Abstract
Age-associated neurodegenerative disorders such as Alzheimer’s disease are a major public health challenge, due to the demographic increase in the proportion of older individuals in society. However, the relatively few currently approved drugs for these conditions provide only symptomatic relief. A major goal of neurodegeneration research is therefore to identify potential new therapeutic compounds that can slow or even reverse disease progression, either by impacting directly on the neurodegenerative process or by activating endogenous physiological neuroprotective mechanisms that decline with ageing. This requires model systems that can recapitulate key features of human neurodegenerative diseases that are also amenable to compound screening approaches. Mammalian models are very powerful, but are prohibitively expensive for high-throughput drug screens. Given the highly conserved neurological pathways between mammals and invertebrates, Caenorhabditis elegans has emerged as a powerful tool for neuroprotective compound screening. Here we describe how C. elegans has been used to model various human ageing-associated neurodegenerative diseases and provide an extensive list of compounds that have therapeutic activity in these worm models and so may have translational potential.
Similar content being viewed by others
Background
Despite decades of intense molecular research and the identification of many specific causative mutations, debilitating neurodegenerative diseases (NDs) including common disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), afflict millions worldwide and remain a significant and unresolved financial and social burden. Indeed, as ageing itself is by far the greatest risk factor for these diseases, this burden is set to increase dramatically as a result of our increasingly ageing population. Given the urgent need for therapies for these devastating and eventually fatal disorders, many researchers have developed animal models of NDs in order to screen for potential new drugs. In this review, we focus on compound screens performed in the nematode worm, Caenorhabditis elegans. We describe various different NDs that have been modelled in worms and list the therapeutic compounds that have been identified for each. In some cases, these compounds have also been shown to be protective in mammalian ND models, suggesting translational potential for human patients. We conclude that the combination of accurate genetic ND worm models with high-throughput automated drug screening platforms is a potentially very efficient strategy for early therapeutic drug discovery for NDs.
Review
An overview of human neurodegenerative diseases
NDs are characterised by progressive neuropsychiatric dysfunction and the loss of structure and function of specific neuronal circuitry that in turn result in behavioural symptoms. NDs can occur on a completely hereditary basis (e.g. Huntington’s disease), or can be hereditary and also appear sporadically in the majority of cases (e.g. AD, PD). In spite of the diversity in the underlying genes involved, inheritance patterns, clinical manifestation and exact sites of neuropathology, the rare, early onset familial (also known as Mendelian) forms and the more prevalent late-onset sporadic forms of different NDs share some common genetic origins and pathological hallmarks, such as the progressive and chronic nature of the disease, the extensive loss of specific neuronal subtypes, synaptic dysfunctions, the formation and deposition of misfolded protein aggregates [1–3]. Research and technological innovations over the past 10 years have made considerable progress in the elucidation of mechanisms of ND initiation and progression that lead to neurodegeneration. Emerging common themes in the pathogenesis of neurodegeneration include: aberrant phosphorylation, palmitoylation and acetylation of disease-causing proteins, protein misfolding, deficient ubiquitin–proteasome system (UPS) or autophagic process to clear disease-causing proteins, altered RNA metabolism, oxidative stress, mitochondrial dysfunction, excitotoxicity, disrupted axonal transport, neuroinflammation and microglial activation [4]. Linkage analysis, high-throughput sequencing and genome-wide association studies (GWAS) have also identified susceptibility genes in many NDs (Table 1) and promise to help unravel even more genes, novel loci and common genetic variants associated with the diverse collection of human NDs. Thus developments of therapeutic interventions that are applicable across the broad spectrum of NDs and target the shared pathogenic mechanisms may offer the best hope for a future neuroprotective therapy.
Caenorhabditis elegans as a model for human neurodegenerative disease
A major challenge to the identification of effective disease-modifying therapies arises from an insufficient knowledge about the contribution of multiple pathways to disease pathogenesis. Mammalian disease models offer in vivo opportunities and extensive similarity to the human brain, but testing the therapeutic value of small molecules in mammalian model systems is extremely expensive and requires time-consuming experimental designs that can be prohibitive. Over the past decades, C. elegans has increasingly been used as a model system to study the underlying molecular mechanisms that give rise to neurodegeneration because of its well-characterised and easily accessible nervous system, short generation time (≈3 days) and lifespan (≈3 weeks), tractability to genetic manipulation, distinctive behavioural and neuropathological defects, coupled with a surprisingly high degree of biochemical conservation compared to humans. Remarkable similarities exist at the molecular and cellular levels between nematode and vertebrate neurons. For example, ion channels, receptors, classic neurotransmitters [acetylcholine, glutamate, γ-aminobutyric acid (GABA), serotonin, and dopamine (DA)], vesicular transporters and the neurotransmitter release machinery are similar in both structure and function between vertebrates and C. elegans [5, 6]. Importantly, the impact of different challenges such as genetic perturbations or exposure to drugs on the survival and function of defined neuronal populations in the C. elegans nervous system can be readily studied in vivo.
To date, various laboratories have developed and characterised a diverse set of C. elegans models of various human NDs, including AD [7], PD [8] and polyglutamine expansion diseases [9] (Table 1). These worm ND models have been developed by over-expressing human ND-associated genes (both wild type and mutant versions) and by mutating or altering the expression level of the orthologous worm genes. Strong parallels were especially observed in the genotype-to-phenotype correlations between the human NDs and the phenotypes of transgenic C. elegans ND models. This supports the validity of the approach as expression of mutant human proteins in C. elegans can closely model a fundamental property of these mutations in humans.
Nevertheless, there are also limitations to using C. elegans to model NDs that must be considered. Although the worm offers huge potential for experimental manipulations, there are aspects of ND pathophysiology that cannot easily be modelled in worms. For example, abundant evidence supports an important role for brain inflammation and microglial cell activation in several NDs, notably AD [10], but there is no microglial equivalent among the 56 glial cells of C. elegans. Clearly, the very simplicity of the worm nervous system that makes it so attractive for studying basic neurobiology is also a disadvantage in that the complexity of the mammalian brain cannot be adequately reflected, and so rodent models will continue to be required to validate any findings from C. elegans ND studies. There are also potential pitfalls of using C. elegans for drug screening, as many compounds do not easily penetrate the worm’s protective cuticle [11] and as biotransformation of compounds by the worms’ E. coli food source may give misleading pharmacological information [12]. Although these potential pitfalls can be mitigated by combining predictive bioaccumulation algorithms [11] with increased dose regimens, and by confirming drug effects using metabolically inactive E. coli, these issues need to be considered when performing drug screens in worms.
Despite the above caveats, C. elegans remains a widely used animal model to identify genes that modify neurodegeneration in vivo. Indeed, genetic screens performed on worm models have identified a wide variety of conserved genes that can suppress or increase disease progression and are thus potential therapeutic drug targets. However, relatively few of these genetic modifiers are common to more than one disease model, despite the shared feature of protein misfolding/aggregation [13, 14]. In addition to its utility for screening for genetic contributors to NDs, C. elegans is a useful pharmacological model for testing potential neuroprotective compounds. Numerous well-characterised ND models have been readily exploited for triaging compounds from large libraries consisting of novel and pre-approved drugs, and for testing the effects of individual drugs, prior to validation in vertebrate models. Potential therapeutics identified via such compound screens using specific worm ND models are shown in Figs. 1, 2, listed in Table 1 and described in detail below.
Structures of compounds with therapeutic effects in C. elegans models of human neurodegenerative diseases. Chemical structures were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov) or MolBase (http://www.molbase.com). AD Alzheimer’s disease, ALS amyotrophic lateral sclerosis, ANCL adult-onset neuronal ceroid lipofuscinosis, FTDP frontotemporal dementia with parkinsonism-17, HD Huntington’s disease, MJD Machado–Joseph disease (spinocerebellar ataxia type 3), PD Parkinson’s disease, Prion prion disease, SMA spinal muscular atrophy
Structures of compounds with therapeutic effects in C. elegans models of human neurodegenerative diseases. Chemical structures were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov) or MolBase (http://www.molbase.com). AD Alzheimer’s disease, ALS amyotrophic lateral sclerosis, ANCL adult-onset neuronal ceroid lipofuscinosis, FTDP frontotemporal dementia with parkinsonism-17, HD Huntington’s disease, MJD Machado–Joseph disease (spinocerebellar ataxia type 3), PD Parkinson’s disease, Prion prion disease, SMA spinal muscular atrophy
Alzheimer’s disease: amyloid-β (Aβ) models
β-Amyloid is the main component of the extracellular plaques found in the brains of Alzheimer’s disease patients. It is widely (though not universally) believed that aggregation of Aβ into oligomeric forms is the main driver of neurodegeneration in Alzheimer’s disease. This has been modelled in nematodes by expressing human Aβ constructs in worm muscle cells [7]. The Aβ-induced paralysis observed in the well-characterised muscle-specific strains has provided a valuable phenotype for straightforward quantification of the effects of treatments on Aβ toxicity and validation of potential therapeutic interventions for Alzheimer’s disease. The C. elegans strain CL2006, which constitutively expresses human Aβ1-42, has been elegantly used to demonstrate the neuroprotective effects of a diverse range of compounds (Table 1; Figs. 1, 2). These include natural products such as specific gingkolides [15], soya isoflavone glycitein [16], the green tea component epigallocatechin gallate [17, 18] and coffee extract [19]; FDA-approved drugs such as tannic acid, bacitracin, rifampicin [20], thioflavin T [21], reserpine [22] and the antidepressant fluoxetine; and polyphenolic compounds such as curcumin and ferulic acid [23, 24]. These treatments conferred considerable life-span extension and cellular stress tolerance [15, 16]. This was a consequence of most compounds attenuating the rate of toxic human Aβ1–42 mediated paralysis, to suppress the Aβ1–42 induced increase in toxic reactive oxygen species and hydrogen peroxide levels, and to inhibit Aβ1–42 oligomerisation and deposition [15, 25]. Recent studies have also demonstrated how the antibiotic tetracycline and its analogues [26], and ethanol extract of Liuwei Dihuang [27] successfully protected the CL4176 inducible Aβ1–42 muscle-specific expression model by inhibiting Aβ1–42 oligomerisation and reducing superoxide production. Oleuropein aglycone, the main polyphenol in extra virgin olive oil, was recently shown to protect against amyloid toxicity in both constitutive and inducible Aβ1–42 models [28]. In addition, two recent large, unbiased yeast-based screens of pharmacological modifiers identified the 8-hydroxyquinoline chemical scaffold (8-OHQ), a class of clinically relevant bioactive metal chelators as neuroprotective compounds that reduced proteotoxicity associated with the aggregation of several ND-specific proteins including TDP-43, α-synuclein, polyglutamine proteins, or Aβ1–42 [29, 30]. Notably, two closely related 8-OHQs–PBT2 and clioquinol, which conferred neuroprotective benefits in mouse models of AD, were further shown to rescue Aβ1–42 toxicity in C. elegans body wall muscle cells [31] and glutamatergic neurons [30]. PBT2 was also effective in improving cognition and reducing Aβ in cerebrospinal fluid in a small Phase IIA trial in AD patients [31].
Tauopathies
In addition to amyloid plaque deposition, Alzheimer’s disease is associated with intraneuronal accumulation of neurofibrillary tangles containing the microtubule-associated protein Tau, which aggregates into insoluble fibrillar deposits when it is hyperphosphorylated [32]. Pathological Tau deposits are also observed in Pick’s disease, corticobasal degeneration, Down’s syndrome and specific types of frontotemporal dementia (FTD) such as frontotemporal dementia with parkinsonism chromosome 17 type (FTDP-17) and frontotemporal lobar dementia (FTLD). Various worm transgenic Tauopathy models expressing mutant human Tau constructs have therefore been generated and yielded complementary findings in regards to the effects of neuronal Tau expression [33–35]. Neurodegeneration in worms expressing transgenic human mutant Tau can be assessed indirectly, using phenotypes such as impaired locomotion and reduced lifespan, but also directly by visualising loss of neuronal cell bodies and neuronal processes in vivo. An example of the latter is shown in Fig. 3, where a human Tau construct containing the FTDP-17-associated V337 M mutation is expressed in all 302 worm neurons via a pan-neuronal C. elegans promoter. In addition, the 26 GABAergic neurons of the worm are specifically labelled by driving green fluorescent protein (GFP) expression from GABA-specific C. elegans promoter. In control worms, a continuous, intact line of GFP fluorescence is seen running along both the ventral and dorsal nerve cords on opposite sides of the animal. In contrast, the mutant Tau transgenic strains exhibits large gaps in these nerve cords where neuronal processes are missing, thus directly demonstrating severe neurodegeneration in the living animal.
A C. elegans genetic model of the Tauopathy, FTDP-17. Triple transgenic worms expressing human V337M mutant Tau protein (Paex-3::V337M Tau), a pharyngeal GFP marker (Pmyo-2::GFP) and a GFP reporter transgene marking the cell bodies and processes of all C. elegans GABAergic neurons (Punc-25::GFP) were compared with control single Punc-25::GFP transgenic worms. All panels are micrographs of representative whole worms. Control (left panels) and Tau V337M expressing worms (right panel) were examined after 1, 5 and 10 days of age. In control worms, intact ventral and dorsal cords were observed at all ages. In contrast, the mutant Tau transgenic GABAergic reporter strain exhibited severe degeneration of neuronal processes. Ventral and dorsal cord gaps (arrows) are disruptions in the continuity of the ventral and dorsal nerve cords, respectively. Scale bar represents 200 μm for all panels except for the bottom two panels, which are high magnifications of the boxed areas of day-10 worms shown above
Using such Tauopathy models, compounds with known anti-aggregation activity like methylene blue, were shown to effectively ameliorate the worms’ motility and neuronal defects [36]. In addition, a novel compound belonging to the aminothienopyridazine class, cmp16, was also shown to rescue these phenotypes and to suppress Tau aggregation in worms [36]. Importantly, aminothienopyridazines are known to suppress Tau aggregation in mammalian cells and so the improved blood–brain barrier permeability of cmp16 suggests that this compound may have significant translational potential. In a recent screen of a library of FDA-approved compounds, dopamine D2 receptor antagonism was identified as a promising strategy for targeting tau-induced neurotoxicity, as antipsychotics such as azaperone, perphenazine, and zotepine improved the phenotypic features of Tauopathy in worms (Table 1; Figs. 1, 2). Azaperone, in particular, effectively ameliorated mutant Tau-induced functional defects and reduced the level of insoluble Tau aggregation [37]. Finally, a recent study reported that the anti-epileptic drug, ethosuximide, could ameliorate the impaired motility and reduced lifespan phenotypes of the Tau V337 M worm FTDP-17 model [38]. Interestingly, ethosuximide’s action in this worm Tau model was independent of its main proposed target in epilepsy, the T-type calcium channel.
Polyglutamine (polyQ) disorders
Expansion of trinucleotide CAG repeats in a variety of different genes leads to neurodegenerative diseases such as Huntington’s disease and spinocerebellar ataxias due to the expression of a polyglutamine tract within the encoded protein. Diverse worm transgenic models where varying lengths of polyQ tracts are expressed in specific sets of neurons, muscle cells and even intestine cells have been widely used to model several aspects of polyQ neurotoxicity, notably to address the mechanisms underlying the impact of aggregation prone proteins on cellular function and to identify novel disease modifiers [39–41]. The progressive nature of polyQ-mediated toxicity, protein aggregation and general severity of phenotype demonstrated in these models is age- and polyQ-tract-length-dependent, recapitulating critical aspects of polyglutamine expansion diseases in patients.
Voisine et al. [42] screened candidate pharmacological compounds utilising a HD model in which the pqe-1 genetic mutant background greatly enhanced toxicity induced by a human Huntingtin construct containing a 150-residue glutamine tract (Htt-Q150). Both lithium chloride and mithramycin alleviated neuronal cell death, while trichostatin A (a class I and class II HDAC inhibitor) provided significant neuroprotection. Using the same HD model, Varma et al. [43] discovered that small molecular inhibitors of metabolism (mitochondrial and glycolytic function) such as rotenone, oligomycin and 4-dinitrophenol rescued neuronal loss and degeneration by activating caspase inhibition and ERK and AKT prosurvival signalling and their efficacy was further validated in cell culture and Drosophila HD models (Table 1; Figs. 1, 2). Resveratrol, a demonstrated activator of sirtuin deacetylases, also effectively alleviated Htt-Q128 toxicity in both worm and neuronal culture models [44]. Recently, treatment of a C. elegans model of SCA3 (spinocerebellar ataxia type 3; also known as Machado-Joseph disease) with 17-(allylamino)-17-demethoxygeldanamycin (17-AAG), an HSP90 inhibitor, successfully decreased the mutant ATXN3 aggregation and improved locomotor activity [39]. Treatment of the same model with valproic acid (VA), another HDAC inhibitor and a well-known anti-epileptic drug, also led to improved locomotor activity accompanied by a decrease in mutant ATXN3 aggregation. Therefore, HDAC inhibitors which promote histone acetylation over deacetylation and which were also known to provide protection against polyQ mediated toxicity in vertebrate and Drosophila neurons may hold promise as a preventive therapy in polyQ diseases.
Other pan-neuronal or neuron specific HD models facilitated the identification of other potential therapeutic interventions, including the anti-cancer agent β-lapachone [45], D. officinarum root extracts [46] and a phenol glycoside salidroside [47], which conferred protection against polyQ neuronal toxicity. Treating C. elegans muscle polyQ models with hydroxylamine, icariin and celecoxib derivatives (NG-094, icariside II and OSU-03012, respectively) ameliorated polyQ-mediated protein aggregation and protected against polyQ proteotoxicity [48–50] (Table 1; Figs. 1, 2). Aspirin, an analgesic agent, was also shown to significantly improve polyQ-mediated animal paralysis, reducing the number of Q35-YFP aggregates and delaying polyQ-dependent acceleration of aging [51].
Parkinson’s disease (PD)
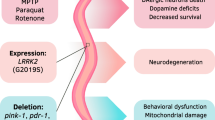
Pathologically, PD is characterised by degeneration of dopaminergic neurons in the substantia nigra and accumulation of Lewy bodies containing aggregated α-synuclein protein. Although most cases are idiopathic, PD can be caused by both environmental (e.g. pesticide exposure) and genetic (e.g. α-synuclein and LRRK2 mutation) effects. Multiple worm PD models, notably the toxin-induced models, have aided in the discovery and validation of potential pharmacological interventions for PD. An example of how dopaminergic neurodegeneration can be directly assessed in vivo in C. elegans is shown in Fig. 4. Here, the eight dopaminergic neurons of the worm are specifically labelled by GFP expression from the promoter of the C. elegans dopamine transporter. In control worms, fluorescent neuronal cell bodies extending long processes are clearly visible in the head (6 neurons) and tail (2 neurons) of the animal. However, treatment with the PD-inducing toxin, 6-hydroxydopamine (6-OHDA), causes the loss of GFP-labelled dopaminergic neuronal cell bodies and/or processes, thus enabling direct visualisation of neurodegeneration.
A C. elegans model of toxin-induced Parkinson’s disease. a Dopaminergic (DA) neuronal cell bodies and neurites in BY250 worms were visualised using an integrated Pdat-1::GFP dopamine transporter marker. C. elegans has eight DA neurons: six are located in the anterior region, which can be subclassified in pairs as two anterior deirid neurons (ADE), two dorsal cephalic neurons (CEP) which are postsynaptic to the ADE neurons and two ventral CEPs that are not postsynaptic to the ADEs; two posterior deirid neurons (PDE) located posteriorly are also shown. Arrows depict the four CEP neuron processes and indicate the ADE and PDE cell bodies in a young worm. Anterior is to the left. b Representative examples of worms scored which display the three characteristic stages of DA neurodegeneration in response to 6-OHDA. Magnification of anterior region of C. elegans shows only the anterior-most DA neurons. WT: in this example, all six anterior DA neurons of this worm appear robust and the dendrites are intact and fully extended. Neuronal process blebbing; cell body rounding: this worm exhibited prominent cell body rounding (asterisk) and dendrite blebbing (arrows); cell body loss: this worm exhibited a complete loss of GFP in most DA neurons as CEP and ADE neurons have all degenerated and are no longer visible in any focal plane, only retention of GFP expression in the remnants of neuron cell bodies and broken neurites. All scale bars represent 20 μm. c Representative images of worms 24 h post-6-OHDA-exposure are presented. BY250 worms treated with ascorbic acid (AA) alone expressed intact and strong GFP in all six DA neurons and dendrites in the heads. However, the majority of BY250 worms incubated with 50 mM 6-OHDA showed a marked GFP expression reduction in the dendrites of ADEs and CEPs, many of the cell somas became round (asterisk) and blebs appeared along the dendrites of CEPs (arrows)
Chemical screens have suggested that compounds which protect mitochondria or increase autophagy protect against α-synuclein toxicity [52, 53]. Braungart et al. [54] performed a focused compound screen using the C. elegans MPTP model of PD and found that lisuride and apomorphine (dopamine receptor agonists), as well as rottlerin (protein kinase C inhibitor) ameliorated the MPTP-induced behavioural defects when present at a low concentration. In addition, nomifensine (dopamine transporter inhibitor), nicotine (acetylcholine receptor agonist), selegiline (monoamine oxidase inhibitor), MPEP (mGluR-5 inhibitor), amantadine, α-lipoic acid (antioxidant) and ascorbic acid (antioxidant) were effective at higher concentrations [53]. In another screen, two mammalian dopamine D2 receptor agonists, bromocriptine and quinpirole, were identified to confer significant neuroprotection independent of dopamine receptors in a 6-OHDA-induced dopaminergic neurodegeneration model of PD [55]. Similarly, a low concentration of acetaminophen (analgesic and antipyretic) was reported by Locke et al. [56] to protect significantly against 6-OHDA toxicity-induced dopaminergic neurodegeneration in P dat-1 ::GFP expressing worms. However, the protection appears to be selective as acetaminophen was not neuroprotective against α-synuclein-induced neurodegeneration at any concentration tested. The anti-epileptic drug, valproic acid provided significant dopaminergic neuroprotection in a C. elegans PD model associated with human α-synuclein overproduction, which was further shown to be mediated through ERK-MAPK signalling [57]. A more recent study has also demonstrated the neuroprotective effects of the naturally occurring polyamine spermidine and phytocompounds such as n-butylidenephthalide, curcumin, N-acetylcysteine and vitamin E on 6-OHDA-induced degeneration of dopaminergic neurons and their ability to attenuate α-synuclein accumulation. n-butylidenephthalide, in particular, had the greatest neuroprotective capacity and was shown to also restore food-sensing behaviour and dopamine levels in both pharmacological and transgenic C. elegans PD models as well as enhancing the life span of 6-OHDA-treated animals [58]. Acetylcorynoline, the major alkaloid component derived from Corydalis bungeana, a traditional Chinese medical herb demonstrated the same neuroprotective effects when applied to the same pharmacological and transgenic C. elegans PD models [59].
Kinase-targeted inhibition of LRRK2 protein activity was recently established as an effective treatment for PD as LRRK2 kinase inhibitors consistently mitigated pathogenesis caused by different LRRK2 mutations. Liu et al. [60] showed that though GW5074, an indoline compound, and sorafenib, a Raf kinase inhibitor, did not have protective effects against α-synuclein- and 6-OHDA-induced toxicity, they increased survival and reduced dopaminergic neurodegeneration in G2019S-LRRK2 transgenic C. elegans and Drosophila. Yao et al. [61] further demonstrated the potency of kinase inhibitors as they were able to pharmacologically rescue both the behavioural deficit and neurodegeneration manifested by the expression of mutant LRRK2 G2019S and R1441C in vivo using two LRRK2 inhibitors, TTT-3002 and LRRK2-IN1, which also potently inhibited in vitro kinase activities of LRRK2 wild-type, R1441C and G2019S at nanomolar to low micromolar concentrations when administered either pre-symptomatically or post-symptomatically. Compounds that have been shown to be protective in the various worm PD models are listed in Table 1 and their chemical structures shown in Figs. 1 and 2.
Amyotrophic lateral sclerosis (ALS)
A number of transgenic lines expressing mutant forms of human SOD1 found in familial ALS patients under a range of promoters have been generated and recapitulated the motor neuron degeneration and paralysis characteristic of ALS patients [102, 103, 105, 108]. Genes recently shown to be mutated in ALS include the DNA/RNA binding proteins TDP-43 and FUS, and C9ORF72, a novel familial and sporadic ALS causative gene. Treatment with methylene blue, an aggregation inhibitor of the phenothiazine class, not only rescued toxic phenotypes (including neuronal dysfunction and oxidative stress) associated with mutant TDP-43 and FUS in C. elegans and zebrafish ALS models [62], but also ameliorated Tau mediated toxicity in a newly established C. elegans model [36]. Using transgenic TDP-43 models, Tauffenberger et al. evaluated 11 compounds previously reported to enhance longevity in C. elegans and resveratrol (polyphenol), rolipram (phosphodiesterase 4 inhibitor), reserpine (antihypertensive), ethosuximide (anti-epileptic), trolox and propyl gallate (antioxidants) were revealed as effective candidates that protected against mutant TDP-43 toxicity in motor neurons [63] (Table 1; Figs. 1, 2). Recent genetic experiments by Kraemer’s group suggested that inhibiting cell division cycle kinase 7 (CDC7) kinase activity reduces phosphorylation of TDP-43 and the consequent neurodegeneration. Small molecule inhibition of CDC-7 by PHA767491 was further shown to robustly reduce TDP-43 phosphorylation and prevent TDP-43 dependent neurodegeneration both in vitro and in vivo [64].
Autosomal dominant adult-onset neuronal ceroid lipofuscinosis (ANCL)
ANCL, also known as autosomal dominant Kufs’ disease and Parry disease, is a rare hereditary disease characterised by intra-neuronal inclusions of autofluorescent lipofuscin-like material and neurodegeneration [65, 66]. Recently, four independent research groups have reported that ANCL is caused by mutations in the DNAJC5 gene that encodes the endogenous neuroprotective synaptic chaperone cysteine string protein (CSP) [67–70]. Our lab has recently developed a C. elegans model of ANCL by using null mutants of the worm DNAJC5 orthologue, dnj-14 [71]. These worms have similar phenotypes to ANCL patients and also to CSP mutants in mice, in terms of reduced lifespan, progressive neuronal dysfunction and neurodegeneration [72]. This evolutionary conservation of CSP’s neuroprotective function suggests that the worm dnj-14 model could have potential for identifying generic neuroprotective interventions rather than disease specific drug targets. Indeed, a focused screen of pharmacological compounds that ameliorated the dnj-14 lifespan and neuronal defects identified the polyphenolic molecule resveratrol, which has been shown to be neuroprotective in a range of animal neurodegeneration models [71]. In contrast to other worm neurodegeneration models [44, 63, 73, 74], however, resveratrol acted in a sir-2.1-independent manner, as sir-2.1; dnj-14 double mutants showed full lifespan rescue by resveratrol. Instead, the mechanism of resveratrol action appeared to be via inhibition of cAMP phosphodiesterase, as the phosphodiesterase inhibitor, rolipram was shown to mimic the effect of resveratrol in rescuing dnj-14 phenotypes [71]. More recently, the anti-epileptic drug ethosuximide has been shown to be protective in the dnj-14 model, acting through a DAF-16/FOXO-dependent mechanism that is distinct from its proposed mechanism of action in epilepsy [38]. Ethosuximide also ameliorates the phenotypes of worm models of FTDP-17 [38] and ALS [63] and reduces protein aggregation in a mouse neuronal cell culture model of Huntington’s disease [38], suggesting that it may have general and evolutionarily conserved neuroprotective properties. Indeed, it has recently been shown that ethosuximide reverses cognitive decline in a rat model of Alzheimer’s disease [75]. Finally, a recent genome-wide transcriptional profiling study of dnj-14 mutants revealed a striking reduction in expression of ubiquitin proteasome system (UPS)-related genes in comparison to wild type control strains [76]. Genes encoding components of multimeric E3 ubiquitin ligases were especially over-represented, suggesting that these may represent potential novel drug targets for treatment of ANCL and perhaps other neurodegenerative diseases.
Translational implications of C. elegans chemical screens
The different screening strategies that have been applied to C. elegans ND models have provided distinct insights into potential therapeutic approaches in patients. These strategies range from robotic automated imaging-based approaches designed for high throughput compound library screening [77] to highly focused screens of a selected small group of compounds that target a common pathological process such as protein aggregation [21]. Large scale screens offer greater coverage of chemical space and so have potential to identify unifying pharmacological themes amongst multiple hits from compound libraries. For example, several different dopamine D2 receptor antagonists were recovered as hits in an unbiased library screen using a Tauopathy model, with genetic techniques then being used to confirm that reduced D2 receptor function is indeed neuroprotective [37]. Whilst this suggests that several currently prescribed atypical anti-psychotic drugs could potentially be re-purposed for treatment of human tauopathies, dosing regimens would need to be carefully considered given reports that the relatively high doses of these medications used to treat aggression and agitation in dementia patients may increase the risk of death [78].
One observation that emerges from our analysis of the large number of studies to date is that very few compounds are therapeutic in multiple C. elegans ND models. Indeed, out of the 72 compounds shown in Figs. 1 and 2, only ethosuximide and resveratrol are effective in more than two ND models and therefore appear to have general neuroprotective activity. This may be due in part to the fact that most published studies have focused on relatively small sets of compounds and so activity across multiple ND models remains to be tested. Nevertheless, it seems certain that this also reflects disease-specific pharmacological actions—for example, Raf kinase inhibition is therapeutic in LRRK2-based PD models, but ineffective in α-synuclein- and 6-OHDA-based PD models [60]. Clearly, effective clinical treatments with such highly disease-specific drugs requires knowledge of the underlying pathophysiological mechanism, which is not always diagnosable in NDs. Drugs such as ethosuximide and resveratrol are therefore potentially very useful, as they may provide general neuroprotective activity regardless of uncertainties regarding molecular pathology. The mechanism of action of ethosuximide and resveratrol remains unclear and controversial [79–81], but both have been linked to increased longevity and healthspan in model organisms [82, 83]. Given that dietary restriction, the best established intervention known to increase longevity and healthspan, is therapeutic in multiple ND models from invertebrates to mice [84], it is clear that slowing the ageing process can confer general neuroprotection. It may be that ethosuximide and resveratrol modulate some of the same conserved neuroprotective mechanisms that decline with age, thus potentially explaining their therapeutic effects in radically different ND models.
Conclusions and future perspectives
The nematode C. elegans has great potential for expediting neuroprotective drug discovery. Its facile genetics and suitability for high-throughput compound screening mean that both target-driven and phenotypic screening approaches can easily be performed (and potentially combined). Although phenotypic screening became unfashionable as a drug discovery paradigm in the post-genomic era, Swinney and Anthony have clearly shown that most new medicines still continue to be discovered via phenotypic screening [85]. This influential work has forced a re-evaluation in the pharma industry and a consequent shift towards phenotypic screening that incorporates available knowledge of targets/mechanisms [86], for which C. elegans is ideally suited. Furthermore, there is increasing evidence that using compound combinations designed to act on multiple molecular targets can be an effective therapeutic strategy—as exemplified by the spectacular success of combination therapy for HIV [87]. Testing of many such drug combinations can be performed rapidly and cheaply using worm models, in contrast to rodent models. In addition, technical developments such as CRISPR [88] now offer the potential to rapidly create new and more accurate C. elegans models of human neurodegenerative diseases, by precisely delivering single-copies of mutant genes identified from patients to appropriate desired locations in the worm genome. Although C. elegans has already facilitated the identification of potential novel therapeutics, the future combination of more accurate genetic models with high-throughput automated drug screening platforms is a potentially very efficient strategy for therapeutic drug discovery for NDs.
References
Muchowski PJ (2002) Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 35(1):9–12
Taylor JP, Hardy J, Fischbeck KH (2002) Toxic proteins in neurodegenerative disease. Science 296(5575):1991–1995
Soto C, Estrada LD (2008) Protein misfolding and neurodegeneration. Arch Neurol 65(2):184–189
Ehrnhoefer DE, Wong BK, Hayden MR (2011) Convergent pathogenic pathways in Alzheimer’s and Huntington’s diseases: shared targets for drug development. Nat Rev Drug Discovery 10(11):853–867
Hardaway JA, Hardie SL, Whitaker SM, Baas SR, Zhang B, Bermingham DP, Lichtenstein AJ, Blakely RD (2012) Forward genetic analysis to identify determinants of dopamine signaling in Caenorhabditis elegans using swimming-induced paralysis. G3 2(8):961–975
Barclay JW, Morgan A, Burgoyne RD (2012) Neurotransmitter release mechanisms studied in Caenorhabditis elegans. Cell Calcium 52(3–4):289–295
Link CD (1995) Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA 92(20):9368–9372
Nass R, Miller DM, Blakely RD (2001) C. elegans: a novel pharmacogenetic model to study Parkinson’s disease. Parkinsonism Relat Disord 7(3):185–191
Satyal SH, Schmidt E, Kitagawa K, Sondheimer N, Lindquist S, Kramer JM, Morimoto RI (2000) Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc Natl Acad Sci USA 97(11):5750–5755
Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129(2):154–169
Burns AR, Wallace IM, Wildenhain J, Tyers M, Giaever G, Bader GD, Nislow C, Cutler SR, Roy PJ (2010) A predictive model for drug bioaccumulation and bioactivity in Caenorhabditis elegans. Nat Chem Biol 6(7):549–557
Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D (2013) Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153(1):228–239
van Ham T, Breitling R, Swertz M, Nollen E (2009) Neurodegenerative diseases: lessons from genome-wide screens in small model organisms. EMBO Mol Med 1(8–9):360–370
Chen X, Burgoyne RD (2012) Identification of common genetic modifiers of neurodegenerative diseases from an integrative analysis of diverse genetic screens in model organisms. BMC Genom 13:71
Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y (2006) Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci 26(50):13102–13113
Gutierrez-Zepeda A, Santell R, Wu Z, Brown M, Wu Y, Khan I, Link CD, Zhao B, Luo Y (2005) Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC neuroscience 6:54
Abbas S, Wink M (2009) Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med 75(3):216–221
Abbas S, Wink M (2010) Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine 17(11):902–909
Dostal V, Roberts CM, Link CD (2010) Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans model of beta-amyloid peptide toxicity. Genetics 186(3):857–866
Lublin A, Isoda F, Patel H, Yen K, Nguyen L, Hajje D, Schwartz M, Mobbs C (2011) FDA-approved drugs that protect mammalian neurons from glucose toxicity slow aging dependent on Cbp and protect against proteotoxicity. PLoS One 6(11):e27762
Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ (2011) Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 472(7342):226–229
Arya U, Dwivedi H, Subramaniam JR (2009) Reserpine ameliorates Abeta toxicity in the Alzheimer’s disease model in Caenorhabditis elegans. Exp Gerontol 44(6–7):462–466
Jagota S, Rajadas J (2012) Effect of phenolic compounds against Abeta aggregation and Abeta-induced toxicity in transgenic C. elegans. Neurochem Res 37(1):40–48
Keowkase R, Aboukhatwa M, Luo Y (2010) Fluoxetine protects against amyloid-beta toxicity, in part via daf-16 mediated cell signaling pathway, Caenorhabditis elegans. Neuropharmacology 59(4–5):358–365
Smith JV, Luo Y (2003) Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J Alzheimers Dis 5(4):287–300
Diomede L, Cassata G, Fiordaliso F, Salio M, Ami D, Natalello A, Doglia SM, De Luigi A, Salmona M (2010) Tetracycline and its analogues protect Caenorhabditis elegans from beta amyloid-induced toxicity by targeting oligomers. Neurobiol Dis 40(2):424–431
Sangha JS, Sun X, Wally OSD, Zhang K, Ji X, Wang Z, Wang Y, Zidichouski J, Prithiviraj B, Zhang J (2012) Liuwei Dihuang (LWDH), a traditional Chinese medicinal formula, protects against β-Amyloid toxicity in transgenic Caenorhabditis elegans. PLoS One 7(8):e43990
Diomede L, Rigacci S, Romeo M, Stefani M, Salmona M (2013) Oleuropein aglycone protects transgenic C. elegans strains expressing Abeta42 by reducing plaque load and motor deficit. PLoS One 8(3):e58893
Tardiff DF, Tucci ML, Caldwell KA, Caldwell GA, Lindquist S (2012) Different 8-hydroxyquinolines protect models of TDP-43 protein, alpha-synuclein, and polyglutamine proteotoxicity through distinct mechanisms. J Biol Chem 287(6):4107–4120
Matlack KE, Tardiff DF, Narayan P, Hamamichi S, Caldwell KA, Caldwell GA, Lindquist S (2014) Clioquinol promotes the degradation of metal-dependent amyloid-beta (Abeta) oligomers to restore endocytosis and ameliorate Abeta toxicity. Proc Natl Acad Sci USA 111(11):4013–4018
McColl G, Roberts BR, Pukala TL, Kenche VB, Roberts CM, Link CD, Ryan TM, Masters CL, Barnham KJ, Bush AI et al (2012) Utility of an improved model of amyloid-beta (Aβ1-42) toxicity in Caenorhabditis elegans for drug screening for Alzheimer’s disease. Mol Neurodegener 7:57
Mandelkow EM, Mandelkow E (1998) Tau in Alzheimer’s disease. Trends Cell Biol 8(11):425–427
Brandt R, Gergou A, Wacker I, Fath T, Hutter H (2009) A Caenorhabditis elegans model of tau hyperphosphorylation: induction of developmental defects by transgenic overexpression of Alzheimer’s disease-like modified tau. Neurobiol Aging 30(1):22–33
Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD (2003) Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci USA 100(17):9980–9985
Miyasaka T, Ding Z, Gengyo-Ando K, Oue M, Yamaguchi H, Mitani S, Ihara Y (2005) Progressive neurodegeneration in C. elegans model of tauopathy. Neurobiol Dis 20(2):372–383
Fatouros C, Pir GJ, Biernat J, Koushika SP, Mandelkow E, Mandelkow E-M, Schmidt E, Baumeister R (2012) Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum Mol Genet 21(16):3587–3603
McCormick AV, Wheeler JM, Guthrie CR, Liachko NF, Kraemer BC (2013) Dopamine D2 receptor antagonism suppresses tau aggregation and neurotoxicity. Biol Psychiatry 73(5):464–471
Chen X, McCue FH, Wong SQ, Kashyap SS, Kraemer BC, Barclay JW, Burgoyne RD, Morgan A (2015) Ethosuximide ameliorates neurodegenerative disease phenotypes by modulating DAF-16/FOXO target gene expression. Mol Neurodegener 10(1):51
Teixeira-Castro A, Ailion M, Jalles A, Brignull HR, Vilaca JL, Dias N, Rodrigues P, Oliveira JF, Neves-Carvalho A, Morimoto RI et al (2011) Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: rescue by the DAF-16 and HSF-1 pathways. Hum Mol Genet 20(15):2996–3009
Brignull HR, Morley JF, Garcia SM, Morimoto RI (2006) Modeling polyglutamine pathogenesis in C. elegans. Methods Enzymol 412:256–282
Faber PW, Voisine C, King DC, Bates EA, Hart AC (2002) Glutamine/proline-rich PQE-1 proteins protect Caenorhabditis elegans neurons from huntingtin polyglutamine neurotoxicity. Proc Natl Acad Sci USA 99(26):17131–17136
Voisine C, Varma H, Walker N, Bates EA, Stockwell BR, Hart AC (2007) Identification of potential therapeutic drugs for huntington’s disease using Caenorhabditis elegans. PLoS One 2(6):e504
Varma H, Cheng R, Voisine C, Hart AC, Stockwell BR (2007) Inhibitors of metabolism rescue cell death in Huntington’s disease models. Proc Natl Acad Sci 104(36):14525–14530
Parker J, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C (2005) Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37(4):349–350
Shin BH, Lim Y, Oh HJ, Park SM, Lee S-K, Ahnn J, Kim DH, Song WK, Kwak TH, Park WJ (2013) Pharmacological activation of Sirt1 ameliorates polyglutamine-induced toxicity through the regulation of autophagy. PLoS One 8(6):e64953
Yang X, Zhang P, Wu J, Xiong S, Jin N, Huang Z (2012) The neuroprotective and lifespan-extension activities of Damnacanthus officinarum extracts in Caenorhabditis elegans. J Ethnopharmacol 141(1):41–47
Xiao L, Li H, Zhang J, Yang F, Huang A, Deng J, Liang M, Ma F, Hu M, Huang Z (2014) Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules 19(6):7757–7769
Haldimann P, Muriset M, Vigh L, Goloubinoff P (2011) The novel hydroxylamine derivative NG-094 suppresses polyglutamine protein toxicity in Caenorhabditis elegans. J Biol Chem 286(21):18784–18794
Cai WJ, Huang JH, Zhang SQ, Wu B, Kapahi P, Zhang XM, Shen ZY (2011) Icariin and its derivative Icariside II extend healthspan via Insulin/IGF-1 pathway in C. elegans. Plos One 6:e28835
Ching TT, Chiang WC, Chen CS, Hsu AL (2011) Celecoxib extends C. elegans lifespan via inhibition of insulin-like signaling but not cyclooxygenase-2 activity. Aging Cell 10(3):506–519
Ayyadevara S, Bharill P, Dandapat A, Hu C, Khaidakov M, Mitra S, Shmookler Reis RJ, Mehta JL (2013) Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid Redox Signal 18(5):481–490
Wolozin B, Gabel C, Ferree A, Guillily M, Ebata A (2011) Watching worms whither: modeling neurodegeneration in C. elegans. Prog Mol Biol Transl Sci 100:499–514
Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C et al (2005) Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem 280(52):42655–42668
Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC (2004) Caenorhabditis elegans MPP + model of Parkinson’s disease for high-throughput drug screenings. Neurodegener Dis 1(4–5):175–183
Marvanova M, Nichols CD (2007) Identification of neuroprotective compounds of Caenorhabditis elegans dopaminergic neurons against 6-OHDA. J Mol Neurosci 31(2):127–137
Locke CJ, Fox SA, Caldwell GA, Caldwell KA (2008) Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson’s disease. Neurosci Lett 439(2):129–133
Kautu BB, Carrasquilla A, Hicks ML, Caldwell KA, Caldwell GA (2013) Valproic acid ameliorates C. elegans dopaminergic neurodegeneration with implications for ERK-MAPK signaling. Neurosci Lett 541:116–119
Fu R-H, Harn H-J, Liu S-P, Chen C-S, Chang W-L, Chen Y-M, Huang J-E, Li R-J, Tsai S-Y, Hung H-S et al (2014) n-Butylidenephthalide protects against dopaminergic neuron degeneration and α-synuclein accumulation in Caenorhabditis elegans models of Parkinson’s Disease. PLoS One 9(1):e85305
Fu R-H, Wang Y-C, Chen C-S, Tsai R-T, Liu S-P, Chang W-L, Lin H-L, Lu C-H, Wei J-R, Wang Z-W et al (2014) Acetylcorynoline attenuates dopaminergic neuron degeneration and α-synuclein aggregation in animal models of Parkinson’s disease. Neuropharmacology 82:108–120
Liu Z, Hamamichi S, Lee BD, Yang D, Ray A, Caldwell GA, Caldwell KA, Dawson TM, Smith WW, Dawson VL (2011) Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson’s disease models. Hum Mol Genet 20(20):3933–3942
Yao C, Johnson WM, Gao Y, Wang W, Zhang J, Deak M, Alessi DR, Zhu X, Mieyal JJ, Roder H et al (2012) Kinase inhibitors arrest neurodegeneration in cell and C. elegans models of LRRK2 toxicity. Hum Mol Genet 22:328–344
Vaccaro A, Patten SA, Ciura S, Maios C, Therrien M, Drapeau P, Kabashi E, Parker JA (2012) Methylene Blue protects against TDP-43 and FUS neuronal toxicity in C. elegans and D. rerio. PLoS One 7(7):e42117
Tauffenberger A, Julien C, Parker JA (2013) Evaluation of longevity enhancing compounds against transactive response DNA-binding protein-43 neuronal toxicity. Neurobiol Aging 34(9):2175–2182
Liachko NF, McMillan PJ, Guthrie CR, Bird TD, Leverenz JB, Kraemer BC (2013) CDC7 inhibition blocks pathological TDP-43 phosphorylation and neurodegeneration. Ann Neurol 74(1):39–52
Haltia M (2003) The neuronal ceroid-lipofuscinoses. J Neuropathol Exp Neurol 62(1):1–13
Haltia M, Goebel HH (2013) The neuronal ceroid-lipofuscinoses: a historical introduction. Biochim Biophys Acta 1832(11):1795–1800
Noskova L, Stranecky V, Hartmannova H, Pristoupilova A, Baresova V, Ivanek R, Hulkova H, Jahnova H, van der Zee J, Staropoli JF et al (2011) Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am J Hum Genet 89(2):241–252
Benitez BA, Alvarado D, Cai Y, Mayo K, Chakraverty S, Norton J, Morris JC, Sands MS, Goate A, Cruchaga C (2011) Exome-sequencing confirms DNAJC5 mutations as cause of adult neuronal ceroid-lipofuscinosis. PLoS One 6(11):e26741
Velinov M, Dolzhanskaya N, Gonzalez M, Powell E, Konidari I, Hulme W, Staropoli JF, Xin W, Wen GY, Barone R et al (2012) Mutations in the gene DNAJC5 cause autosomal dominant Kufs disease in a proportion of cases: study of the Parry family and 8 other families. PLoS One 7(1):e29729
Cadieux-Dion M, Andermann E, Lachance-Touchette P, Ansorge O, Meloche C, Barnabe A, Kuzniecky RI, Andermann F, Faught E, Leonberg S et al (2013) Recurrent mutations in DNAJC5 cause autosomal dominant Kufs disease. Clin Genet 83(6):571–575
Kashyap SS, Johnson JR, McCue HV, Chen X, Edmonds MJ, Ayala M, Graham ME, Jenn RC, Barclay JW, Burgoyne RD et al (2014) Caenorhabditis elegans dnj-14, the orthologue of the DNAJC5 gene mutated in adult onset neuronal ceroid lipofuscinosis, provides a new platform for neuroprotective drug screening and identifies a SIR-2.1-independent action of resveratrol. Hum Mol Genet 23(22):5916–5927
Burgoyne RD, Morgan A (2015) Cysteine string protein (CSP) and its role in preventing neurodegeneration. Semin Cell Dev Biol 40:153–159
Bizat N, Peyrin JM, Haik S, Cochois V, Beaudry P, Laplanche JL, Neri C (2010) Neuron dysfunction is induced by prion protein with an insertional mutation via a Fyn kinase and reversed by sirtuin activation in Caenorhabditis elegans. J Neurosci 30(15):5394–5403
Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE (2009) Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int 54(2):111–118
Tiwari SK, Seth B, Agarwal S, Yadav A, Karmakar M, Gupta SK, Choubey V, Sharma A, Chaturvedi RK (2015) Ethosuximide induces hippocampal neurogenesis and reverses cognitive deficits in amyloid-beta toxin induced Alzheimer’s rat model via PI3 K/Akt/Wnt/beta-catenin pathway. J Biol Chem 290:28540–28558
McCue HV, Chen X, Barclay JW, Morgan A, Burgoyne RD (2015) Expression profile of a Caenorhabditis elegans model of adult neuronal ceroid lipofuscinosis reveals down regulation of ubiquitin E3 ligase components. Sci Rep 5:14392
Sleigh JN, Buckingham SD, Esmaeili B, Viswanathan M, Cuppen E, Westlund BM, Sattelle DB (2011) A novel Caenorhabditis elegans allele, smn-1(cb131), mimicking a mild form of spinal muscular atrophy, provides a convenient drug screening platform highlighting new and pre-approved compounds. Hum Mol Genet 20(2):245–260
Schneider LS, Dagerman KS, Insel P (2005) Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 294(15):1934–1943
Crunelli V, Leresche N (2002) Block of thalamic T-type Ca(2+) channels by ethosuximide is not the whole story. Epilepsy Curr Am Epilepsy Soc 2(2):53–56
Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL et al (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148(3):421–433
Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, SY S, Lamming DW et al (2013) Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339(6124):1216–1219
Evason K, Huang C, Yamben I, Covey DF, Kornfeld K (2005) Anticonvulsant medications extend worm life-span. Science 307(5707):258–262
Baur JA (2010) Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev 131(4):261–269
Pani G (2015) Neuroprotective effects of dietary restriction: evidence and mechanisms. Semin Cell Dev Biol 40:106–114
Swinney DC, Anthony J (2011) How were new medicines discovered? Nat Rev Drug Discovery 10(7):507–519
Swinney DC (2013) The contribution of mechanistic understanding to phenotypic screening for first-in-class medicines. J Biomol Screen 18(10):1186–1192
Weiss RA (2008) Special anniversary review: 25 years of human immunodeficiency virus research: successes and challenges. Clin Exp Immunol 152(2):201–210
Frokjaer-Jensen C (2013) Exciting prospects for precise engineering of Caenorhabditis elegans genomes with CRISPR/Cas9. Genetics 195(3):635–642
Fay DS, Fluet A, Johnson CJ, Link CD (1998) In vivo aggregation of beta-amyloid peptide variants. J Neurochem 71(4):1616–1625
Link CD, Johnson CJ, Fonte V, Paupard M-C, Hall DH, Styren S, Mathis CA, Klunk WE (2001) Visualization of fibrillar amyloid deposits in living, transgenic Caenorhabditis elegans animals using the sensitive amyloid dye, X-34. Neurobiol Aging 22(2):217–226
Yatin SM, Yatin M, Aulick T, Ain KB, Butterfield DA (1999) Alzheimer’s amyloid beta-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: protective effect of vitamin E. Neurosci Lett 263(1):17–20
Drake J, Link CD, Butterfield DA (2003) Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging 24(3):415–420
Florez-McClure ML, Hohsfield LA, Fonte G, Bealor MT, Link CD (2007) Decreased insulin-receptor signaling promotes the autophagic degradation of beta-amyloid peptide in C. elegans. Autophagy 3(6):569–580
Link CD, Taft A, Kapulkin V, Duke K, Kim S, Fei Q, Wood DE, Sahagan BG (2003) Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol Aging 24(3):397–413
Aparecida Paiva F, de Freitas Bonomo L, Ferreira Boasquivis P, Borges Raposo de Paula IT, Guerra JF, Mendes Leal W, Silva ME, Pedrosa ML, Oliveira Rde P (2015) Carqueja (Baccharis trimera) Protects against oxidative stress and beta-amyloid-induced toxicity in Caenorhabditis elegans. Oxid Med Cell Longev 2015:740162
Link CD (2006) C. elegans models of age-associated neurodegenerative diseases: lessons from transgenic worm models of Alzheimer’s disease. Exp Gerontol 41(10):1007–1013
Dosanjh LE, Brown MK, Rao G, Link CD, Luo Y (2010) Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-β. J Alzheimers Dis 19(2):681–690
Treusch S, Hamamichi S, Goodman JL, Matlack KES, Chung CY, Baru V, Shulman JM, Parrado A, Bevis BJ, Valastyan JS et al (2011) Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast. Science 334(6060):1241–1245
Hornsten A, Lieberthal J, Fadia S, Malins R, Ha L, Xu X, Daigle I, Markowitz M, O’Connor G, Plasterk R et al (2007) APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci USA 104(6):1971–1976
Ewald CY, Cheng R, Tolen L, Shah V, Gillani A, Nasrin A, Li C (2012) Pan-neuronal expression of APL-1, an APP-related protein, disrupts olfactory, gustatory, and touch plasticity in Caenorhabditis elegans. J Neurosci 32(30):10156–10169
Oeda T, Shimohama S, Kitagawa N, Kohno R, Imura T, Shibasaki H, Ishii N (2001) Oxidative stress causes abnormal accumulation of familial amyotrophic lateral sclerosis-related mutant SOD1 in transgenic Caenorhabditis elegans. Hum Mol Genet 10(19):2013–2023
Wang J, Farr GW, Hall DH, Li F, Furtak K, Dreier L, Horwich AL (2009) An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet 5(1):e1000350
Witan H, Kern A, Koziollek-Drechsler I, Wade R, Behl C, Clement AM (2008) Heterodimer formation of wild-type and amyotrophic lateral sclerosis-causing mutant Cu/Zn-superoxide dismutase induces toxicity independent of protein aggregation. Hum Mol Genet 17(10):1373–1385
Murakami A, Kojima K, Ohya K, Imamura K, Takasaki Y (2002) A new conformational epitope generated by the binding of recombinant 70-kd protein and U1 RNA to anti-U1 RNP autoantibodies in sera from patients with mixed connective tissue disease. Arthritis Rheum 46(12):3273–3282
Gidalevitz T, Krupinski T, Garcia S, Morimoto RI (2009) Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet 5(3):e1000399
Silva DF, Esteves AR, Oliveira CR, Cardoso SM (2011) Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Curr Alzheimer Res 8(5):563–572
Zhang T, Mullane PC, Periz G, Wang J (2011) TDP-43 neurotoxicity and protein aggregation modulated by heat shock factor and insulin/IGF-1 signaling. Hum Mol Genet 20(10):1952–1965
Li J, Huang KX, Wd L (2013) WD: Establishing a novel C. elegans model to investigate the role of autophagy in amyotrophic lateral sclerosis. Acta Pharmacol Sin 34(5):644–650
Liachko NF, Guthrie CR, Kraemer BC (2010) Phosphorylation promotes neurotoxicity in a Caenorhabditis elegans model of TDP-43 proteinopathy. J Neurosci 30(48):16208–16219
Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, Petrucelli L, Link CD (2010) Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet 19(16):3206–3218
Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci 99(16):10417–10422
Wang H, Lim PJ, Yin C, Rieckher M, Vogel BE, Monteiro MJ (2006) Suppression of polyglutamine-induced toxicity in cell and animal models of Huntington’s disease by ubiquilin. Hum Mol Genet 15(6):1025–1041
Yamanaka K, Okubo Y, Suzaki T, Ogura T (2004) Analysis of the two p97/VCP/Cdc48p proteins of Caenorhabditis elegans and their suppression of polyglutamine-induced protein aggregation. J Struct Biol 146(1–2):242–250
Parker J, Connolly J, Wellington C, Hayden M, Dausset J, Neri C (2001) Expanded polyglutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death. Proc Natl Acad Sci USA 98(23):13318–13323
Lejeune F-X, Mesrob L, Parmentier F, Bicep C, Vazquez-Manrique R, Parker JA, Vert J-P, Tourette C, Neri C (2012) Large-scale functional RNAi screen in C. elegans identifies genes that regulate the dysfunction of mutant polyglutamine neurons. BMC Genom 13(1):91
Teixeira-Castro A, Ailion M, Jalles A, Brignull HR, Vilaça JL, Dias N, Rodrigues P, Oliveira JF, Neves-Carvalho A, Morimoto RI, Maciel P (2011) Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: rescue by the DAF-16 and HSF-1 pathways. Hum Mol Genet 20:2996–3009
Christie NT, Lee AL, Fay HG, Gray AA, Kikis EA (2014) Novel polyglutamine model uncouples proteotoxicity from aging. PLoS One 9(5):e96835
Hamamichi S, Rivas RN, Knight AL, Cao S, Caldwell KA, Caldwell GA (2008) Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc Natl Acad Sci USA 105(2):728–733
van Ham TJ, Thijssen KL, Breitling R, Hofstra RMW, Plasterk RHA, Nollen EAA (2008) C. elegans model identifies genetic modifiers of α-Synuclein inclusion formation during aging. PLoS Genet 4(3):e1000027
Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G (2003) Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem 86(1):165–172
Settivari R, LeVora J, Nass R (2009) The divalent metal transporter homologues SMF-1/2 mediates dopamine neuron sensitivity in Caenorhabditis elegans models of manganism and Parkinson’s disease. J Biol Chem M109.051409
Cao S, Gelwix CC, Caldwell KA, Caldwell GA (2005) Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci 25(15):3801–3812
Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, Tsunoda M, Mitani S, Iwatsubo T (2006) Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J Biol Chem 281(1):334–340
Buttner S, Broeskamp F, Sommer C, Markaki M, Habernig L, Alavian-Ghavanini A, Carmona-Gutierrez D, Eisenberg T, Michael E, Kroemer G et al (2014) Spermidine protects against alpha-synuclein neurotoxicity. Cell Cycle 13(24):3903–3908
Karpinar DP, Balija MB, Kugler S, Opazo F, Rezaei-Ghaleh N, Wender N, Kim HY, Taschenberger G, Falkenburger BH, Heise H et al (2009) Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J 28(20):3256–3268
Kuwahara T, Koyama A, Koyama S, Yoshina S, Ren C-H, Kato T, Mitani S, Iwatsubo T (2008) A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in α-synuclein transgenic C. elegans. Hum Mol Genet 17(19):2997–3009
Kuwahara T, Tonegawa R, Ito G, Mitani S, Iwatsubo T (2012) Phosphorylation of α-synuclein protein at Ser-129 reduces neuronal dysfunction by lowering its membrane binding property in Caenorhabditis elegans. J Biol Chem 287(10):7098–7109
Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH, Segal L, Raghavan K, Matsumoto K et al (2009) LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci 29(29):9210–9218
Yao C, El Khoury R, Wang W, Byrd TA, Pehek EA, Thacker C, Zhu X, Smith MA, Wilson-Delfosse AL, Chen SG (2010) LRRK2-mediated neurodegeneration and dysfunction of dopaminergic neurons in a Caenorhabditis elegans model of Parkinson’s disease. Neurobiol Dis 40(1):73–81
Bizat N, Peyrin J-M, Haïk S, Cochois V, Beaudry P, Laplanche J-L, Néri C (2010) Neuron dysfunction is induced by prion protein with an insertional mutation via a Fyn kinase and reversed by Sirtuin activation in Caenorhabditis elegans. J Neurosci 30(15):5394–5403
Park K-W, Li L (2011) Prion protein in Caenorhabditis elegans: distinct models of anti-BAX and neuropathology. Prion 5(1):28–38
Nussbaum-Krammer CI, Park K-W, Li L, Melki R, Morimoto RI (2013) Spreading of a prion domain from cell-to-cell by vesicular transport in Caenorhabditis elegans. PLoS Genet 9(3):e1003351
Lakowski B, Hekimi S (1998) The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci USA 95(22):13091–13096
Levitan D, Greenwald I (1998) Effects of SEL-12 presenilin on LIN-12 localization and function in Caenorhabditis elegans. Development 125(18):3599–3606
Wittenburg N, Eimer S, Lakowski B, Rohrig S, Rudolph C, Baumeister R (2000) Presenilin is required for proper morphology and function of neurons in C. elegans. Nature 406(6793):306–309
Springer W, Hoppe T, Schmidt E, Baumeister R (2005) A Caenorhabditis elegans Parkin mutant with altered solubility couples alpha-synuclein aggregation to proteotoxic stress. Hum Mol Genet 14(22):3407–3423
Sämann J, Hegermann J, von Gromoff E, Eimer S, Baumeister R, Schmidt E (2009) Caenorhabditits elegans LRK-1 and PINK-1 act antagonistically in stress response and neurite outgrowth. J Biol Chem 284(24):16482–16491
Briese M, Esmaeili B, Fraboulet S, Burt EC, Christodoulou S, Towers PR, Davies KE, Sattelle DB (2009) Deletion of smn-1, the Caenorhabditis elegans ortholog of the spinal muscular atrophy gene, results in locomotor dysfunction and reduced lifespan. Hum Mol Genet 18(1):97–104
Nass R, Miller DM, Blakely RD (2001) C. elegans: a novel pharmacogenetic model to study Parkinson’s disease. Parkinsonism Relat Disord 7(3):185–191
Ruan Q, Harrington AJ, Caldwell KA, Caldwell GA, Standaert DG (2010) VPS41, a protein involved in lysosomal trafficking, is protective in Caenorhabditis elegans and mammalian cellular models of Parkinson’s disease. Neurobiol Dis 37(2):330–338
Liu J, Banskota AH, Critchley AT, Hafting J, Prithiviraj B (2015) Neuroprotective effects of the cultivated Chondrus crispus in a C. elegans model of Parkinson’s disease. Mar Drugs 13(4):2250–2266
Caldwell KA, Tucci ML, Armagost J, Hodges TW, Chen J, Memon SB, Blalock JE, DeLeon SM, Findlay RH, Ruan Q et al (2009) Investigating bacterial sources of toxicity as an environmental contributor to dopaminergic neurodegeneration. PLoS One 4(10):e7227
Authors’ contributions
XC, JWB, RDB and AM conceived and designed the study. XC and AM wrote the manuscript, with input from JWB and RDB. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the BBSRC and AgeUK for funding our research into worm models of neurodegeneration. We thank Dr. Brian Kraemer (University of Washington, USA) for providing the CK49 and CZ1200 strains shown in Fig. 3; and the Caenorhabditis Genetics Center for providing the BY250 strain created by Dr. Randy Blakely (Vanderbilt University, USA) shown in Fig. 4.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, X., Barclay, J.W., Burgoyne, R.D. et al. Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chemistry Central Journal 9, 65 (2015). https://doi.org/10.1186/s13065-015-0143-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-015-0143-y