Abstract
Background
Kidney failure and dialysis treatment have a large impact on a patient’s life. Patients experience numerous, complex symptoms and usually have multiple comorbid conditions. Despite the multitude of problems, patients often have priorities for improvement of specific aspects of their functioning, which would be helpful for clinicians to become informed of. This highlights a clear need for patient-centered care in this particular patient group, with routine screening as a vital element to timely recognize symptoms and tailored treatment to match individual patients’ needs and priorities. By also providing feedback on patient’s screening results to the patient itself, the patient is empowered to actively take control in one’s mostly uncontrollable disease process. The current paper describes the study design of a multicenter randomized controlled trial evaluating the effectiveness of the “E-HEealth treatment in Long-term Dialysis” (E-HELD) intervention. This therapist-guided Internet-based cognitive-behavioral therapy (ICBT) intervention is focused on and personalized to the myriad of problems that dialysis patients experience and prioritize.
Methods
After a screening procedure on adjustment problems, 130 eligible dialysis patients will be randomized to care as usual or the E-HELD intervention. Patients will complete questionnaires on distress (primary outcome measure), several domains of functioning (e.g., physical, psychological, social), potential predictors and mediators of treatment success, and the cost-effectiveness of the intervention, at baseline, 6-month follow-up, and 12-month follow-up. In addition, to take account of the personalized character of the intervention, the Personalized Priority and Progress Questionnaire (PPPQ) will be administered which is a personalized instrument to identify, prioritize, and monitor individual problems over time.
Discussion
The present study design will provide insight in the effectiveness of tailored ICBT in patients with kidney failure who are treated with dialysis. When proven effective, the screening procedure and the subsequent ICBT intervention could be implemented in routine care to detect, support, and treat patients struggling with adjustment problems.
Trial registration
NL63422.058.17 [Registry ID: METC-LDD]
NL7160 [Netherlands Trial Register; registered on 16 July 2018]
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
Title {1} | E-HEalth treatment in Long-term Dialysis (E-HELD): study protocol for a multicenter randomized controlled trial evaluating Internet-based cognitive-behavioral therapy in dialysis patients |
Trial registration {2a and 2b} | Netherlands Trial Register (NL7160). Registered on July 16, 2018. |
Protocol version {3} | Version 7.0. Date: January 29, 2020. |
Funding {4} | This work was supported by the Dutch Kidney Foundation (SWO 16.07). |
Author details {5a}c | Judith Tommel, Health, Medical and Neuropsychology unit, Institute of Psychology, Faculty of Social and Behavioural Sciences, Leiden University, Leiden, the Netherlands. Andrea W. M. Evers, Health, Medical and Neuropsychology unit, Institute of Psychology, Faculty of Social and Behavioural Sciences, Leiden University, Leiden, the Netherlands. Henk W. van Hamersvelt, Department of Nephrology, Radboud Institute for Health Sciences, Radboud university medical center, Nijmegen, the Netherlands. Sandra van Dijk, Health, Medical and Neuropsychology unit, Institute of Psychology, Faculty of Social and Behavioural Sciences, Leiden University, Leiden, the Netherlands. Niels H. Chavannes, Public Health and Primary Care, Leiden University Medical Center, Leiden, the Netherlands. Lieke Wirken, Health, Medical and Neuropsychology unit, Institute of Psychology, Faculty of Social and Behavioural Sciences, Leiden University, Leiden, the Netherlands. Luuk B. Hilbrands, Department of Nephrology, Radboud Institute for Health Sciences, Radboud university medical center, Nijmegen, the Netherlands. Henriët van Middendorp, Health, Medical and Neuropsychology unit, Institute of Psychology, Faculty of Social and Behavioural Sciences, Leiden University, Leiden, the Netherlands. |
Name and contact information for the trial sponsor {5b} | Health, Medical and Neuropsychology unit, Institute of Psychology, Faculty of Social and Behavioural Sciences, Leiden University, Wassenaarseweg 52, 2333 AK Leiden, the Netherlands |
Role of sponsor {5c} | The funder and sponsor played no role in the design of the study, the collection, management, analysis, and interpretation of data and in writing the manuscript. |
Introduction
Background and rationale {6a}
Chronic kidney disease (CKD) is a growing, public health threat with an estimated global prevalence of 11–13% [1]. Risk factors of CKD include older age, diabetes mellitus, hypertension, cardiovascular disease, and obesity [2]. The key characteristic of CKD is a gradual loss of kidney function. When kidney function is declined to less than 15% of normal functioning, most patients become dependent on life-sustaining renal replacement therapy—dialysis or kidney transplantation. This final stage of CKD is referred to as kidney failure and is related to high symptom burden and a low quality of life (QOL) [3,4,5]. Symptoms include fatigue, sleep disturbances, itch, muscle cramps, pain, worrying, depressed mood, social difficulties, and changes in sexual functioning [5,6,7,8,9]. Together with high comorbidity in most patients, this large amount of symptoms makes it difficult for clinicians to detect the symptoms most troubling to patients, which could lead to symptoms being underrecognized or underestimated [10]. This is particularly problematic since increased symptom burden is found to be one of the most important predictors of dialysis patients’ low QOL [11, 12].
To improve the recognition of symptoms, routine questionnaire screenings are vital to detect symptoms that would be overlooked otherwise. Additionally, routine screening would facilitate patient-clinician communication by making it easier for patients to discuss their difficulties and needs [9, 13]. To simplify screening procedures, an online screening tool could be efficient, reduces missing values, and offers the possibility to present feedback in a way that is easy to understand, for example, by visualizing patients’ results [9, 14]. Subsequently, when results indicate a need for support, clinicians can offer a tailored treatment plan to meet individual patient needs.
With regard to the dialysis population, the value of tailoring treatment to individual patient needs is supported by a previous study of our group that found a high variety of reported problems and priorities for improvement between male and female patients, different age groups, and dialysis types [15]. In addition to these differences in priorities, the dialysis population is known for its high heterogeneity and comorbidity, which necessitates a personalized approach [16]. Moreover, besides increased motivation, adherence, and patient satisfaction, tailored or personalized treatment is found to result in stronger and longer-lasting effects compared to standardized treatment [17,18,19,20].
Cognitive-behavioral therapy (CBT) is a well-established treatment for psychological problems and psychiatric disorders and is increasingly used in patients with chronic conditions [21, 22]. In patients with chronic conditions, CBT can be used to support patients in adjustment to illness, including feelings of helplessness and loss of control, coping with uncertainty about the future, and adjusting to the need of medical or emotional support [23]. Other indications for CBT involve comorbid psychiatric disorders, difficulties in adherence to treatment, and problems related to illness behaviors which entails the way people perceive and act on their physical symptoms [23, 24]. With regard to dialysis patients, CBT can be applied to support patients in adjusting to and coping with physical problems that are inherent to kidney failure (e.g., fatigue, itch, pain), the dialysis treatment, or comorbid conditions, such as diabetes or cardiovascular diseases. Promising results are found for CBT to improve dialysis patients’ adjustment to these physical problems as shown by improved functioning on the physical aspects of health-related quality of life (HRQOL) [25,26,27] and decreased symptoms of fatigue [28]. Considering psychological functioning, CBT is found to be effective in treating anxiety and depression and improving the mental aspects of HRQOL in dialysis patients [29,30,31].
Despite the benefits of CBT for patients with a chronic condition, some patients experience barriers that prevent them from participating in psychological interventions, such as time constraints, a lack of availability of services, financial problems, problems in transportation to care [32], and the remaining stigma on mental illness [33, 34]. A solution to overcome these barriers is to offer CBT online. In Internet-based CBT (ICBT), patients have the freedom to select the time and place that best suits their schedule, while following treatment at home lowers the threshold of seeking psychological support [35, 36]. Especially for dialysis patients—considering their numerous visits to the clinic, mobility problems, and high disease burden—this would make CBT more accessible, convenient, and less burdensome. In addition, for therapists, offering CBT online is time-efficient and enhances flexibility which, in turn, results in reduced costs and shorter waiting lists [36].
ICBT interventions usually consist of guided self-help formats in which patients are supported by a therapist through short messages, telephone, or videoconference [37]. ICBT is found to be as effective as face-to-face CBT in improving both mental (e.g., anxiety and depression) [38, 39] and physical functioning (e.g., disease-specific quality of life, irritable bowel syndrome symptoms, headache, pain severity, disability, and fatigue) [37,38,39] in several somatic conditions. Compared to self-guided interventions, interventions guided by a therapist are found to result in better outcomes [40, 41]: therapist support does not only increase adherence and prevents drop-out [35], it also allows for therapists to tailor the intervention to individual patient needs, which increases the effectiveness of (I)CBT [19, 35].
When it comes to kidney failure, no controlled, adequately powered studies have been performed to study the effectiveness of tailored ICBT in the dialysis population. Only two small, single-arm studies and one feasibility and acceptability randomized controlled trial were performed, focusing on one particular symptom (e.g., depression). The single-arm studies found significant improvements in depression [42, 43]. One of the studies also found improvements in anxiety, general distress, and mental HRQOL, but found no improvements in disability and disease burden [42]. With regard to feasibility, challenges concerning patients’ computer literacy, the acceptability of distress screenings, and perceived treatment need were reported [42, 44]. Hence, for ICBT to succeed, it was urged to be attentive of a patient’s computer literacy and to embed screening procedures early on in dialysis patients’ care pathway to get patients accustomed to screenings and psychosocial support [44]. Despite these challenges, patients who completed the intervention found ICBT to be highly acceptable, worth their time, and felt more confident that they could manage their symptoms. In addition, preliminary results indicated promising effects for the cost-effectiveness of ICBT [42].
Given the lack of adequately controlled and powered studies, more research is needed to evaluate the effectiveness of ICBT in the dialysis population. In the current study, we will evaluate effectiveness of the “E-HEealth treatment in Long-term Dialysis” (E-HELD) intervention. This therapist-guided ICBT intervention is focused on and personalized to the myriad of problems that dialysis patients experience.
Objectives {7}
The current paper addresses the design and objectives of a multicenter randomized controlled trial that aims to evaluate the effectiveness of the E-HELD intervention—a guided ICBT intervention tailored to the individual needs of patients with kidney failure treated with dialysis. It is hypothesized that, compared to care as usual, the E-HELD intervention will result in a lower impact of the disease on daily life, with distress being the primary study outcome. To take account of the personalized character of the intervention, next to generic outcome measures, a personalized outcome measure will be included to identify, prioritize, and monitor individual problems over time and incorporates the most relevant HRQOL domains of dialysis patients. Also, socio-demographic and clinical characteristics, potential predictors and mediators of treatment success (self-efficacy, self-management, illness cognitions, personality traits, therapeutic relationship), and the cost-effectiveness of the intervention will be assessed.
Trial design {8}
This protocol is a two-arm, parallel-group multicenter randomized controlled superiority trial (RCT), with an allocation ratio of 1:1. Participants will be randomly assigned to one of two groups: (1) the control group that will receive care as usual or (2) the intervention group that will receive 3–4 months of ICBT treatment.
Methods: participants, interventions and outcomes
Study setting {9}
The study will be conducted in two academic hospitals (Radboud university medical center, Nijmegen; Leiden University Medical Center, Leiden), two non-academic hospitals (VieCuri Medical Centre, Venlo; Bernhoven Hospital, Uden), and two dialysis centers (Dialysis Center Groningen, Groningen; Ravenstein Dialysis Centre, Ravenstein). All study sites are located in the Netherlands.
Eligibility criteria {10}
Inclusion and exclusion criteria
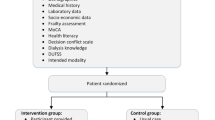
See Fig. 1 for an overview of the participant timeline. The inclusion criteria are age of 18 years and older, being treated for kidney failure with hemodialysis or peritoneal dialysis for at least 3 months, and being fluent in Dutch language. Patients will be excluded when they have serious comorbid physical (life expectancy <12 months) or psychiatric conditions (i.e., DSM diagnosis), recent major stressful life events unrelated to kidney failure, and cognitive problems that would interfere with completing the questionnaires or participating in the ICBT treatment. In addition, patients will be excluded when they have a scheduled kidney transplantation within the upcoming 12 months, when they are currently receiving psychological treatment, and when they do not have access to a computer or Internet.
Screening of adjustment problems
Patients who meet the in- and exclusion criteria are invited to participate in the study and complete the screening and baseline questionnaires. If the screening results show adjustment problems, patients are invited to participate in the RCT. Adjustment problems are defined by physical problems, limitations in daily life, and distress. The choice for the included adjustment problems is based on a previous study on patient priorities conducted by our group [15]. See Table 1 for extended information on the study outcomes and their assessment points.
Physical problems
Physical problems include pain, fatigue, and itch. Pain is measured by the subscale pain of the RAND Short Form-36 Health Status Inventory (RAND SF-36) [47]. Fatigue is measured by the Shortened Fatigue Questionnaire (SFQ) [49], which is a 4-item shortened version of the Checklist Individual Strength (CIS) [49]. Itch is measured by the Impact of Chronic Skin Disease on Daily Life (ISDL) [50].
Limitations in daily life
Limitations in daily life include limitations in daily and social activities. Both are measured by subscales of the RAND SF-36 [47]—limitations in daily activities by the subscale role limitations due to physical problems and limitations in social activities by the subscale social functioning.
Distress
Distress will be measured by a combination of depression and anxiety using the Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS) [45]. This 16-item questionnaire combines the Patient Health Questionnaire depression scale (PHQ-9) [64] and the Generalized Anxiety Disorder 7-item Scale (GAD-7) [65] as a composite measure of depression and anxiety. Using this composite score, distress can be measured with one single outcome measure without needing to specify between anxiety and depression. Higher scores indicate higher levels of distress.
Personal Profile Chart
Patients’ scores on the screening questionnaires are visualized in a Personal Profile Chart, using traffic light colors (i.e., red, orange, green). See Fig. 2 for an illustration of the Personal Profile Chart and Table 2 for an overview of the cut off points that were used to indicate red, orange, and green scores.
For the domains physical problems and limitations in daily life, percentile scores from a comparable dialysis population are used to formulate cut off points. The percentile scores are based on a sample of 172 Dutch dialysis patients [66]. Scores of the 25% of patients scoring worst on the screening measure are visualized in red and indicate frequent problems. Scores between the top 25 and 50% are visualized in orange and indicate occasional problems. Scores below the top 50% are visualized in green and indicates no or rare problems.
For distress, existing generic cut off points for severe, moderate, and mild problems are used to determine red, orange, and green scores [64, 65]. Generic cut off scores were used since distress scores of this sample were comparable to that of the general population [66].
When a patient’s Personal Profile Chart shows minimally 2 orange domains and/or 1 red domain, the patient is considered eligible for participation in the RCT. Patients who have 1 or 2 red domains on emotional problems—indicating severe symptoms of depression or anxiety—will be excluded and referred to their GP. For more information on the development of the Personal Profile Chart, see the development and treatment protocol of the e-health care pathway for the CKD and dialysis population [67].
Who will take informed consent? {26a}
During consultation or during dialysis, the nephrologist or (research) nurse will inform eligible patients about the study, answer questions, and invite patients to participate in the study. Patients will receive the patient information form, a folder about the study, and the informed consent form. Patients consenting in participation will hand in their signed informed consent form for the screening questionnaires at the hospital/dialysis center or return them by mail using the pre-stamped return envelope. After completing the screening questionnaires, patients who show adjustment problems—as indicated by the screening questionnaires—are invited to participate in the RCT. To participate in this part of the study, patients sign another informed consent form and return it by mail (see Fig. 1 for the participant timeline).
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Not applicable, this trial does not have biological specimens.
Interventions
Explanation for the choice of comparators {6b}
Participants will be randomly assigned to one of the two groups: (1) the control group that will receive care as usual or (2) the intervention group that will receive 3–4 months of ICBT treatment in addition to usual care. In the Netherlands, usual care for dialysis patients is based on a multidisciplinary approach by a team consisting of nephrologists, dialysis nurses, and specialized social workers that uses complaints and disease-specific PROMS (patient-reported outcome measures) as a base for guidance for medical and psychosocial problems experienced by individual patients. Specialized social workers have to work according to the Dutch Quality Standard for social workers on a dialysis unit. When deemed necessary, additional consultation by a medical psychologist or psychiatrist is routinely available in all Dutch dialysis centers. More information on the Dutch guidelines on renal replacement therapy can be found at https://www.nefrovisie.nl/richtlijnen-indicatoren/.
Intervention description {11a}
Procedure E-HELD intervention
Participants allocated to the intervention condition will receive tailored ICBT. The ICBT intervention is guided by a trained psychologist, referred to as the “e-Coach.” All e-Coaches participating in this study have a master’s degree in health or clinical psychology and are supervised by a senior clinical psychologist with post-academic training in CBT. Treatment starts with a face-to-face intake meeting with the e-Coach. When a face-to-face meeting is not possible, the e-Coach will schedule a digital, videoconferencing meeting instead. During the intake, the patient will receive feedback on the screening results using the Personal Profile Chart in which the results are visualized. Based on the discussion of patient’s results, the patient and the e-Coach will determine patient’s top priorities for improvement and formulate treatment goals. Subsequently, the e-Coach will tailor the intervention so that it meets the patient’s needs. In addition, the e-Coach will explain the online environment and explores the patient’s wishes with regard to the intensity and the form of the treatment (e.g., the amount of exercises, a wish for additional phone calls, or a preference for reading texts or practical exercises). Subsequently, the patient will work on the homework assignments for 3 to 4 months.
Characteristics E-HELD intervention
The E-HELD intervention includes modules on coping with fatigue, itch, pain, physical disabilities, negative mood, social relations, and lifestyle. In consultation between patient and e-Coach, one or two modules will be chosen based on the screening results and patient’s priorities. Consequently, every patient’s treatment has a unique character, especially since within each module different exercises can be chosen. Exercises include several homework assignments such as reading psychoeducational texts and completing personal assignments (e.g., keeping a diary of daily activities) and exercises (e.g., relaxation). Using a message box in the online environment, the e-Coach will provide weekly personalized feedback on the exercises and send motivational messages. The patient can respond to the e-Coach’s messages or ask questions via the same message box. After 3 to 4 months, the e-Coach will contact the patient for an end-of-treatment consultation to end treatment with a final module on setting long-term goals and preventing relapse.
All modules consist of evidence-based cognitive-behavioral techniques that target risk and resilience factors and were developed from standardized CBT protocols of patients with various chronic somatic conditions [68,69,70]. Studies on psoriasis and rheumatoid arthritis using this intervention showed promising effects on improved physical functioning and a reduced impact of disease on daily life [70, 71]. For the current study, we tailored the intervention to the needs of dialysis patients based on the results of a previous study on patient priorities [15]. Additionally, we adjusted the itch module making it suitable for dialysis patients and added lifestyle modules to support patients in adherence to medication, physical exercise, diet restrictions, and to quit smoking. For more information on the development of the intervention for the CKD and dialysis population, please see the development and treatment protocol [67].
Criteria for discontinuing or modifying allocated interventions {11b}
When a patient’s health significantly worsens over the course of the study, the e-Coach will discuss together with the patient to either discontinue treatment, set the treatment on hold with the possibility to continue in the future, or continue treatment in an adjusted manner (e.g., lower the amount of assignments).
Strategies to improve adherence to interventions {11c}
We will regularly ask patients to their needs and wishes in order to optimize the feasibility and the tailored character of the treatment, making the treatment personally relevant for patients. To track adherence, the e-Coach will monitor completed assignments, modules, and the amount of chat messages.
Relevant concomitant care permitted or prohibited during the trial {11d}
Not applicable. Usual care will be continued during the course of the trial.
Provisions for post-trial care {30}
This study does not provide post-trial care.
Outcomes {12}
At baseline, information on socio-demographic and clinical characteristics (age, sex, education level, employment and marital status, dialysis type and duration, comorbidity, and current or previous psychological treatment) will be collected. The Charlson Comorbidity Index [72]—a measure for comorbid conditions with weighted scores for the condition—will be calculated based on patients’ medical records. In addition, we will assess computer and Internet use and treatment expectancies regarding the ICBT treatment by means of self-report. For extended information on the study outcomes and the assessment moments, see Table 1.
Primary outcome: distress
The primary outcome is distress, as measured by a combination of anxiety and depression using the Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS) [45]. The 16-item PHQ-ADS combines the PHQ-9 [64] and the GAD-7 [65] as a composite measure of depression and anxiety. Higher scores indicate higher levels of depression and anxiety symptoms. Distress will be assessed at baseline, 6 months follow-up (i.e., post-treatment for the intervention group), and 12 months follow-up (i.e., 6 months post-treatment for the intervention group).
Secondary outcomes
All secondary outcomes will be assessed at baseline, 6 months follow-up, and 12 months follow-up (Table 1).
Personalized outcome assessment
The Personalized Priority and Progress Questionnaire (PPPQ) [46] is a personalized instrument to identify, prioritize, and monitor individual problems over time. The baseline measurement consists of 8 items on several domains of functioning that have proven relevant for dialysis patients in a previous study in the Netherlands, including physical health (e.g., fatigue, pain, itch), mental health (e.g., anxiety, depression), social functioning (e.g., dependence on others), and daily activities (e.g., work, hobbies) [15]. In addition, the PPPQ includes 1 item on priorities: patients are asked to select the domains they prioritize for improvement by making a top 3. The follow-up measurement assesses the amount of progress in the areas of functioning over the past 6 months (8 items). Additionally, patients indicate on which of these areas they actively worked in the previous months (1 item). Higher scores on the follow-up measurement indicate improved functioning.
Domains of functioning
Health-related quality of life
The RAND Short Form-36 Health Status Inventory (RAND SF-36) [47] will be used to measure health-related quality of life (HRQOL). The RAND SF-36 contains 36 items that can be summarized into two summary scales: the physical component score (PCS) and the mental component score (MCS). PCS consists of the subscales physical functioning, role limitations due to physical problems, pain, and general health. MCS includes the subscales emotional wellbeing, role limitations due to emotional problems, social functioning, and vitality. Higher scores indicate a better HRQOL. The Hays norm-based scoring algorithm will be applied to transform raw scores into T-scores (M = 50 ± 10 in the general population) [47].
Symptom burden
The Dialysis Symptom Index (DSI) [48] assesses physical and emotional symptom burden in patients receiving dialysis. The DSI includes 30 items on the presence of symptoms. When present, symptom severity is rated on a 5-point Likert scale. Higher scores indicate higher symptom severity.
Fatigue
The subjctive experience of fatigue subscale of the Checklist Individual Strength (CIS) [49] will be used to assess fatigue. This subscale contains 8 items, with higher scores indicating more fatigue.
Itch
The itch subscale of the Impact of Chronic Skin Disease on Daily Life (ISDL) [50] will be used to assess itch. This subscale contains 4 items including an 11-point VAS (0 = no itch, 10 = worst itch ever). Higher scores indicate more itch.
Social functioning
Social functioning will be assessed by the subscales perceived support (5 items), actual support (3 items), and mutual visits (2 items) of the Inventory for Social Resilience [51]. Higher scores indicate better social functioning.
Lifestyle behavior
Relevant lifestyle behaviors for dialysis patients will be assessed using 8 self-constructed items. Items on diet, fluid restrictions, medication adherence, and exercise are answered on a 4-point Likert scale (1 = always, 4 = never). An example item is “I take my medication as prescribed.” Smoking behavior and weight are examined by yes/no questions (“I smoke,” “I have a healthy weight”). In addition, patients note their height and weight for BMI calculations.
Other outcomes
All other outcomes will be assessed at baseline, 6 months follow-up, and 12 months follow-up (Table 1).
Psychological parameters
Self-efficacy
Self-efficacy will be measured by the Self-Efficacy for Managing Chronic Disease 6-ltem Scale (SEMCD-6) [52]. Higher scores indicate better self-efficacy.
Self-management
The Partners in Health Scale (PiH) [53] assesses chronic condition self-management knowledge and behaviors. The version of the PiH used in the study is adjusted to CKD and consists of 13 items. In this version, the illness is referred to as “kidney disease” and includes an additional item on dealing with the emotional consequences of having a kidney disease. Higher scores indicate better self-management.
Illness cognitions
Illness cognitions are measured by the Illness Cognition Questionnaire (ICQ) [54]. Illness cognitions reflect ways of reevaluating the aversive character of a chronic disease. The 18-item ICQ includes three generic illness cognitions: helplessness (emphasizing the aversive meaning of the disease), acceptance (diminishing the aversive meaning by learning how to cope with the disease), and perceived benefits (adding positive meaning to the disease, e.g., personal growth). Higher scores reflect higher levels of the illness cognitions.
Optimism
The Life Orientation Test – Revised (LOT-R) [55] is used to assess optimism. The LOT-R includes 10 items. Higher scores indicate more optimism.
Extraversion and neuroticism
The subscales extraversion and neuroticism of the Dutch version [57] of the Eysenck Personality Questionnaire – Revised Short Scale (EPQ-RSS) [56] will be used to measure both personality traits. Both subscales include 12 items, with higher scores indicating more extraversion and neuroticism.
Therapeutic relationship
The Internet-specific Therapeutic Relationship Questionnaire (ITRQ) [58] measures the Internet-specific aspects of the therapeutic relationship during Internet-based interventions. The ITRQ includes two subscales: Internet-specific time and attention (i.e., time lag aspects in communication and the sufficiency of the therapist’s attention), and Internet-specific reflection and comfort (i.e., sharing information with the therapist and the treatment environment being a patient’s home). The ITRQ includes 8 items, with higher scores indicating a better therapeutic relationship. In contrast to the other measures, the ITRQ will be completed only by patients allocated to the intervention condition. Assessment will take place at post-treatment.
Cost-effectiveness
Health-related quality of life for economic evaluations
The Five-level EQ-5D (EQ-5D-5L) [59] measures HRQOL on five dimensions of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Scores of this 5-item questionnaire can be translated into quality-adjusted life years (QALYs) for health technology assessments and economic evaluations [60].
Productivity losses and medical costs
Two questionnaires of the Institute for Medical Technology Assessment (iMTA) will be used for the measurement of costs in economic evaluations: the iMTA Productivity Cost Questionnaire (iPCQ) [61] and the iMTA Medical Consumption Questionnaire (iMCQ) [62]. The iPCQ assesses productivity losses and includes 12 items which will be presented based on the patient’s answers. The iMCQ is a generic instrument for measuring medical costs. The iMCQ includes questions on the type and frequency of treatment, contact with health care providers, hospitalization, and medication use. The iMCQ includes 31 items—items will be presented based on the answers on the previous items.
Capability wellbeing
The ICEpop CAPability measure for Adults (ICECAP-A) [63] is a measure for capability wellbeing for the general adult population for use in economic evaluations. It includes 5 items on stability, attachment, autonomy, achievement, and enjoyment.
Participant timeline {13}
See Fig. 1 for the SPIRIT figure with the participant timeline.
Sample size {14}
The power calculation is based on the difference in change of distress from baseline to follow-up between the intervention and the control group. The effect size was based on previous RCTs on (Internet-based) CBT treatment in dialysis patients with a focus on distress-related outcomes (e.g., depression) indicating medium effects [73, 74]. In the absence of studies focusing on a broader spectrum of adjustment problems such as the current study, we used these studies for an estimation of the effect size despite the fact that they focused solely on distress. When using an alpha level of .05 and an effect size of 0.50 (Cohen’s d moderate effect), and taking account of potential dropouts, effects for 2 groups of 65 patients yield a power of at least .80.
Recruitment {15}
Patient recruitment will take place in several centers in the Netherlands: Radboud university medical center, Nijmegen; Leiden University Medical Center, Leiden; VieCuri Medical Centre, Venlo; Bernhoven Hospital, Uden; Dialysis Center Groningen, Groningen; and Ravenstein Dialysis Centre, Ravenstein. Patients will be recruited from February 2019 until November 2021. Depending on the rate of inclusion, we will contact other centers as well. After screening on the inclusion and exclusion criteria, nephrologists or (research) nurses recruit eligible patients during consultation or dialysis treatment. After 3–6 months, screening will be repeated to include newly started dialysis patients.
Assignment of interventions: allocation
Sequence generation {16a}
Eligible patients (i.e., signs of adjustment problems as indicated by the screening questionnaires) will be randomized to the intervention or control group on a 1:1 allocation ratio using computer-generated random numbers. To reduce predictability of a random sequence, a blocking procedure will be used including random block sizes of 4 and 6. In addition, randomization will be stratified by sex, dialysis type, and study site.
Concealment mechanism {16b}
Randomization will be performed by an independent data manager who is not involved in the study.
The document describing the allocation sequence is not accessible for researchers involved in the study. Only the data manager has access to this document.
Implementation {16c}
The data manager will generate the allocation sequence. The data manager will inform the researcher of the allocated group to which the participant is randomized. Subsequently, the researcher will inform the participant by telephone and will explain the next steps of the study.
Assignment of interventions: blinding
Who will be blinded {17a}
This is an open-label study. Due to the nature of the intervention (ICBT) and the control condition (care as usual), blinding is not required or possible.
Procedure for unblinding if needed {17b}
Blinding will not occur (see previous point).
Data collection and management
Plans for assessment and collection of outcomes {18a}
Participants complete a set of online questionnaires at home. The first assessment is at baseline, the second at 6-month follow-up (i.e., post-treatment for the intervention group) being the primary outcome measure for the analyses, and the third and last assessment is at 12-month follow-up (i.e., 6 months post-treatment for the intervention group). See Table 1 for an overview of the specific questionnaires for each assessment and Fig. 1 for the participant timeline.
Plans to promote participant retention and complete follow-up {18b}
The assessments consist of online questionnaires that participants can complete at home, at a moment that suits them. To split up completion time, participants can pause the questionnaires at any moment and continue completion within the next 14 days. To promote participant retention in the intervention condition, we will tailor the treatment to the participant’s wishes, needs, and abilities. In addition, the e-Coaches will regularly ask patients for feedback to make adjustments if necessary. This could mean, for example, that for a given patient treatment will include less psychoeducational texts, more practical exercises, and additional phone calls with the e-Coach therapist.
Data management {19}
Participant identification codes will be used to link data to participants. The file containing the linking between participant numbers and personal data (e.g., name, date of birth) will be managed by the researchers and will be locked for access by others. All the information collected in this trial will be stored in a secured locker. Electronic data will be stored on the central server of the Health, Medical, and Neuropsychology unit of Leiden University, which will be backed up daily. Collected data (personal data from informed consents and medical files, self-report data from questionnaires) will be stored for a period of 15 years.
Confidentiality {27}
Participant identification codes will be used to link data to participants. The file containing the linking between participant numbers and personal data (e.g., name, date of birth) will be managed by the researchers and will be locked for access by others.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable, this trial does not have biological specimens.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Descriptive statistics (means, standard deviations, medians, and interquartile ranges) of relevant variables will be calculated. In case of non-normally distributed variables, transformations will be applied to allow parametric statistics. Baseline data of the intervention and control condition will be compared to assess similarity. Additionally, several t-tests will be performed on the 6-month follow-up data, both on the primary and secondary outcomes.
To examine differences in the primary outcome measure change in distress between patients in the intervention and control condition, a linear mixed-model analysis of variance will be conducted. Linear mixed-effects modeling has superior qualities with regard to missing values and makes use of all available data, making this a full-intention-to-treat analysis. Models will be fitted with full information maximum likelihood estimation. Between-group effects at post-treatment and follow-up will be analyzed with baseline scores of dependent variables as covariates. Time will be operationalized as a continuous variable, because post-treatment assessment will vary across participants as a result of different intervention length. Fixed linear effects of time and condition will be included and random effects of intercept.
Similar mixed-model analyses of variance will be conducted to examine differences in change in the scores on the secondary outcome measurements between the two conditions. In addition, correlational and regression analyses will be performed to assess the potential role of demographic and clinical characteristics and psychological parameters (e.g., treatment expectancies, therapeutic relationship) on the effects of the ICBT intervention.
Interim analyses {21b}
As part of master’s degree education offered at Leiden University, students write their thesis within the context of ongoing research projects and are, for example, involved in the data collection of these projects. We want to offer students the possibility of doing an analysis on the baseline data in a small subsample of participants.
Methods for additional analyses (e.g., subgroup analyses) {20b}
There are no subgroup analyses planned.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
The primary outcome will be assessed using an intention-to-treat analysis. In addition, we will repeat the analyses with a per protocol analysis using a sample of patients who fully completed the intervention.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
The pseudonymized dataset and syntaxes can be made available by the corresponding author upon reasonable request and in accordance with the transfer guidelines of Leiden University.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The study is centrally organized and coordinated by JT, AWME, and HvM from Leiden University. The principal investigators of the participating centers (HWvH, AG, MMHH, YRPdW, RW, AAMJH) are responsible for the coordination of the study within their centers.
Composition of the data monitoring committee, its role and reporting structure {21a}
Monitoring of the conduct of the study will be performed by an independent data manager who is not involved in the study. The data manager will check whether the data that have been entered in the database match the source data by random sampling and guarantee the anonymity of the personal data. As per the protocol that has been set up for data management in the Health, Medical and Neuropsychology unit of Leiden University, the data manager will be responsible for checking the data during the recruitment period as well as the final check of the data at the end of the study.
Adverse event reporting and harms {22}
All adverse events (AEs) reported spontaneously by the participant or observed by the investigator or staff will be recorded. Serious adverse events (SAEs) will be reported by the study staff to the accredited METC that approved the protocol, within 7 days of first knowledge for SAEs that result in death or are life threatening followed by a period of maximum 8 days to complete the initial preliminary report. All other SAEs will be reported within a period of maximum 15 days after the sponsor has first knowledge of the serious adverse events. Reporting suspected unexpected serious adverse reactions (SUSARs) is not applicable, since this study does not include a medicinal product.
Frequency and plans for auditing trial conduct {23}
The study staff will submit a summary of the progress of the trial to the accredited METC once a year. Information will be provided on the date of inclusion of the first subject, numbers of subjects included, and numbers of subjects that have completed the trial, serious adverse events/ serious adverse reactions, other problems, and amendments.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
All protocol amendments will be approved of by the METC prior to implementation. If relevant, participants will be informed of protocol modifications.
Dissemination plans {31a}
Results will be reported in national and international conferences and submitted for publication in peer-reviewed journals.
Discussion
ICBT is increasingly used in patients with chronic somatic conditions to treat psychological problems such as anxiety and depression and to support patients in adjustment to illness, treatment adherence, and self-management. Results from meta-analyses and systematic reviews suggest promising effects for both psychosocial and disease-specific outcomes of ICBT in several chronic somatic conditions, especially when guided and tailored to specific patient needs [37,38,39]. However, when it comes to the dialysis population, almost no adequately powered studies and no tailored studies are available.
The few studies that have focused on dialysis patients found improvements in anxiety, depression, and HRQOL, but found mixed results on the experienced burden of kidney disease on daily life and no effects on disability [42, 43]. In terms of the feasibility of the ICBT interventions, challenges were found with regard to computer literacy. Nonetheless, positive results were found with regard to the acceptability of the treatment [42, 44]. Since these results were derived from underpowered and/or non-controlled studies, more research is needed to evaluate the effectiveness of ICBT in dialysis patients.
The current study will assess the effectiveness of a guided ICBT intervention that is specifically developed for dialysis patients and that will be tailored to individual patients’ needs using a multicenter randomized controlled trial. To evaluate the effectiveness of the intervention, we will primarily look at distress, but furthermore take the personalized character of the treatment into account by examining effects by means of a personalized outcome instrument and several secondary HRQOL variables. In addition, the potential role of demographic, clinical, and psychological parameters in determining treatment outcome will be assessed, as well as the cost-effectiveness of the intervention. When the ICBT intervention proves to be effective, the intervention could be implemented in standard care and patients could be offered a personalized treatment supporting them in the problems they prioritize for improvement.
A unique feature of this study is the use of an innovative screening tool to detect patients with an increased risk for adjustment problems such as physical problems, limitations in daily life, and distress. The domains included in the screening tool were based on a previous study of our group in which we evaluated patients’ most prominent problems from a patient perspective [15], to ensure that the screening tool detects what matters most to patients. In addition, the screening tool includes validated screening questionnaires and makes use of disease-specific norm scores, allowing clinicians and patients to compare the individual patient’s scores to other dialysis patients. In previous research, dialysis patients have indicated that they feel reference scores would help to better understand the meaning of their results and indicated to find it highly valuable to receive feedback on their results [9]. To meet these needs, patients’ results will be visualized in a Personal Profile Chart. This chart presents feedback on patients’ results in a way that is easy to understand by using traffic light colors and text boxes with additional explanations [67]. By sending these to all patients who filled out the screening questionnaires, irrespective of whether or not they were eligible for the intervention, patients are provided with feedback on their functioning and are encouraged to discuss this Profile Chart with their nephrologist during a regular consultation.
Another strength of the intervention are the multiple options for tailoring. The ICBT intervention includes modules focusing on issues that patients have indicated to be a priority for improvement as found by a previous study [15], making this intervention a suitable and personally meaningful option for every dialysis patient. Additionally, the intervention will be guided by a therapist, as research showed that therapist-guided interventions yield better results and allows for tailoring of the intervention [19, 40, 75]. At the start of the intervention, the e-Coach uses the patient’s Personal Profile Chart to determine treatment goals together with the patient and, subsequently, tailors the intervention to the patient’s needs. During treatment, the e-Coach continues to tailor the intervention by monitoring whether the form and intensity of the intervention still matches the patient’s preferences and abilities and to make adjustments if necessary.
There are several limitations to consider. As found in previous studies on ICBT in dialysis patients, challenges with computer literacy are likely to occur. This could result in lower inclusion rates due to patients not having access to a computer or Internet. In addition, when patients are enrolled in the study, poor computer skills could lead to drop-out when participating in the study appears to be too difficult. To minimize the risk of drop-out, the therapists who guide the intervention will be highly attentive of these difficulties and will make adjustments if the intervention appears to be too difficult. For example, therapists could choose to communicate via telephone instead of online text messages. Another risk for drop-out is patients’ high disease burden. This risk could be minimized in the same way: regularly monitoring whether ICBT treatment matches the patient’s abilities and making adjustments if necessary (e.g., lowering the intensity).
In conclusion, the present study design will provide insight in the effectiveness of a patient-priorities tailored ICBT intervention in patients with kidney failure who are treated with dialysis. When tailored ICBT proves to be a valuable and effective intervention, the screening procedure and the subsequent ICBT intervention could be implemented in routine care to detect, support, and treat patients struggling with adjustment problems.
Trial status
Recruitment started in February 2019 and will be ongoing till October 2021. The current protocol is version 7 of 29-1-2020.
Availability of data and materials {29}
The dataset used and analyzed in this trial will be made available from the corresponding author upon reasonable request.
Abbreviations
- AEs:
-
Adverse events
- BMI:
-
Body mass index
- CBT:
-
Cognitive-behavioral therapy
- CIS:
-
Checklist Individual Strength
- CKD:
-
Chronic kidney disease
- DSI:
-
Dialysis Symptom Index
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- E-HELD:
-
E-HEealth treatment in Long-term Dialysis
- EPQ-RSS:
-
Eysenck Personality Questionnaire – Revised Short Scale
- EQ-5D-5L:
-
Five-level EQ-5D
- GAD-7:
-
Generalized Anxiety Disorder 7-item Scale
- GFR:
-
Glomerular filtration rate
- GP:
-
General practitioner
- HRQOL:
-
Health-related quality of life
- ICBT:
-
Internet-based cognitive-behavioral therapy
- ICECAP-A:
-
ICEpop CAPability measure for Adults
- ICQ:
-
Illness Cognition Questionnaire
- ISDL:
-
Impact of Chronic Skin Disease on Daily Life
- iMTA:
-
Institute for Medical Technology Assessment
- iMCQ:
-
iMTA Medical Consumption Questionnaire
- iPCQ:
-
iMTA Productivity Cost Questionnaire
- ISR:
-
Inventory for Social Resilience
- ITRQ:
-
Internet-specific Therapeutic Relationship Questionnaire
- LOT-R:
-
Life Orientation Test – Revised
- MCS:
-
Mental component score
- METC:
-
Medical Research Ethics Committee
- METC-LDD:
-
Medical Ethical Committee Leiden-Den Haag-Delft
- PCS:
-
Physical component score
- PiH:
-
Partners in Health Scale
- PHQ-9:
-
Patient Health Questionnaire depression scale
- PHQ-ADS:
-
Patient Health Questionnaire Anxiety and Depression Scale
- PPPQ:
-
Personalized Priority and Progress Questionnaire
- QALYs:
-
Quality-adjusted life-years
- QOL:
-
Quality of life
- RAND SF -36:
-
RAND Short Form-36 Health Status Inventory
- RCT:
-
Randomized controlled trial
- SAE:
-
Serious adverse events
- SEMCD-6:
-
Self-Efficacy for Managing Chronic Disease 6-ltem Scale
- SFQ:
-
Shortened Fatigue Questionnaire
- SUSARs:
-
Suspected unexpected serious adverse reactions
- VAS:
-
Visual analog scale
References
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765.
Saran R, Robinson B, Abbott K, Agodoa L, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3):A7.
Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057–64.
Almutary H, Bonner A, Douglas C. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care. 2013;39(3):140–50.
Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99.
Cukor D, Cohen SD, Peterson RA, Kimmel PL. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042–55.
Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(4):623–35.
Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT. Physical symptoms and quality of life in patients on chronic dialysis: results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD). Nephrol Dial Transplant. 1999;14(5):1163–70.
van der Willik EM, Hemmelder MH, Bart HA, van Ittersum FJ, Hoogendijk-van den Akker JM, Bos WJW, et al. Routinely measuring symptom burden and health-related quality of life in dialysis patients: first results from the Dutch registry of patient-reported outcome measures. Clin Kidney J. 2021;14(6):1535–44.
Weisbord SD, Fried LF, Mor MK, Resnick AL, Unruh ML, Palevsky PM, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960–7.
Davison S, Jhangri G, Johnson J. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69(9):1621–5.
Thong MSY, van Dijk S, Noordzij M, Boeschoten EW, Krediet RT, Dekker FW, et al. Symptom clusters in incident dialysis patients: associations with clinical variables and quality of life. Nephrol Dial Transplant. 2008;24(1):225–30.
Anderson NE, Calvert M, Cockwell P, Dutton M, Kyte D. The use of patient-reported outcomes in patients treated with maintenance hemodialysis: a perspective. Am J Kidney Dis. 2019;74(3):399–406.
Benze G, Nauck F, Alt-Epping B, Gianni G, Bauknecht T, Ettl J, et al. PROutine: a feasibility study assessing surveillance of electronic patient reported outcomes and adherence via smartphone app in advanced cancer. Ann Palliat Med. 2019;8(2):104–11.
Tommel J, Evers AWM, van Hamersvelt HW, Jordens R, van Dijk S, Hilbrands LB, et al. “What matters to you?”: the relevance of patient priorities in dialysis care for assessment and clinical practice. Semin Dial. 2022:1–11.
Vandecasteele SJ, Tamura MK. A patient-centered vision of care for ESRD: dialysis as a bridging treatment or as a final destination? J Am Soc Nephrol. 2014;25(8):1647–51.
van Middendorp H, Evers AW. The role of psychological factors in inflammatory rheumatic diseases: from burden to tailored treatment. Best Pract Res Clin Rheumatol. 2016;30(5):932–45.
Evers AW, Gieler U, Hasenbring MI, van Middendorp H. Incorporating biopsychosocial characteristics into personalized healthcare: a clinical approach. Psychother Psychosom. 2014;83(3):148–57.
Johansson R, Sjoberg E, Sjogren M, Johnsson E, Carlbring P, Andersson T, et al. Tailored vs. standardized internet-based cognitive behavior therapy for depression and comorbid symptoms: a randomized controlled trial. PLoS One. 2012;7(5):e36905.
Johansen MA, Berntsen GKR, Schuster T, Henriksen E, Horsch A. Electronic symptom reporting between patient and provider for improved health care service quality: a systematic review of randomized controlled trials. Part 2: Methodological Quality and Effects. J Med Internet Res. 2012;14(5):e126.
Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26(1):17–31.
White CA. Cognitive behavioral principles in managing chronic disease. West J Med. 2001;175(5):338.
Halford J, Brown T. Cognitive–behavioural therapy as an adjunctive treatment in chronic physical illness. Adv Psychiatr Treat. 2009;15(4):306–17.
Mechanic D. The concept of illness behavior. J Chronic Dis. 1962;15(2):189–94.
Lii YC, Tsay SL, Wang TJ. Group intervention to improve quality of life in haemodialysis patients. J Clin Nurs. 2007;16(11c):268–75.
Tsay SL, Lee YC, Lee YC. Effects of an adaptation training programme for patients with end-stage renal disease. J Adv Nurs. 2005;50(1):39–46.
Duarte PS, Miyazaki MC, Blay SL, Sesso R. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int. 2009;76(4):414–21.
Picariello F, Hudson JL, Moss-Morris R, Macdougall IC, Chilcot J. Examining the efficacy of social-psychological interventions for the management of fatigue in end-stage kidney disease (ESKD): a systematic review with meta-analysis. Health Psychol Rev. 2017;11(2):197–216.
Ng CZ, Tang SC, Chan M, Tran BX, Ho CS, Tam WW, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavioral therapy for hemodialysis patients with depression. J Psychosom Res. 2019;126:109834.
Lee M-C, Wu S-FV, Hsieh N-C, Tsai J-M. Self-management programs on eGFR, depression, and quality of life among patients with chronic kidney disease: a meta-analysis. Asian Nurs Res (Korean Soc Nurs Sci). 2016;10(4):255–62.
Nadort E, Schouten RW, Witte SH, Broekman BF, Honig A, Siegert CE, et al. Treatment of current depressive symptoms in dialysis patients: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2020;67:26–34.
Mojtabai R, Olfson M, Sampson NA, Jin R, Druss B, Wang PS, et al. Barriers to mental health treatment: results from the National Comorbidity Survey Replication (NCS-R). Psychol Med. 2011;41(8):1751.
Corrigan PW, Druss BG, Perlick DA. The impact of mental illness stigma on seeking and participating in mental health care. Psychol Sci Public Interest. 2014;15(2):37–70.
Looper KJ, Kirmayer LJ. Perceived stigma in functional somatic syndromes and comparable medical conditions. J Psychosom Res. 2004;57(4):373–8.
Andersson G, Titov N. Advantages and limitations of Internet-based interventions for common mental disorders. World Psychiatry. 2014;13(1):4–11.
Griffiths F, Lindenmeyer A, Powell J, Lowe P, Thorogood M. Why are health care interventions delivered over the internet? A systematic review of the published literature. J Med Internet Res. 2006;8(2):e10.
Cuijpers P, Van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med. 2008;31(2):169–77.
van Beugen S, Ferwerda M, Hoeve D, Rovers MM, Spillekom-van Koulil S, van Middendorp H, et al. Internet-based cognitive behavioral therapy for patients with chronic somatic conditions: a meta-analytic review. J Med Internet Res. 2014;16(3):e88.
Mehta S, Peynenburg VA, Hadjistavropoulos HD. Internet-delivered cognitive behaviour therapy for chronic health conditions: a systematic review and meta-analysis. J Behav Med. 2019;42(2):169–87.
Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):329–42.
Spek V, Cuijpers P, Nyklícek I, Riper H, Keyzer J, Pop V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med. 2007;37(3):319–28.
Chan R, Dear BF, Titov N, Chow J, Suranyi M. Examining internet-delivered cognitive behaviour therapy for patients with chronic kidney disease on haemodialysis: a feasibility open trial. J Psychosom Res. 2016;89:78–84.
Hernandez R, Burrows B, Wilund K, Cohn M, Xu S, Moskowitz JT. Feasibility of an Internet-based positive psychological intervention for hemodialysis patients with symptoms of depression. Soc Work Health Care. 2018;57(10):864–79.
Hudson JL, Moss-Morris R, Game D, Carroll A, McCrone P, Hotopf M, et al. Improving distress in dialysis (iDiD): a feasibility two-arm parallel randomised controlled trial of an online cognitive behavioural therapy intervention with and without therapist-led telephone support for psychological distress in patients undergoing haemodialysis. BMJ Open. 2016;6(4):e011286.
Kroenke K, Wu J, Yu Z, Bair MJ, Kean J, Stump T, et al. Patient health questionnaire anxiety and depression scale: initial validation in three clinical trials. Psychosom Med. 2016;78(6):716–27.
Tommel J, Cardol CK, Evers AW, van Dijk S, van Middendorp H. The Personalized Prioity and Progress Questionnaire (PPPQ): a personalized outcome instrument for use in clincial practice and clinical trials. Manuscript in prep 2021.
Hays RD, Sherbourne CD, Mazel RM. The rand 36-item health survey 1.0. Health Econ. 1993;2(3):217–27.
Weisbord SD, Fried LF, Arnold RM, Rotondi AJ, Fine MJ, Levenson DJ, et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manag. 2004;27(3):226–40.
Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38(5):383–92.
Evers A, Duller P, Van De Kerkhof P, Van der Valk P, De Jong E, Gerritsen M, et al. The Impact of Chronic Skin Disease on Daily Life (ISDL): a generic and dermatology-specific health instrument. Br J Dermatol. 2008;158(1):101–8.
Van Dam-Baggen R, Kraaimaat F. De Inventarisatielijst Sociale Betrokkenheid (ISB): een zelfbeoordelingslijst om sociale steun te meten [The Inventory for Social Support (ISB): A self-report inventory for the measurement of social support]. Gedragstherapie. 1992;25(1):27–46.
Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–62.
Petkov J, Harvey P, Battersby M. The internal consistency and construct validity of the partners in health scale: validation of a patient rated chronic condition self-management measure. Qual Life Res. 2010;19(7):1079–85.
Evers AW, Kraaimaat FW, van Lankveld W, Jongen PJ, Jacobs JW, Bijlsma JW. Beyond unfavorable thinking: the illness cognition questionnaire for chronic diseases. J Consult Clin Psychol. 2001;69(6):1026.
Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67(6):1063.
Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Scales (EPS Adult): Comprising the EPQ-Revised (EPQ-R), EPQ-R Short Scale, Impulsiveness (IVE) Questionnaire. London: Hodder & Stoughton; 1991.
Sanderman R, Arrindell WA, Ranchor AV, Eysenck HJ, Eysenck SBG. Het meten van persoonlijkheid met de Eysenck Personality Questionnaire (EPQ): Een handleiding. Groningen: UMCG/Rijksuniversiteit Groningen; 2012.
Ferwerda M, van Beugen S, van Riel PC, van de Kerkhof PC, de Jong EM, Smit JV, et al. Measuring the therapeutic relationship in internet-based interventions. Psychother Psychosom. 2016;85(1):47–9.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
Versteegh MM, Vermeulen KM, Evers SM, De Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19(4):343–52.
Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart-van RL. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health. 2015;18(6):753–8.
iMTA Productivity and Health Research Group. Manual iMTA Medical Cost Questionnaire (iMCQ). Rotterdam: iMTA, Erasmus University Rotterdam; 2018.
Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res. 2012;21(1):167–76.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Tommel J, Evers AW, van Hamersvelt HW, Jordens R, van Dijk S, Hilbrands LB, et al. Predicting health-related quality of life in dialysis patients: factors related to negative outcome expectancies and social support. Patient Educ Couns. 2021;104(6):1474–80.
Cardol CK, Tommel J, van Middendorp H, Ciere Y, Sont JK, Evers AW, et al. Detecting and treating psychosocial and lifestyle-related difficulties in chronic disease: development and treatment protocol of the E-GOAL eHealth care pathway. Int J Environ Res Public Health. 2021;18(6):3292.
Evers AWM, Kraaimaat FW, Van Riel PLCM, De Jong AJL. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain. 2002;100:141–53.
Evers AW, Duller P, De Jong EM, Otero ME, Verhaak CM, van der Valk PG, et al. Effectiveness of a multidisciplinary itch-coping training programme in adults with atopic dermatitis. Acta Derm Venereol. 2009;89(1):57–63.
van Beugen S, Ferwerda M, Spillekom-van Koulil S, Smit JV, Zeeuwen-Franssen ME, Kroft EB, et al. Tailored therapist-guided internet-based cognitive behavioral treatment for psoriasis: a randomized controlled trial. Psychother Psychosom. 2016;85(5):297–307.
Ferwerda M, van Beugen S, van Middendorp H, Spillekom-van Koulil S, Donders ART, Visser H, et al. A tailored-guided internet-based cognitive-behavioral intervention for patients with rheumatoid arthritis as an adjunct to standard rheumatological care: results of a randomized controlled trial. Pain. 2017;158(5):868–78.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Warmerdam L, van Straten A, Twisk J, Riper H, Cuijpers P. Internet-based treatment for adults with depressive symptoms: randomized controlled trial. J Med Internet Res. 2008;10(4):e44.
Xing L, Chen R, Diao Y, Qian J, You C, Jiang X. Do psychological interventions reduce depression in hemodialysis patients?: a meta-analysis of randomized controlled trials following PRISMA. Medicine. 2016;95(34):e4675.
Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E. Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014;13(3):288–95.
Acknowledgements
We would like to thank the patients for participating in the study. We would also like to thank Katja Cardol for her role in the development of the Personal Profile Chart and adjusting the intervention to the needs of patient with a chronic kidney disease; Milon van Vliet for her work as e-Coach; Yvette Ciere for her role in the development of the Personal Profile Chart; and André Gaasbeek, Marc Hermans, Yvonne de Waal, Ralf Westerhuis, and Daan Hollander for their role as principal investigator of the participating centers.
Funding
This trial was funded by the Dutch Kidney Foundation (SWO 16.07). The funder does not have a role in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
JT is responsible for writing the draft manuscript, supported by HvM and AWME. AWME, HvM, and HWvH are the principal investigators of this trial; they led the protocol development and obtained funding for the grant that supports this study. JT, AWME, HWvH, SvD, NC, LW, LBH, and HvM participated in the design of the study and contributed intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate {24}
The study has been developed in accordance with the Helsinki Declaration. Ethical approval was assigned by the Medical Ethical Committee Leiden-Den Haag-Delft (METC-LDD), with protocol number NL63422.058.17. All participating patients will provide written informed consent.
Consent for publication {32}
This manuscript does not contain personal identifiers or any other personal data from patients. All non-identifiable data are available, on request, from the corresponding author.
Competing interests {28}
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tommel, J., Evers, A.W.M., van Hamersvelt, H.W. et al. E-HEalth treatment in Long-term Dialysis (E-HELD): study protocol for a multicenter randomized controlled trial evaluating personalized Internet-based cognitive-behavioral therapy in dialysis patients. Trials 23, 477 (2022). https://doi.org/10.1186/s13063-022-06392-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06392-9