the most active compound with IC50 value of 44.34 μg/ml. For Hela cancer cell line, the same compounds were active. Compounds 7 and 17 were more active than DCF and compound 17 was the most active compound with IC50 value of 30.92 μg/ml. In case of lovo cancer cell line, all seven compounds were more active than DCF. Compound 2 was the most active compound with IC50 value of 28.82 μg/ml. Finally, the activity towards breast cancer cell line (MDA-MB231) was better than that of DCF for the aforementioned compounds except for compound 14. Compound 17 again was the most active compound with IC50 value of 26.54 μg/ml. In the light of biological results, we can see that the 4,7-dichloroquinoline 1 showed moderate anticancer activity that were enhanced upon converting it to 1-(4-(7-chloloquinoline-4-ylamino) phenyl)ethanone 2. The activity still exists upon preparation of (E)-1-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino) phenyl)-3-(dimethylamino) prop-2-en-1-one 4. Further preparation of the sulfonamide derivatives 5–21 using various sulfa drugs only succeeded to obtain active derivatives with the guanidine derivative 7, the thiazole derivative 11, the pyrimidine derivative 14 and the 5-methoxypyrimidine derivative 17. Combination with diaryl sulfone moieties as in compounds 22 and 23 did not yield active compounds.
Molecular docking
Phosphoinositide 3-kinases (PI3K) comprises an important class of enzymes that phosphorylates the 3 hydroxyl group of inisitol and play a major role in signal transduction through the cell cycle. Targeting PI3K by inhibitors has become a well-known strategy in seeking for new anticancer agents [28]. Quinolinesulfonamide derivatives were reported to express good inhibitory activity on PI3K enzyme [16]. In our present investigation and in a trial to suggest the mechanism of action of the active compounds, molecular docking of compounds 1, 2, 4, 7, 11, 14 and 17 was performed on the active site of PI3K to explore their binding modes to amino acids of the active site of the enzyme. The protein data bank file (PDB: 3S2A) was selected for this purpose. The file contains PI3K enzyme co-crystallized with a quinoline ligand. All docking procedures were achieved by MOE (Molecular Operating Environment) software 10.2008 provided by chemical computing group, Canada. Docking on the active site of PI3K enzyme was performed for all synthesized compounds. Docking protocol was verified by redocking of the cocrystallized ligand in the vicinity of the active site of the enzyme with energy score (S) = −29.8249 kcal/mol and root mean standard deviation (RMSD) = 1.9094 (Fig. 3). The quinoline ligand interacts with the active site of PI3K by six interactions: Val 882 with a hydrogen bond of 2.90 Å, Tyr 867 with a hydrogen bond of 3.33 Å, Asp 864 with a hydrogen bond of 3.33 Å, Lys 833 with a hydrogen bond of 3.33 Å, Ser 806 with a hydrogen bond of 3.74 Å and Asp 841 with a hydrogen bond of 2.79 Å through a water molecule. All the docked compounds were fit in the active site of enzyme. Energy scores (S) as well as amino acids interactions were listed in Table 2. The best docking score was achieved by compound 17 with a value = −27.1666 kcal/mol. Compound 17 interacted with Val 822 with a hydrogen bond of 3.20 Å, with Asp 964 with a hydrogen bond of 2.48 Å, with Ser 806 with a hydrogen bond of 3.38 Å and finally with His 984 with a hydrogen bond of 2.70 Å (Figs. 4, 5).
Experimental
Chemistry
Melting points (uncorrected) were determined in open capillary on a Gallen Kamp melting point apparatus (Sanyo Gallen Kamp, UK). Precoated silica gel plates (Kieselgel 0.25 mm, 60 F254, Merck, Germany) were used for thin layer chromatography. A developing solvent system of chloroform/methanol (8:2) was used and the spots were detected by ultraviolet light. IR spectra (KBr disc) were recorded using an FT-IR spectrophotometer (Perkin Elmer, USA). 1H-NMR spectra were scanned on an NMR spectrophotometer (Bruker AXS Inc., Switzerland), operating at 500 MHz for 1H- and 125.76 MHz for 13C. Chemical shifts are expressed in δ-values (ppm) relative to TMS as an internal standard, using DMSO-d 6 as a solvent. Elemental analyses were done on a model 2400 CHNSO analyser (Perkin Elmer, USA). All the values were within ±0.4 % of the theoretical values. All reagents used were of AR grads.
(E)-1-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-(dimethylam-ino)prop-2-en-1-one (4)
1-(4-(7-chloroquinoline-4-ylamino)phenyl)ethanone 2 (2.97 g, 0.01 mol) and dimethylformamide-dimethylacetal (1.19 g, 0.01 mol) was added into dry xylene (30 mL). Reaction was refluxed for 10 h, and the solid product recrystallized from ethanol to give 4.
Yield, 89 %; m.p.268.1 °C. IR: 3100 (arom.), 2966, 2856 (aliph.), 1696 (CO), 1618 (CN), 776 (CCl).). 1HNMR: 2.4 [s, 3H, N(CH3)2], 3.6 [s, 1H, N-CH3], 5.4, 6.5 [2d, 2H, CH = CH quinoline, J = 7.1, 7.3 Hz], 6.1,7.4 [2d, 2H, CH = CH, J = 7.5, 7.4 Hz], 6.9–7.6 [m, 3H, Ar–H]. 13CNMR: 36.3, 44.5 (2), 91.5, 114.6, 115.3, 116.9, 121.4 (2), 131.7, 132.8 (2), 133.0, 135.9, 136.6, 141.4, 146.2, 152.5, 161.4, 166.4, 191.3. MS m/z (%): 365 (M+) (2.84), 74 (100). Anal.Calcd. For C21H20ClN3O (365.86): C, 68.94; H, 5.51; N, 11.49. Found: C, 68.66; H, 5.22; N, 11.74.
Synthesis of sulfonamide derivatives 5–21
4-(7-chloro-1-methylquinolin-4-(1H)-ylideneamino) phenyl-3-(dimethylamino)-prop-2-en-1-one 4 (3.65 g, 0.01 mol) and sulfa-drugs (0.012 mol) was added into ethanol (10 mL) and acetic acid (5 mL). The mixture was refluxed for 18 h. The solid product formed was recrystallized from dioxane to give 5–21.
4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino) phenyl)-3-oxoprop-1-en-ylamino)benzenesulfonamide (5)
Yield, 88 %; m.p. 299.0 °C. IR: 3381, 3209 (NH2, NH), 3078 (arom.), 2937, 2869 (aliph.), 1635 (CO), 1593 (CN), 1373, 1182 (SO2), 867 (CCl). 1HNMR: 3.6 [s, 3H, N-CH3], 6.2, 7.3 [2d, 2H, 2CH quinoline, J = 7.2 Hz], 6.1, 7.6 [2d, 2H, CH = CH, J = 7.4 Hz], 7.7–8.6 [m, 13H, Ar–H + SO2NH2], 12.0 [s, 1H, NH]. 13CNMR: 40.5, 95.1, 99.8, 104.9 (2), 112.5, 115.4, 116.2, 119.5 (2), 125.8 (2), 127.9, 128.2 (2), 133.8, 137.6, 138.4, 143.1, 144.6, 146.7, 152.5, 172.5, 189.3. MS m/z (%): 492 (M+) (4.72), 91 (100). Anal. Calcd. For C25H21ClN4O3S (492.98): C, 60.91; H, 4. 29; N, 11.36. Found: C, 61.19; H, 4.52; N, 11.01.
N-(4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)phenylsulfonyl)acetamide(6)
Yield, 76 %; m.p. 310.0 °C. IR: 3367 (NH), 3066 (arom.), 2939, 2877 (aliph.), 1724, 1635 (2CO), 1593 (CN), 1369,1184 (SO2), 833 (CCl). 1HNMR: 2.0 [s, 3H, COCH3], 3.5 [s, 3H, N-CH3], 6.3, 7.3 [2d, 2H, 2CH quinoline, J = 7.4 Hz], 6.6, 7.6 [2d, 2H, CH = CH, J = 7.6 Hz], 7.7–8.6 [m, 12H, Ar–H + SO2NH], 12.0 [s, 1H, NH]. 13CNMR: 23.6, 40.5, 97.8, 101.3, 112.7(2), 115.1, 116.0, 119.5, 120.2 (2), 125.9 (2), 128.1, 129.5 (2), 130.2, 134.6, 142.8 (2), 144.5, 146.9, 150.0, 152.4, 163.1, 186.7, 189.6. MS m/z (%): 535 (M+) (9.36), 74 (100). Anal. Calcd. For C27H23ClN4O4S (535.01): C, 60.61; H, 4.33; N, 10.47. Found: C, 60.29; H, 4.59; N, 10.19.
N-carbamimidoyl-4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)- phenyl)-3-oxoprop-1-enylamino)benzenesulfonamide (7)
Yield, 81 %; m.p. 146.6 °C. IR: 3431, 3336, 3209 (NH2, NH), 3100 (arom.), 2957, 2858 (aliph.), 1635 (CO), 1593 (CN), 1373, 1178 (SO2), 827 (CCl). 1HNMR: 3.4 [s, 3H, NCH3], 6.2, 7.6 [2d, 2H, 2CH quinoline, J = 7.3 Hz], 6.1, 7.4 [2d, 2H, CH = CH, J = 7.4 Hz], 7.7–8.6 [m, 13H, Ar–H + NH2], 9.4 [s, 1H, NH imino], 10.3, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 40.5, 94.9, 99.4, 112.8 (2), 115.2, 116.1, 119.5, 120.2 (2), 125.8 (2), 127.8, 129.5 (2), 131.2, 133.8, 134.6, 138.0, 142.9, 144.8, 145.1, 158.2, 158.5, 172.8, 189.2. MS m/z (%): 535 (M+) (7.74), 76 (100). Anal. Calcd. For C26H23ClN6O3S (535.02): C, 58.37; H, 4. 33; N, 15.71. Found: C, 58.55; H, 4.09; N, 15.47.
4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino) phenyl)-3-oxoprop-1-en-ylamino)-N-(3-methylisoxazol-5-yl)benzenesulfonamide (8)
Yield, 86 %; m.p. 192.5 °C. IR: 3446, 3215 (NH), 3088 (arom.), 2970, 2883 (aliph.), 1635 (CO), 1616 (CN), 1369,1159 (SO2), 821 (CCl). 1HNMR: 2.3 [s, 3H, CH3], 3.4 [s, 3H, NCH3], 6.1, 7.3 [2d, 2H, 2CH quinoline, J = 7.7 Hz], 6.6, 7.6 [2d, 2H, CH = CH, J = 7.4 Hz], 6.7 [s, 1H, CH isoxazole], 7.7–8.5 [m, 12H, Ar–H + SO2NH], 12.0 [s,1H, NH]. 13CNMR: 12.4, 40.5, 95.5, 100.4, 104.7, 113.0 (2), 115.5, 116.3, 119.5, 120.1 (2), 125.8, 129.2 (2), 132.9 (2), 133.7, 134.6, 142.8, 144.9, 145.2, 146.8, 147.4, 153.7, 154.3, 158.5, 170.5, 186.9. MS m/z (%): 574 (M+) (1.62), 58 (100). Anal. Calcd. For C29H24ClN5O4S (574.05): C, 60.68; H, 4. 21; N, 12.20. Found: C, 60.39; H, 4.54; N, 12.49.
4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino) phenyl)-3-oxoprop-1-en-ylamino)-N- (3,4-dimethylisoxazol-5-yl)benzenesulfonamide (9)
Yield, 77 %; m.p. 212.1 °C. IR: 3381, 3230 (NH), 3099 (arom.), 2926, 2819, 2763 (aliph.), 1635 (CO), 1589 (CN), 1373, 1180 (SO2), 810 (CCl). H1 NMR: 1.9, 2.6 [2s, 6H, 2CH3], 3.4 [s, 3H, NCH3], 6.2, 7.3 [2d, 2H, 2CH quinoline, J = 7.6 Hz], 6.6, 7.5 [2d, 2H, CH = CH, J = 7.5 Hz], 7.6–8.6 [m, 11H, Ar–H], 10.4, 12.0 [2s,2H, NH +SO2NH]. 13CNMR: 6.4, 10.8, 40.5, 95.5, 100.3, 102.9, 104.4 (2), 115.5, 116.4, 119.2, 120.7 (2), 126.1, 127.3 (2), 129.5 (2), 133.6, 134.1, 135.2, 142.9, 144.4, 145.4, 147.7, 157.4, 157.9, 161.5, 172.5, 189.3. MS m/z (%): 588 (M+) (11.22), 55 (100). Anal. Calcd. For C30H26ClN5O4S (588.08): C, 61.27; H, 4. 46; N, 11.91. Found: C, 61.01; H, 4.17; N, 11.64.
4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino) phenyl)-3-oxoprop-1-en-ylamino)-N-(1-phenyl-1H-pyrazol-5-yl)benzenesulfonamide (10)
Yield, 80 %; m.p. 94.3 °C. IR: 3417, 3230 (NH), 3064 (arom.), 2966, 2827 (aliph.), 1635 (CO), 1591 (CN), 1373, 1180 (SO2), 763 (CCl). 1HNMR: 3.4 [s, 3H, NCH3], 6.2, 7.5 [2d, 2H, 2CH quinoline, J = 7.5 Hz], 6.5, 7.2 [2d, 2H, CH = CH, J = 7.7 Hz], 7.8–8.6 [m, 18H, Ar–H], 10.2, 12.0 [2s, 2H, NH +SO2NH]. 13CNMR: 40.5, 97.3, 100.0, 103.5, 111.6 (2), 113.0, 116.2, 118.6, 123.7 (2), 124.7 (2), 125.1, 129.0 (2), 129.1, 129.2 (2), 129.3 (2), 129.4, 129.5, 135.1, 136.2, 137.7, 138.9, 140.2, 142.7, 144.3, 146.1, 156.8, 172.4, 186.8. MS m/z (%): 635 (M+) (4.43), 103 (100). Anal. Calcd. For C34H27ClN6O3S (635.13): C, 64.30; H, 4. 28; N, 13.23. Found: C, 64.56; H, 4.52; N, 13.49.
4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino) phenyl)-3-oxoprop-1-en-ylamino)-N-(thiazol-2-yl) benzenesulfonamide (11)
Yield, 69 %; m.p. 172.7 °C. IR: 3341, 3219 (NH), 3101 (arom.), 2937, 2869 (aliph.), 1635 (CO), 1589 (CN), 1373, 1180 (SO2), 773 (CCl). 1HNMR): 3.4 [s, 3H, N-CH3], 5.8, 7.6 [2d, 2H, 2CH quinoline, J = 7.0 Hz], 6.2, 7.2 [2d, 2H, CH = CH, J = 7.3 Hz], 6.6, 6.8 [2d, 2CH thiazole, J = 7.9 Hz], 7.7–8.6 [m, 11H, Ar–H], 10.2, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 40.5, 95.1, 99.8, 108.5, 112.9(2), 115.3, 116.2, 119.5, 120.1 (2), 125.9, 128.3 (2), 129.5 (2), 133.0, 134.6, 135.7, 136.9, 143.0, 144.6, 145.1, 146.9, 152.6, 168.4, 172.5, 186.6. MS m/z (%): 576 (M+) (8.99), 101 (100). Anal. Calcd. For C28H22ClN5O3S2 (576.09): C, 58.38; H, 3.85; N, 12.16. Found: C, 58.23; H, 4.11; N, 12.46.
4-(E)-3-(4-(E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino) phenyl)-3-oxoprop-1-en-ylamino)-N-(5-methyl-1,3,4-thiadiazol-2-yl)benzenesulfonamide (12)
Yield, 82 %; m.p. 304.3 °C. IR: 3246, 3115 (NH), 3088 (arom.), 2937, 2859 (aliph.), 1635 (CO), 1589 (CN), 1383, 1182 (SO2), 769 (CCl). 1HNMR: 2.4 [s, 3H, CH3 thiadiazole], 3.4 [s, 3H, N-CH3], 6.2, 7.6 [2d, 2H, 2CH quinoline, J = 7.6 Hz], 6.6, 7.2 [2d, 2H, CH = CH, J = 7.8 Hz], 7.7–8.5 [m, 11H, Ar–H], 10.3, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 16.4, 40.5, 95.2, 99.9, 115.4 (2), 116.3, 120.2, 120.4, 125.2 (2), 127.9, 128.2 (2), 129.5 (2), 133.1, 134.8, 135.3, 143.0, 143.8, 144.6, 144.8, 152.1, 154.7, 168.3, 172.4, 189.3. MS m/z (%): 591 (M+) (25.7), 178 (100). Anal. Calcd. For C28H23ClN6O3S2 (591.10): C, 56.89; H, 3.92; N, 14.22. Found: C, 56.59; H, 3.68; N, 14.49.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(pyridin-2-yl)benzenesulfonamide (13)
Yield, 91 %; m.p. 177.1 °C. IR: 3323, 3219 (NH), 3080 (arom.), 2939, 2849 (aliph.), 1654 (CO), 1596 (CN), 1375, 1178 (SO2), 773 (CCl). 1HNMR: 3.4 [s, 3H, NCH3], 6.2, 7.6 [2d, 2H, 2CH quinoline, J = 7.6 Hz], 6.6, 7.3 [2d, 2H, CH = CH, J = 7.1 Hz], 7.7–8.6 [m, 15H, Ar–H],10.3, 12.0 [2s, 2H, NH +SO2NH]. 13CNMR: 40.5, 95.3, 100.0, 104.9, 112.9 (2), 113.7, 115.3, 116.4, 119.5, 120.2 (2), 128.2, 129.5 (2), 132.9 (2), 133.7, 134.4, 135.7, 140.3, 142.9, 143.9, 144.6, 145.2, 146.7, 152.4, 153.4, 172.5, 186.6. MS m/z (%): 570 (M+) (18.2), 79 (100). Anal. Calcd. For C30H24ClN5O3S (570.06): C, 63.21; H, 4. 24; N, 12. 29. Found: C, 63.47; H, 4.52; N, 12.55.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(pyrimidin-2-yl)benzenesulfonamide (14)
Yield, 65 %; m.p. 212.9 °C. IR: 3367, 3179 (NH), 3078 (arom.), 2937, 2870 (aliph.), 1635 (CO), 1577 (CN), 1375,1178 (SO2), 883 (CCl). 1HNMR: 3.4 [s, 3H, N-CH3], 6.2, 7.3 [2d, 2H, 2CH quinoline, J = 7.4 Hz], 6.6, 7.6 [2d, 2H, CH = CH, J = 7.5 Hz], 7.0–8.6 [m, 15H, Ar–H + SO2NH], 12.0 [s, 1H, NH]. 13CNMR: 40.5, 95.5, 100.3, 112.6 (2), 115.9, 116.0, 119.5, 120.2 (2), 125.8, 128.1 (2), 130.3 (2), 132.9, 133.7, 134.3, 134.6, 142.8, 144.3, 145.2, 146.9, 157.6 (2), 157.7, 158.6, 172.5, 186.6. MS m/z (%): 571 (M+) (33.2), 158 (100). Anal. Calcd. For C29H23ClN6O3S (571.05): C, 60.99; H, 4. 06; N, 14.72. Found: C, 61.28; H, 4.32; N, 14.47.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(4-methylpyrimidin-2-yl)benzenesulfonamide (15)
Yield, 78 %; m.p. 274.8 °C. IR: 3366, 3259 (NH), 3076 (arom.), 2962, 2870 (aliph.), 1635 (CO), 1562 (CN), 1373, 1182 (SO2), 773 (CCl). 1HNMR: 2.3 [s, 3H, CH3], 3.4 [s, 3H, NCH3], 6.2, 7.6 [2d, 2H, 2CH quinoline, J = 7.3 Hz], 6.6, 7.3 [2d, 2H, CH = CH, J = 7.4 Hz], 7.5–8.5 [m, 13H, Ar–H], 10.3, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 23.7, 40.5, 95.4, 100.2, 104.9, 112.4 (2), 114.9, 115.2, 115.8, 119.6 (2), 128.2, 129.5 (2), 130.5 (2), 132.9, 134.4, 134.6, 142.8, 144.3, 145.3, 146.7, 152.4, 157.4, 158.0, 168.6, 172.5, 186.6. MS m/z (%): 585 (M+) (9.36), 172 (100). Anal.Calcd. For C30H25ClN6O3S (585.08): C, 61.59; H, 4.31; N, 14.36. Found: C, 61.29; H, 4.59; N, 14.09.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide (16)
Yield, 91 %; m.p. 97.9 °C. IR: 3354, 3239 (NH), 3055 (arom.), 2947, 2861 (aliph.), 1635 (CO), 1593 (CN), 1371, 1180 (SO2), 864 (CCl). 1HNMR: 2.2 [s, 6H, 2CH3], 3.4 [s, 3H, NCH3], 5.8, 7.2 [2d, 2H, 2CH quinoline, J = 7.3 Hz], 6.6, 7.7 [2d, 2H, CH = CH, J = 7.5 Hz], 7.8–8.5 [m, 13H, Ar–H + SO2NH], 12.0 [s, 1H, NH]. 13CNMR: 23.4 (2), 40.2, 95.3, 100.1, 104.7, 112.3 (2), 113.8, 114.6, 115.4, 120.6 (2), 125.7, 129.4 (2), 130.8 (2), 132.9, 133.7, 134.8, 144.8, 145.0, 146.9, 157.1, 167.7, 167.8 (2), 172.7, 189.3. MS m/z (%): 599 (M+) (2.71), 109 (100). Anal. Calcd. For C31H27ClN6O3S (599.10): C, 62.15; H, 4. 54; N, 14.03. Found: C, 62.36; H, 4.19; N, 14.29.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(5-methoxypyrimidin-2-yl)benzenesulfonamide (17)
Yield, 84 %; m.p. 264.5 °C. IR: 3396, 3221 (NH), 3101 (arom.), 2979, 2865 (aliph.), 1637 (CO), 1593 (CN), 1371, 1178 (SO2), 862 (CCl). 1HNMR: 3.4 [s, 3H, NCH3], 3.9 [s, 3H, OCH3], 5.9, 7.4 [2d, 2H, 2CH pyrimidine, J = 7.1 Hz], 6.2, 7.3 [2d, 2H, 2CH quinoline, J = 7.8 Hz], 6.6, 7.6 [2d, 2H, CH = CH, J = 7.4 Hz], 7.7–8.6 [m, 11H, Ar–H], 10.3, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 40.5, 56.7, 95.4, 100.2, 105.0 (2), 112.6, 115.1, 116.0, 119.6 (2), 125.8, 128.2 (2), 129.8 (2), 130.1, 133.7, 134.6, 142.8, 144.2, 144.9, 145.3, 149.9, 151.7, 152.4, 153.3, 172.5, 186.6, 186.9. MS m/z (%): 601 (M+) (11.87), 74 (100). Anal. Calcd. For C30H25ClN6O4S (601.08): C, 59.95; H, 4.19; N, 13.98. Found: C, 60.23; H, 3.81; N, 13.69.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(2,6-dimethoxypyrimidin-4-yl)benzenesulfonamide (18)
Yield, 87 %; m.p. 232.6 °C. IR: 3387, 3201 (NH), 3097 (arom.), 2980, 2839 (aliph.), 1635 (CO), 1589 (CN), 1352, 1178 (SO2), 771 (CCl). 1HNMR: 3.4 [s, 3H, N-CH3], 3.7 [s, 6H, 2OCH3], 5.9 [s, 1H, CH pyrimidine], 6.2, 7.3 [2d, 2H, 2CH quinoline, J = 7.5 Hz], 6.6, 7.2 [2d, 2H, CH = CH, J = 7.8 Hz], 7.4–8.5 [m, 11H, Ar–H], 10.3, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 40.5, 54.1, 54.9, 85.1, 95.6, 100.4, 104.9 (2), 115.4, 116.2, 119.5, 120.2 (2), 128.1, 129.8 (2), 132.7 (2), 132.9, 133.7, 134.6, 142.7, 144.2, 144.9, 145.2, 152.3, 160.8, 161.0, 164.7, 172.0, 186.6. MS m/z (%): 631 (M+) (34.47), 154 (100). Anal. Calcd. For C31H27ClN6O5S (631.10): C, 59.00; H, 4.31; N, 13.32. Found: C, 58.76; H, 4.62; N, 13.03.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(5,6-dimethoxypyrimidin-4-yl)benzenesulfonamide (19)
Yield, 83 %; m.p. 110.5 °C. IR: 3365, 3230 (NH), 3095 (arom.), 2941, 2863 (aliph.), 1635 (CO), 1577 (CN), 1375, 1159 (SO2), 773 (CCl). 1HNMR: 3.4 [s, 3H, N-CH3], 3.6, 3.8 [2s, 6H, 2OCH3], 6.2, 7.2 [2d, 2H, 2CH quinoline, J = 7.6 Hz], 6.6, 7.6 [2d, 2H, CH = CH, J = 7.7 Hz], 7.7–8.4 [m, 11H, Ar–H], 8.5 [s, 1H, CH pyrimidine], 10.3, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 40.5, 54.2, 56.5, 95.3, 100.1, 112.6 (2), 115.8, 119.4, 120.8 (2), 127.9, 129.5 (2), 130.2, 133.0 (2), 133.8, 134.7, 142.9, 144.7, 145.1, 146.9, 149.8, 150.9, 152.0, 154.3, 161.7, 172.5, 186.6. MS m/z (%): 631 (M+) (22.13), 189 (100). Anal. Calcd. For C31H27ClN6O5S (631.10): C, 59.00; H, 4.31; N, 13.32. Found: C, 59.31; H, 4.04; N, 13.10.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(1H-indazol-6-yl)benzenesulfonamide (20)
Yield, 89 %; m.p. 100.1 °C. IR: 3374, 3231 (NH), 3086 (arom.), 2978, 2848 (aliph.), 1635 (CO), 1589 (CN), 1363, 1151 (SO2), 819 (CCl). 1HNMR: 3.4 [s, 3H, N-CH3], 5.8, 6.6 [2d, 2H, 2CH quinoline, J = 7.2 Hz], 6.2, 6.8 [2d, 2H, CH = CH, J = 7.5 Hz], 7.0–8.5 [m, 16H, Ar–H + SO2NH], 10.8, 12.0 [2s, 2H, 2NH]. 13CNMR: 40.5, 91.1, 95.5, 100.4, 113.0, 115.1 (2), 115.4, 116.3, 119.5, 119.6, 119.8, 120.0, 120.6, 125.8, 129.0 (2), 129.8 (2), 132.1, 132.8, 133.5, 137.3, 140.7, 143.6, 144.3, 145.3, 146.8, 147.0, 154.3, 173.4, 189.8. MS m/z (%): 609 (M+) (51.63), 117 (100). Anal. Calcd. For C32H25ClN6O3S (609.10): C, 63.10; H, 4.14; N, 13.80. Found: C, 62.76; H, 4.40; N, 14.18.
4-((E)-3-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)-3-oxoprop-1-enylamino)-N-(quinoxalin-2-yl)benzenesulfonamide (21)
Yield, 66 %; m.p. 209.9 °C. IR: 3334, 3212 (NH), 3064 (arom.), 2981, 2863 (aliph.), 1635 (CO), 1591 (CN), 1375, 1178 (SO2), 767 (CCl). 1HNMR: 3.4 [s, 3H, NCH3], 6.2, 7.3 [2d, 2H, 2CH quinoline, J = 7.0 Hz], 6.6, 7.2 [2d, 2H, CH = CH, J = 7.3 Hz], 7.5–8.6 [m, 16H, Ar–H], 10.3, 12.0 [2s, 2H, NH + SO2NH]. 13CNMR: 40.5, 95.5, 100.3, 112.7 (2), 115.1, 116.0, 119.5,120.2 (2), 125.1, 126.3, 127.2, 127.3, 129.1, 130.1 (2), 131.1 (2), 132.8, 133.0, 133.8, 134.7, 138.0, 138.1, 139.2, 140.3, 142.7, 144.3, 149.7, 152.1, 169.6, 186.7. MS m/z (%): 621 (M+) (10.76), 177 (100). Anal. Calcd. For C33H25ClN6O3S (621.11): C, 63.81; H, 4.06; N, 13.53. Found: C, 63.49; H, 4.34; N, 13.23.
(E)-3-(4-(4-aminophenylsulfonyl)phenylamino)-1-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)prop-2-en-1-one (22)
Compound 4 (3.65gm, 0.01 mol) and dapson (2.48 g, 0.01 mol) was added into ethanol (10 mL) and acetic acid (5 mL). The reaction was refluxed for 9 h and the solid obtained while hot was recrystallized from dioxane to give 22.
Yield, 69 %; m.p. 95.2 °C. IR: 3446, 3348, 3213 (NH2, NH), 3100 (arom.), 2956, 2838 (aliph.), 1635 (CO), 1591 (CN), 1369, 1180 (SO2), 821 (CCl). 1HNMR: 3.4 [s, 3H, NCH3], 5.9 [s, 2H, NH2], 6.1, 7.4 [2d, 2H, 2CH quinoline, J = 7.8 Hz], 6.5, 6.6 [2d, 2H, CH = CH, J = 7.9 Hz], 7.5–8.6 [m, 15H, Ar–H], 12.0 [s, 1H, NH]. 13CNMR: 40.5, 95.5, 100.3, 113.3 (2), 113.4, 115.8 (2), 116.6, 119.3, 125.8 (2), 128.9 (4), 129.6 (2), 132.9 (3), 133.7, 135.9, 142.8, 144.2, 145.2, 146.9, 152.4, 154.3, 172.5, 186.6. MS m/z (%): 569 (M+) (19.87), 90 (100). Anal. Calcd. For C31H25ClN4O3S (569.07): C, 65.43; H, 4.43; N, 9.85. Found: C, 65.13; H, 4.71; N, 9.57.
(2E,2′E)-3,3′-(4,4′-sulfonylbis(4,1-phenylene)bis(azanediyl))bis(1-(4-((E)-7-chloro-1-methylquinolin-4(1H)-ylideneamino)phenyl)prop-2-en-1-one) (23)
Compound 4 (7.30 gm, 0.02 mol) and Dapson (2.48 g, 0.01 mol) was added into ethanol (20 mL) containing acetic acid (10 mL). Reaction was refluxed for 12 h and the solid obtained while hot was recrystallized from acetic acid to give 23.
Yield, 60 %; m.p. 186.9 °C. IR: 3143 (NH), 3078 (arom.), 2964, 2842 (aliph.), 1635 (CO), 1570 (CN), 1375, 1180 (SO2), 819 (CCl). 1HNMR: 3.4 [s, 6H, 2N-CH3], 6.2, 7.3 [2d, 4H, 4CH quinoline, J = 7.7 Hz], 6.6, 7.2 [2d, 4H, 2CH = CH, J = 7.8 Hz], 7.4–8.5 [m, 22H, Ar–H], 9.3, 12.0 [2s, 2H, 2NH]. 13CNMR: 40.5 (2), 95.8 (2), 100.7 (2), 104.9 (2), 113.4 (4), 115.8 (2), 116.7 (2), 119.6 (4), 125.8 (4), 129.7 (4), 132.8 (4), 133.6 (2), 134.6 (2), 142.6 (2), 144.0 (2), 145.9 (2), 146.7 (2), 152.3 (2), 172.5 (2), 186.7. MS m/z (%): 889 (M+) (6.48), 272 (100). Anal. Calcd. For C50H38Cl2N6O4S (889.85): C, 67.49; H, 4.30; N, 9.44. Found: C, 67.83; H, 4.66; N, 9.12.
Anticancer screening
The cytotoxic activity in vitro of the novel synthesized compounds was measured using the sulforhodamine B stain (SRB) assay and the method of Skehan et al. [29]. The in vitro anticancer screening was done at pharmacognosy Department, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Cells were plated in 96-multiwell plate (104 cells/well) for 24 h before treatment with the compound(s) to allow attachment of cell to the wall of the plate. Test compounds were dissolved in dimethylsulfoxide. Different concentrations of the compound under test (10, 25, 50, and 100 μΜ) were added to the cell monolayer. Triplicate wells were prepared for each individual concentration. Monolayer cells were incubated with the compound(s) for 48 h at 37 °C and in an atmosphere of 5 % CO2. After 48 h, cells were fixed, washed and stained for 30 min with 0.4 % (Wt/vol) SRB dissolved in 1 % acetic acid. Excess unbound dye was removed by four washes with 1 % acetic acid and attached stain was recovered with Trise-EDTA buffer. Color intensity was measured using an enzyme-linked immunosorbent assay ELISA reader. Optical density was read at 510 nm. The relation between the surviving fraction and drug concentration was plotted to get the survival curve after the specified time The molar concentration required for 50 % inhibition of cell viability (IC 50 ) was calculated and compared to the reference drug 2′,7′-dichlorofluorescein (DCF). The results are given in Table 1.
Molecular docking
“All the molecular modeling studies were carried out on an Intel Pentium 1.6 GHz processor, 512 MB memory with Windows XP operating system using Molecular Operating Environment (MOE, 10.2008) software. All the minimizations were performed with MOE until a RMSD gradient of 0.05 kcal mol−1 Å−1 with MMFF94X force field and the partial charges were automatically calculated. The protein data bank file (PDB: 3S2A) was selected for this purpose. The file contains PI3K enzyme co-crystallized with a quinoline ligand obtained from protein data bank. The enzyme was prepared for docking studies where: (i) Ligand molecule was removed from the enzyme active site. (ii) Hydrogen atoms were added to the structure with their standard geometry. (iii) MOE Alpha Site Finder was used for the active sites search in the enzyme structure and dummy atoms were created from the obtained alpha spheres. (iv) The obtained model was then used in predicting the ligand enzymes interactions at the active site”.
Conclusion
In summary, we had synthesized a novel series of benzene-sulfonamide derivatives. Seven products 1, 2, 4, 7, 11, 14 and 17 presented sound anticancer activity hostile to lung (A594 Raw), hela, and colorectal (lovo) cancer cell lines with better or comparable activity to DCF. Moreover, molecular docking for these active compounds showed proper fitting on the active site of PI3K enzyme suggesting their action as inhibitors for this enzyme but more investigation should be carried out in the future to explore precisely the mechanism of the action of the synthesized derivatives.
References
Gopal M, Shenoy S, Doddamani LS (2003) Antitumor activity of 4-amino and 8-methyl-4-(3diethylamino propylamino)pyrimido[4′,5′:4,5]thieno (2,3-b) quinolines. J Photochem Photobiol B 72:69–78
Kim YH, Shin KJ, Lee TG, Kim E, Lee MS, Ryu SH, Suh PG (2005) G2 arrest and apoptosis by 2-amino-N-quinoline-8-yl-benzenesulfonamide (QBS), a novel cytotoxic compound. Biochem Pharmacol 69:1333–1341
Zhao YL, Chen YL, Chang FS, Tzeng CC (2005) Synthesis and cytotoxic evaluation of certain 4-anilino-2-phenylquinoline derivatives. Eur J Med Chem 40:792–797
Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA (1966) plant antitumor agents. I. the isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc 88:3888–3890
Ma ZZ, Hano Y, Nomura T, Chen YJ (1997) Two new pyrroloquinzolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles 46:541–546
Kobayash J, Cheng J, Nakamura H, Ohizumi Y, Hirata Y, Sasaki T, Ohta T, Nozoe S (1988) Ascididemin, a novel pentacyclic aromatic alkaloid with potent antileukemic activity from the okinawan tunicate Didemnum sp. Tetrahedron Lett 29:1177–1180
Utsugi T, Aoyagi K, Asao T, Okazaki S, Aoyagi Y, Sano M, Wierzba K, Yamada Y (1997) Antitumor activity of a novel quinoline derivative, TAS-103, with inhibitory effects on topoisomerases I and II. Jpn J Cancer Res 88:992–1002
Jonckers TH, van Miert S, Cimanga K, Bailly C, Colson P, De Pauw-Gillet MC, van den Heuvel H, Claeys M, Lemière F, Esmans EL, Rozenski J, Quirijnen L, Maes L, Dommisse R, Lemière GL, Vlietinck A, Pieters L (2002) Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J Med Chem 45:3497–3508
Gireesh KK, Rashid A, Chakraborti S, Panda D, Manna T (2012) CIL-102 binds to tubulin at colchicine binding site and triggers apoptosis in MCF-7 cells by inducing monopolar and multinucleated cells. BiochemPharmacol 84:633–645
Peczyńska-Czoch W, Pognan F, Kaczmarek L, Boratyński J (1994) Synthesis and structure-activity relationship of methyl-substituted indolo[2,3-b]quinolines: novel cytotoxic, DNA topoisomerase II inhibitors. J Med Chem 37:3503–3510
Martirosyan A, Rahim-Bata R, Freeman A, Clarke C, Howard R, Strobl J (2004) Differentiation-inducing quinolines as experimental breast cancer agents in the MCF-7 human breast cancer cell model. Biochem Pharmacol 68:1729–1738
Mól W, Matyja M, Filip B, Wietrzyk S (2008) Synthesis and antiproliferative activity in vitro of novel (2-butynyl)thioquinolines. Bioorg Med Chem 16:8136–8141
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug. Discov. 7:168–181
Supuran CT, Scozzafava A (2007) Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 15:4336–4350
Supuran CT, Scozzafava A, Casini A (2003) Carbonic anhydrase inhibitors. Med Res Rev 23:146–189
Knight SD, Schmidt SJ (2008) Smithkline Beecham corporation. US Patent: US 8138347
Smart BE (2001) Fluorine substituent effects (on bioactivity). J Fluor Chem 109:3–11
Al-Dosari MS, Ghorab MM, Alsaid MS, Nissan YM (2013) Discovering some novel 7-chloroquinolines carrying a biologically active benzenesulfonamide moiety as a new class of anticancer agents. Chem Pharm Bull 61:50–58
Al-Dosari MS, Ghorab MM, Alsaid MS, Nissan YM, Ahmed AB (2013) Synthesis and anticancer activity of some novel trifluoromethylquinolines carrying a biologically active benzenesulfonamide moiety. Eur J Med Chem 69:373–383
Ghorab MM, Ragab FA, Heiba HI, Nissan YM, Ghorab WM (2012) Novel brominated quinoline and pyrimidoquinoline derivatives as potential cytotoxic agents with synergistic effects of γ-radiation. Arch Pharm Res 35:1335–1346
Ghorab MM, Alsaid MS, Nissan YM (2012) Dapson in heterocyclic chemistry, part V: synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active dihydropyridine, dihydroisoquinoline, 1,3-dithiolan, 1,3-dithian, acrylamide, pyrazole, pyrazolopyrimidine and benzochromenemoieties. Chem Pharm Bull 60:1019–1028
Ghorab MM, Ceruso M, AlSaid MS, Nissan YM, Arafa RK, Supuran CT (2014) Novel sulfonamides bearing pyrrole and pyrrolopyrimidine moieties as carbonic anhydrase inhibitors: synthesis, cytotoxic activity and molecular modeling. Eur J Med Chem 87:186–196
Ghorab MM, Ceruso M, AlSaid MS, Nissan YM, Supuran CT (2014) Carbonic anhydrase inhibitors: synthesis, molecular docking, cytotoxic and inhibition of the human carbonic anhydrase isoforms I, II, IX, XII with novel benzenesulfonamides incorporating pyrrole, pyrrolopyrimidine and fused pyrrolopyrimidine moieties. Biooorg Med Chem 22:3684–3695
Ghorab MM, Alsaid MS, Nissan YM (2013) Anti breast cancer of some novel pyrrolo and pyrrolopyrimidine derivatives bearing a biologically active sulfonamide moiety. Life Sci J 10:2170–2183
Ghorab MM, Alsaid MS, Nissan YM (2014) Synthesis and molecular docking of some novel anticancer sulfonamides carrying a biologically active pyrrole and pyrrolopyrimidine moieties. Acta Pol Pharmaceutica 71:603–614
Ferrer R, Lobo G, Gamboa N, Rodrigues J, Abramjuk C, Jung K, Leien M, Charris JE (2009) Synthesis of [(7-Chloroquinolin-4-yl)amino]chalcones: potential antimalarial and anticancer agents. Sci Pharm 77:725
Ghorab MM, AlSaid MS, Ghabbour HA, Fun H (2014) Design, synthesis, X-ray crystallographic study and anticancer activity of novel 4-(7-chloro-1-methylquinolin-4-(1H)-ylideneamino)-phenyl-3-(dimethylamino)-prop-2-en-1-. Asian J Chem 26:8497–8500
Maira SM (2016) PI3K inhibitors for cancer treatment: five years of preclinical and clinical research after BEZ235. Mol Cancer Ther 2011:10
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Authors’ contributions
MMG, MSA designed and contributed in synthesis. MSA carried out biological screening. YMN carried out molecular docking study. AAA contributed in experimental interpretation. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-VPP-302.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ghorab, M.M., Alsaid, M.S., Al-Dosari, M.S. et al. Novel chloroquinoline derivatives incorporating biologically active benzenesulfonamide moiety: synthesis, cytotoxic activity and molecular docking. Chemistry Central Journal 10, 18 (2016). https://doi.org/10.1186/s13065-016-0164-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-016-0164-1