Abstract
Background
Estrogen promotes breast cancer development and progression mainly through estrogen receptor (ER). However, blockage of estrogen production or action prevents development of and suppresses progression of ER-negative breast cancers. How estrogen promotes ER-negative breast cancer development and progression is poorly understood. We previously discovered that deletion of cell cycle inhibitors p16Ink4a (p16) or p18Ink4c (p18) is required for development of Brca1-deficient basal-like mammary tumors, and that mice lacking p18 develop luminal-type mammary tumors.
Methods
A genetic model system with three mouse strains, one that develops ER-positive mammary tumors (p18 single deletion) and the others that develop ER-negative tumors (p16;Brca1 and p18;Brca1 compound deletion), human BRCA1 mutant breast cancer patient-derived xenografts, and human BRCA1-deficient and BRCA1-proficient breast cancer cells were used to determine the role of estrogen in activating epithelial-mesenchymal transition (EMT), stimulating cell proliferation, and promoting ER-negative mammary tumor initiation and metastasis.
Results
Estrogen stimulated the proliferation and tumor-initiating potential of both ER-positive Brca1-proficient and ER-negative Brca1-deficient tumor cells. Estrogen activated EMT in a subset of Brca1-deficient mammary tumor cells that maintained epithelial features, and enhanced the number of cancer stem cells, promoting tumor progression and metastasis. Estrogen activated EMT independent of ER in Brca1-deficient, but not Brca1-proficient, tumor cells. Estrogen activated the AKT pathway in BRCA1-deficient tumor cells independent of ER, and pharmaceutical inhibition of AKT activity suppressed EMT and cell proliferation preventing BRCA1 deficient tumor progression.
Conclusions
This study reveals for the first time that estrogen promotes BRCA1-deficient tumor initiation and progression by stimulation of cell proliferation and activation of EMT, which are dependent on AKT activation and independent of ER.
Similar content being viewed by others
Background
Estrogen plays a critical role in promoting breast cancer development and progression in addition to normal breast development [1, 2]. Although estrogen acts mainly through the estrogen receptor (ER), ovariectomy, blockage of estrogen action, or inhibition of estrogen synthesis can prevent the development of and suppress the progression of ER-negative breast cancers [3, 4]. In addition to stimulation of proliferation and induction of DNA damage in both ER-positive and ER-negative cells [1, 2], estrogen activates an epithelial-mesenchymal transition (EMT) program in ER-positive breast cancer cells to promote their stemness and invasiveness in vitro [5, 6]. Notably, it has recently been reported that estrogen promotes the number and function of ER-negative breast cancer stem cells (CSCs) through paracrine signaling produced in ER-positive cells in response to estrogen [7, 8]. Whether and how estrogen promotes ER-negative breast cancer development and progression are poorly understood.
Breast cancer comprises, among others, three main subtypes: human epidermal growth factor receptor 2 (HER2)-positive, ER-positive luminal, and ER-negative basal-like breast cancers (BLBCs) [9]. Luminal-type tumors respond to hormone therapy. BLBCs are poorly differentiated and the most lethal, which is partly due to their enrichment of CSCs that are thought to drive clinical relapse and metastasis [10, 11]. The CSCs can be generated from luminal tumor cells by the EMT program [12, 13]. BLBCs may originate from luminal progenitors and contain a number of distinct cell types including cells that express luminal, basal, and mesenchymal biomarkers [14,15,16,17,18,19]. More than half of BLBCs are associated with functional loss of BRCA1 caused by germline or somatic mutation, or promoter methylation [9, 20, 21]. We and others have demonstrated that depletion of Brca1 in mice activates EMT and induces highly heterogeneous BLBCs [18, 22, 23]. Most importantly, only a part of the cells in both human and mouse BRCA1-deficient tumors exhibit mesenchymal features [18, 22,23,24], suggesting that a subset of BRCA1-deficient tumor cells have undergone EMT. Whether and how estrogen activates EMT in BRCA1-deficient, ER-negative basal-like tumor cells to promote their tumor initiation and progression remain elusive.
The phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway regulates cell proliferation, survival, metabolism, EMT, and stem cell fate [25,26,27], and is aberrantly activated in 77% of breast cancers [9], including BRCA1-deficient disease [28]. BRCA1 deficiency activates the PI3K/AKT pathway in immortalized fibroblasts and tumor cells by accumulating nuclear AKT [29]. Estrogen activates the PI3K/AKT pathway in both an ER-dependent and ER-independent manner [30, 31]. Estrogen also promotes the survival of Brca1-deficient tumor cells and mammary epithelial cells (MECs) [30]. Inhibition of the PI3K/AKT pathway reduces proliferation of Brca1-deficient mouse embryonic fibroblasts (MEFs) suppressing the growth of tumors generated by Brca1-deficient MEFs [28]. Whether estrogen promotes EMT and proliferation of ER-negative BRCA1-deficient tumor cells through activation of the PI3K/AKT pathway remains elusive.
The RB family of proteins (RB, p107, p130) that are phosphorylated and inactivated by CDK4 and CDK6 (CDK4/6), control the G1-to-S transition of the cell cycle. CDK4/6 are inhibited by inhibitors of CDK4/6 (INK4) including p16INK4A (p16) and p18INK4C (p18) [32]. Inactivation of the INK4-CDK4/6-RB pathway is a common event in breast cancers [9, 32]. p16 is inactivated in ~ 30% of and p18 expression is frequently reduced in human breast cancers [9, 32]. RB is a major target for genomic disruption in BRCA1 mutant human breast cancers, and most BRCA1-deficient BLBCs carry a dysfunctional INK4-CDK4/6-RB pathway [9, 33, 34]. All widely used BRCA1 mutant breast cancer cell lines have deletions in either RB or p16 [35, 36], reflecting the importance of inactivation of the INK4-CDK4/6-RB pathway in the proliferation of BRCA1-deficient tumor cells. We and others have reported that mice lacking p18 or Rb and p107 develop luminal-type mammary tumors [37, 38], suggesting a role of the RB pathway in controlling luminal tumorigenesis. BRCA1 deficiency in human and mouse MECs activates INK4-CDK4/6-RB pathway, inducing premature senescence [19, 39, 40]. We demonstrated that deletion of either p16 or p18 in mice rescues the premature senescence of MECs caused by Brca1 deficiency, and that p16;Brca1 and p18;Brca1 double-mutant mice develop BLBCs with EMT features [19, 23, 39]. These mutant mice provide unique mouse models to study the role of Brca1 in the suppression of EMT and basal-like mammary tumorigenesis.
In this report, we used p16;Brca1 and p18;Brca1 double-mutant mammary tumors and human BRCA1 mutant breast cancer patient-derived xenografts (PDX) to determine the function and mechanism of estrogen in promoting ER-negative BRCA1-deficient tumor initiation and progression. We demonstrate that estrogen promotes BRCA1-deficient tumor initiation and progression by stimulation of cell proliferation and activation of EMT, which are dependent on AKT activation, but independent of ER.
Methods
Mice, histopathologic analysis, and immunostaining
The generation of p18−/−, p18−/−;Brca1MGKO (p18−/−; Brca1f/f;MMTV-Cre or p18−/−;Brca1f/−;MMTV-Cre) and p16−/−;Brca1MGKO (p16−/−;Brca1f/f;MMTV-Cre or p16−/−; Brca1f/−;MMTV-Cre) mice has been described previously [23, 37, 39]. The Institutional Animal Care and Use Committee at the University of Miami approved all animal procedures. Histopathologic analysis and immunohistochemical analysis (IHC) were performed as described previously [19, 23, 37]. The primary antibodies used were Ck14 (Thermal Scientific), ERα (Santa Cruz), p-Akt (Ser473), p-4E-bp1 (Thr37/46), vimentin (Vim), E-cadherin (E-cad), p-Fra1 (Ser265) (Cell Signaling), Ki67 and fibronectin (Fn) (Abcam). Immunocomplexes were detected using the Vectastain ABC DAB kit according to the manufacturer’s instructions (Vector Laboratories) or by using Alexa Fluor 488-conjugated or Alexa Fluor 594-conjugated secondary antibodies (Biolegend). The positive results of IHC were quantified by the H score, as previously described [41].
Mammary and tumor cell preparation, fluorescence-activated cell sorting (FACS) analysis, cell sorting, and tumorsphere formation assay
Mammary glands and tumors were dissected from female mice and cell suspensions were prepared as previously described [19, 23, 37]. For surface marker analysis; mammary and tumor cells were isolated, stained, and analyzed as previously described [23, 37]. Briefly, cells were stained with anti-CD24-PE (BD Pharmingen), anti-CD29-FITC (Biolegend, San Diego, CA, USA), biotinylated and allophycocyanin (APC)-conjugated CD45, CD31 and TER119 antibodies (BD Pharmingen), and Violet dye (Dead Cell Stain, ThermoFisher Scientific). For intracellular staining of Vim, 1 × 106 cells were fixed with 4% paraformaldehyde and permeabilized with 90% methanol. Anti-Vim was added as primary antibody and Alexa Fluor 594 anti-rabbit (Invitrogen) as secondary antibody. For intracellular staining of ERα, cells were fixed and permeabilized with the Cytofix/Cytoperm fixation/permeabilization kit (BD Pharmingen). ERα staining was performed according to the manufacturer’s instructions. For bromodeoxyuridine (BrdU) incorporation, tumor cells were labeled with 10 μM BrdU (Sigma) for 1.5 h, fixed with 75% ethanol, and permeabilized with 2 M HCl. The cells were then stained with fluorescein isothiocyanate (FITC)-conjugated anti-BrdU (Cell Signaling) and propidium iodide (PI) and analyzed by flow cytometry. FACS was performed using the LSR-Fortessa machine (BD Pharmingen). Data analysis was performed using Kaluza software (Beckman Coulter). To isolate tumor cells depleted of hematopoietic and endothelial cells, we stained mammary tumor cells with biotinylated and APC-conjugated CD45, CD31 and TER119 antibodies and Violet dye. Lin− (CD45−, CD31−, TER119−) living (Violet dye negative) cells were sorted on a BD FACS SORP Aria-IIu machine. For tumorsphere formation assay, 30,000 p18−/−; Brca1MGKO mammary tumor cells were plated in triplicate ultra-low attachment plates with or without addition of 17β-estradiol (E2, Sigma) in serum-free DMEM-F12 supplemented with B27, epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF) as described [23, 37]. The number and size of tumorspheres formed were calculated after 7 days.
Cell culture, treatment, cell viability assay and western blot analysis
MCF7 and SUM149 cells were cultured per American Type Culture Collection (ATCC) recommendations. Primary murine mammary tumor cells were cultured in phenol-free DMEM/F12 (Gibco), with 10% charcoal-stripped FBS (Gibco), 10 μg/ml insulin, and 10 ng/ml EGF. For treatment of estrogen, AZD5363, and 4OHT, tumor cells were cultured in 10% charcoal-stripped FBS in the presence of E2, AZD5363 (ApexBio Technology), 4OHT (Sigma), or dimethyl sulfoxide (DMSO) for the indicated times and then collected for further analysis. To determine cell viability, 50,000 cells were plated in 24-well plates and treated with DMSO or drugs at the indicated concentrations for 5 days. Viable cell numbers were determined on day 1, day 3, and day 5 by an automatic cell counter (Bio-rad) with trypan blue exclusion. For western blot, tissue and cell lysates were prepared as previously reported [19, 23, 37]. Primary antibodies used are as follows: HSP90 (Santa Cruz), Gapdh (Ambion), ERα (Santa cruz), Brca1, p-Akt (Ser473), p-4E-bp1 (Thr37/46), p-mTor (Ser2248), p-Gsk3β (Ser9), E-cad, Vim, Snail, Slug, p-Fra1 (Ser 265), p-Rb (ser780) (Cell Signaling) and Fn (Abcam).
Transplantation, analysis of metastasis, and tumor treatment
For mammary tumor cell transplantation, cells were suspended in a 50% solution of Matrigel (BD) and then inoculated into the left and/or right inguinal mammary fat pads (MFPs) of 6–8-week-old female NSG mice (Jackson Laboratory) respectively. At 6 or 7 weeks after transplantation, animals were euthanized and mammary tumors were dissected for histopathological, immunohistochemical, and biochemical analyses. For PDX tumor tissue transplantation, 4 mm × 2 mm tissue fragments prepared from BRCA1 mutant PDX tumors (TM00091, Jax lab) were transplanted into MFPs of 4-week-old female NSG mice. At 4 weeks after transplantation, or when tumor volume reached the maximal size that the Institutional Animal Care and Use Committee (IACUC) allowed, animals were euthanized and tumors were analyzed. For estrogen treatment in vivo, 0.72 mg E2 (SE-121, IRA, Sarasota, FL, USA) or placebo beeswax pellet was implanted subcutaneously in mice receiving tumor cell or tissue transplants. To analyze the estrogen-induced metastasis from mammary tumors, metastatic p18−/−;Brca1MGKO tumor cells were inoculated into the MFPs of 4-week-old female NSG mice in which either E2 or placebo beeswax pellet was implanted subcutaneously. When newly generated tumors reached the maximum size (1.3 cm3) allowed by the IACUC in 3–6 weeks, or the mice became moribund, the lungs and other major organs were examined for detection of metastasis. For quantification of the number of metastatic nodules in the lungs, fixed lung tissues of all five lobes were sagittally sectioned at 200-μm intervals. At least three sections for each lobe were prepared and stained with H.E. The metastatic nodules in each lobe of lung tissue were confirmed by H.E. staining, counted under a microscope, and averaged. The number of nodules in all lobes was then calculated. For AZD5363 treatment of pre-existing mammary tumors, p18−/−;Brca1MGKO tumor cells were transplanted into MFPs of NSG mice and allowed to reach ~ 250 mm3 in size. Mice were then treated with AZD5363 ((150 mg/kg solubilized in a 10% DMSO 25% w/v (2-Hydroxypropyl)-β-cyclodextrin buffer (Sigma)) or vehicle by oral gavage once a day. The tumor size was measured daily with a caliper. Tumor volumes were calculated as:
V = a × b2/2
where “a” is the largest diameter and “b” is the smallest. Statistical significance was evaluated using the two-tailed t test.
Statistical analysis
All data are presented as the mean ± SD for at least three repeated individual experiments for each group. Quantitative results were analyzed by the two tailed Fisher exact test or two-tailed Student’s t test. P < 0.05 was considered statistically significant.
Results
Deletion of Brca1 in p16 and p18 null epithelia results in ER-negative mammary tumors
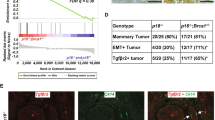
We previously discovered that the expression of p16 and p18 along with senescence markers was increased in MECs of Brca1+/− (heterozygous germline deletion of Brca1) and Brca1MGKO (specific deletion of Brca1 in epithelia) mice [19, 23, 39]. p18−/− mice in the Balb/c background developed ER-positive luminal-type mammary tumors [37] whereas p18−/−;Brca1+/− double mutant mice formed ER-negative basal-like mammary tumors [19]. We generated p16−/−;Brca1MGKO and p18−/−;Brca1MGKO double-mutant mice in the Balb/c-B6 mixed background. We found that 47% of p18−/−, 73% of p18−/−;Brca1MGKO, 60% of p16−/−;Brca1MGKO, and 8% of Brca1MGKO mice in the Balb/c-B6 mixed background developed mammary tumors, yet no p16−/− mice developed mammary tumors at similar ages (Table 1 and Additional file 1: Figure S1). Though the mammary tumor incidence of p18−/− mice in the Balb/c-B6 mixed background was lower than those in the Balb/c background (47% vs. 83%) (Table 1 and Reference [19, 37]), p18−/− tumors in the Balb/c-B6 mixed background were also predominantly ERα-positive, Ck5/CK14 negative tumors (Table 1 and Additional file 1: Figure S1). These results indicate that loss of p18 induces ER-positive mammary tumors independent of mouse genetic background. Further analysis revealed that only 18% (n = 11) of p18−/−;Brca1MGKO and none (n = 6) of p16−/−;Brca1MGKO tumors were positive for ERα. These rates were significantly lower than that of p18−/− tumors (71%, n = 7). Consistent with these results, 82% of p18−/−;Brca1MGKO and all of p16−/−;Brca1MGKO tumors were positive for Ck5 or Ck14, whereas only 29% of p18−/− tumors were positive for Ck5 or Ck14 (Table 1 and Additional file 1: Figure S1). These data confirm the role of Brca1 in the suppression of ER-negative, basal-like tumorigenesis in an epithelium autonomous manner.
Deletion of Brca1 stimulates the tumor-initiating potential of the tumor cells
We transplanted 106 primary tumor cells from three individual p16−/−;Brca1MGKO and four p18−/−;Brca1MGKO tumors into MFPs of NSG mice respectively and found that all of these cells efficiently generated tumors in 6–7 weeks. Furthermore, we found that as few as 4000 p16−/−;Brca1MGKO or p18−/−;Brca1MGKO tumor cells efficiently generated tumors in the same period (Table 2 and data not shown). In contrast, as many as 107 tumor cells from three individual p18−/− tumors did not generate tumors in 6–7 weeks when transplanted (experiment 1 in Table 2 and data not shown). Since p16−/− mice did not develop mammary tumors, we were unable to determine the tumor initiating potential of p16-/- mammary tumor cells by transplantation assay. These results suggest that the deletion of Brca1 enhances the tumor-initiating potential of tumor cells, which is consistent with our previous finding that loss of Brca1 activates EMT and induces ER-negative basal-like mammary tumors with enriched CSCs [23].
Generation of transplantable ER-negative Brca1-deficient mammary tumor models
In mammary and tumor cells ERα expression is restricted to the CD24+CD29low luminal population while the CD24+CD29high population is ERα negative while also enriched with basal and stem cells [42, 43]. Consistently, we previously reported that 40–60% of the cells in most of the ER-positive p18−/− tumors and less than 10% of the cells in most of the ER-negative p18−/−;Brca1MGKO tumors were CD24+CD29low [23, 37]. We detected a predominant CD24+CD29high population containing 60–81% of the cells in most of the p18−/−;Brca1MGKO and p16−/−;Brca1MGKO tumors (Additional file 2: Figure S2A) [23]. We chose and further characterized two individual p18−/−;Brca1MGKO tumors in addition to two individual p16−/−;Brca1MGKO tumors in which ERα was undetectable by western blot and IHC (Table 1, Additional file 1: Figure S1, and Fig. 3c); there were more than 75% CD24+CD29high cells and less than 2% CD24+CD29low cells (Additional file 2: Figure S2A and data not shown). Taking advantage of the tumors predominantly composed of CD24+CD29high cells, we transplanted 6 × 104 FACS-sorted Lin− cells (depleted of hematopoietic and endothelial cells) from a p18−/−;Brca1MGKO tumor and a p16−/−;Brca1MGKO tumor into eight MFPs of NSG mice (four for each cell type). After 1–2 months all recipient mice produced mammary tumors that were ERα negative (placebo group in Table 3, Additional file 2: Figure S2B, C, and data not shown). We cultured these tumor cells and found that most (> 90%) of the cells maintained their CD24+CD29high feature and less than 2% of the cells were CD24+CD29low, whereas, as a control, about half of the p18−/− tumor cells were CD24+CD29low (Additional file 2: Figure S2D). We determined expression of ERα by FACS and found that all cells derived from four individual p18−/−;Brca1MGKO and p16−/−;Brca1MGKO tumors were ERα negative whereas, as controls, 59% of MCF7 and 43% of p18−/− tumor cells were ERα positive, respectively (Additional file 2: Figure S2E). We transplanted 1 × 106 cultured cells from each of the two individual p18−/−;Brca1MGKO and p16−/−;Brca1MGKO tumors into MFPs of NSG mice. We found all recipient mice also generated ERα-negative mammary tumors, which were indistinguishable from tumors generated by Lin− primary tumor cells in respect to ERα negativity (placebo group in Table 3, Additional file 2: Figure S2F, G, and data not shown). In summary these results demonstrate that transplantation of representative p18−/−;Brca1MGKO and p16−/−;Brca1MGKO tumor cells into MFPs of NSG mice generate reproducible ER-negative tumors, which build a model system to study BRCA1-deficient mammary tumor development and progression.
Estrogen promotes Brca1-deficient mammary tumor initiation and metastasis
To determine the role of estrogen in Brca1-deficient mammary tumorigenesis, we transplanted Brca1-proficient and Brca1-deficient mammary tumor cells into MFPs of NSG mice with or without estrogen (17β-estradiol, E2) supplement. We found with E2 supplement, a half (3 out of 6) and a quarter (1 out of 4) of the mice that received two individual p18−/− tumor cell transplants yielded small tumors, respectively (experiment 2 in Table 2 and Additional file 3: Figure S3). Importantly, all p18−/−;Brca1MGKO and p16−/−;Brca1MGKO tumor cells yielded significantly larger tumors in NSG mice when transplanted with E2 supplement compared with those with placebo treatment (Table 3). IHC and western blot analysis confirmed the expression of Brca1 and ERα in regenerated mammary tumors by p18−/− tumor cells and lack of Brca1 and ERα in regenerated tumors by p18−/−;Brca1MGKO cells with E2 supplement (Additional file 3: Figure S3 and Additional file 4: Figure S4B, C). Pathologic analysis revealed that relative to placebo-treated tumors, E2-treated p18−/−;Brca1MGKO and p16−/−; Brca1MGKO tumors were poorly differentiated, more aggressive (increased necrosis, squamous metaplasia, spindle cells, nuclear-cytoplasm ratio, and mitotic indices) and contained highly heterogeneous cell types, whereas, E2-treated p18−/− tumors retained well-differentiated glandular structures (Fig. 1a and Additional file 3: Figure S3). Notably, one out of four p18−/−;Brca1MGKO tumors that were treated with E2 metastasized to the lungs and liver (Table 3 and Fig. 1a). We found that 51% (25/49) of DMSO-treated tumorspheres were smaller than 50 μm whereas 63% (47/74) of E2-treated tumorspheres were larger than 50 μm. The number of E2-treated tumorspheres that were larger than 100 μm or that were 50–100 μm was significantly more than that of DMSO-treated tumorspheres. Notably, the increased number of p18−/−;Brca1MGKO tumorspheres was not inhibited by 4OHT, an ER antagonist (Fig. 1b). These data suggest that E2 stimulates Brca1-deficient CSCs independent of ER. Together, these results demonstrate that in addition to the increased tumor-initiating potential of ER-positive Brca1-proficient tumor cells, estrogen enhances the number of Brca1-deficient CSCs and promotes ER-negative basal-like tumor initiation and progression.
Estrogen promotes Brca1-deficient mammary tumor initiation and progression. a Representative H.E staining of regenerated p18−/−;Brca1MGKO mammary tumors treated with or without E2. Note that the metastasized tumors in lung and liver and the highly heterogeneous tumor cell types in E2-treated mammary tumors. Boxed areas, a, b, c, and d, are enlarged to show the four different group of cells in E2-treated tumors. Spindle cells (black arrows), cells with high nuclear-cytoplasm ratio (green arrows), and mitotic cells (red arrows) are indicated. b p18−/−;Brca1MGKO mammary tumor cells were analyzed by tumorsphere formation assay in the presence of E2, 4OHT, dimethyl sulfoxide (DMSO), or E2 + 4OHT. The number of spheres large than 30 μm was quantified; *p < 0.05 between the E2-treated group and DMSO-treated group; **p < 0.05 between the group treated with E2 + 4OHT and the 4OHT-treated group. c BRCA1 mutant patient-derived xenograft (PDX) tumors were transplanted into the mammary fat pads of NSG mice along with E2 pellets or placebo pellets under the skin. Tumor size was measured and plotted after 4 weeks of transplantation. Data are represented as mean ± SD for tumors in each group (n = 3). d Representative H.E staining of BRCA1 mutant PDX tumors treated with or without E2. Note the clear boundary between placebo-treated tumor tissue and surrounding tumor-free tissue (left), and the E2-treated tumor front (indicated by arrows) invading the surrounding muscle tissue (right)
Consistent with the findings derived from E2-treated p18−/−;Brca1MGKO and p16−/−;Brca1MGKO tumors, we also found that BRCA1 mutant PDX tumor development was significantly enhanced by E2 treatment relative to that by placebo (Fig. 1c). E2-treated BRCA1 mutant PDX tumors were also less differentiated, but more heterogeneous and invasive than placebo-treated tumors (Fig. 1d). These data further support the finding that estrogen promotes human BRCA1-deficient tumor initiation and progression.
To further investigate the role of estrogen in Brca1-deficient mammary tumor progression, we isolated a highly metastatic p18−/−;Brca1MGKO tumor cell line from a mammary tumor (tumor B) that was metastasized to lung. We then transplanted metastatic p18−/−;Brca1MGKO tumor cells into the MFPs of NSG mice with the supplement of either E2 or placebo pellet. We found that all newly generated mammary tumors metastasized to lung in 3–6 weeks. Notably, E2-treated mice with mammary tumors developed significantly more metastatic nodules in their lungs when compared with placebo-treated animals (115 ± 62 vs 241 ± 75, p < 0.05) (Fig. 2a-c, and Additional file 5: Figure S5). IHC analysis revealed that metastasized tumors in lung were negative for ER (Fig. 2d). These results confirm that estrogen promotes ER-negative Brca1-deficient mammary tumor metastasis.
Estrogen promotes Brca1-deficient mammary tumor metastasis. a-d We inoculated 1 × 106 metastatic p18−/−;Brca1MGKO tumor (donor B) cells into the mammary fat pads of 4-week-old female NSG mice in which either E2 or placebo Beeswax pellet was implanted subcutaneously. When newly generated tumors reached maximum size (1.3 cm3) allowed by the IACUC in 3–6 weeks, or the mice became moribund, lungs were examined for gross appearance (a), H.E. staining (b), and quantification of the number of metastatic nodules (c). M, metastatic nodules. Data in (c) are mean ± SD for the numbers of metastatic nodules detected in all lobes of the lungs in each group (n = 4). d Representative immunohistochemical analysis of lung metastasis with antibodies against estrogen receptor (ER)α, p-fos-related antigen 1 (Fra1), and vimentin (Vim)
Estrogen activates EMT in a subset of Brca1-deficient tumor cells
Estrogen has been found to activate EMT and stimulate the function of ER-negative breast CSCs through paracrine signaling produced in ER-positive cells in response to estrogen [7, 8]. However, clinically hormone therapy prevents development and suppresses the progression of BRCA1-deficient, ER-negative breast cancers [3, 4]. Inspired by the finding that a fraction of BRCA1-deficient human and mouse tumor cells harbor EMT features [16,17,18,19, 22,23,24, 39, 44], and the finding that E2 enhanced the heterogeneity of p18−/−;Brca1MGKO and p16−/−;Brca1MGKO tumors with elevated squamous metaplasia and spindle-shaped cells (typical morphological characteristics of mesenchymal cells), we hypothesized that estrogen promotes EMT and stimulates BRCA1-deficient, ER-negative tumorigenesis independent of ER. We found by western blot analysis that E2-treated p18−/−;Brca1MGKO tumors expressed a higher level of EMT marker (Vim) and EMT-inducing transcription factors (EMT-TFs) including p-Fra1 and Snail, than placebo-treated tumors. Importantly, we noticed by IHC that the number of cells that are positive for EMT markers and EMT-TFs was drastically enhanced in E2-treated p18−/−;Brca1MGKO tumors than in placebo-treated counterparts (Fig. 3a and Additional file 4: Figure S4A). We confirmed, as expected, that metastasized mammary tumors in lung were positive for Vim and p-Fra1 (Fig. 2d). These data suggest that estrogen activates EMT in a subset of ER-negative Brca1-deficient epithelial tumor (carcinoma) cells that have not undergone EMT, leading to an increase in the fraction of mesenchymal-like cells in Brca1-deficient tumors.
Estrogen activates epithelial-mesenchymal transition in Brca1-deficient and estrogen receptor (ER)-negative tumor cells. a, b p18−/−;Brca1MGKO (a) and BRCA1 mutant patient-derived xenograft (PDX) (b) tumors treated with E2 or placebo were analyzed by immunohistochemical staining and western blot. Samples in (a) were derived from four different tumors developed in four individual mice. c The expression of genes indicated in the regenerated p18−/− tumors treated with E2 and primary p18−/− tumors was determined by western blot; p18−/−;Brca1MGKO tumor was used as a control. d, e p18−/−;Brca1MGKO tumor cells were treated with 50 nM E2 and dimethyl sulfoxide (DMSO) for 72 h and 144 h, respectively, and analyzed by fluorescence-activated cell sorting (FACS) (d) and western blot (e). The percentages of vimentin (Vim)-positive cells in (d) are indicated. f p18−/−; Brca1MGKO tumor cells were treated with 50 nM E2 or DMSO for 120 h and stained with anti-Vim. g, h p18−/−;Brca1MGKO tumor cells were treated with DMSO or 50 nM E2 in the presence or absence of 5 μM 4OHT for 72 h and analyzed by western blot (g) and FACS (h). i SUM149 breast cancer cells were treated with DMSO or 50 nM for 72 h and analyzed by FACS. The percentages of VIM-positive cells are indicated
We examined BRCA1 mutant PDX tumors and discovered that more than 80% of tumor cells expressed a high level of VIM without treatment (Fig. 3b). These data are supported by the findings in both mouse [23] and human mammary tumors [24, 44] that most BRCA1 mutant tumors are VIM positive and some of them are predominantly composed of VIM-positive cells. Though we failed to detect a further increase in the number of VIM-positive cells by E2-treatment in BRCA1 mutant PDX tumors (Fig. 3b), the number of tumor cells that are positive for fibronectin, another key marker of mesenchymal cells, and for SNAIL and SLUG was drastically enhanced by E2 relative to the placebo (Fig. 3b). These results indicate that estrogen also activates EMT in a subset of human BRCA1 mutant tumor cells.
As no tumor formed after the transplantation of p18−/− tumor cells without an E2 supplement (Table 2), we determined EMT markers in E2-treated tumors and primary tumors. We found no significant change in the expression of E-cad, p-Fra1, and Snail in E2-treated p18−/− tumors compared to p18−/− primary tumors (Fig. 3c, Additional file 4: Figure S4C, and data not shown). Notably, except for a slight increase in Slug in a longer treatment, the expression of E-cad, Vim, Snail, and p-Fra1 in p18−/− tumor cells in culture was not affected by E2 either, whereas, the expression of Vim, Snail, and Slug in p18−/−;Brca1MGKO tumor cells was clearly enhanced by E2 under the same culture conditions (Fig. 3e, g and Additional file 6: Figure S6A-D). Together, these results indicate that estrogen promotes EMT in Brca1-deficient, but not in Brca1-proficient, tumor cells.
Because the number of cells with mesenchymal features varies in different BRCA1-deficient tumors, we screened a panel of primary p18−/−;Brca1MGKO tumor cells by FACS for expression of Vim and identified at least two types of p18−/−;Brca1MGKO tumor cells, and in one of these, Vim-positive cells constituted 30–65% of the total tumor cells (type 1) and in the other, Vim-positive cells constituted > 90% of the total cells (type 2) (Fig. 3d-h and Additional file 6: Figure S6C). Due to the enrichment of EMT-promoting factors including transforming growth factors (TGFs) in serum, culture immortalized MECs in serum in vitro activates EMT [45]. Therefore, we cultured tumor cells in charcoal-stripped FBS with minimal hormones and cytokines (http://www.thermofisher.com) and performed all of our in vitro experiments using either primary or early passaged (before passage 3) tumor cells.
We treated p18−/−;Brca1MGKO tumor cells with E2 in multiple time periods and observed by western blot that E2 stimulated expression of Vim and EMT-TFs including p-Fra1 and/or Snail/Slug in both type of cells (Fig. 3d-h, Additional file 6: Figure S6), indicating E2 promotes EMT in Brca1-deficient tumor cells in vitro. As the majority of BRCA1-deficient tumors contain a fraction of cells that are positive for VIM and/or other mesenchymal markers, and only a small number of human BRCA1-deficient tumors, i.e. metaplastic breast cancers, are predominantly composed of mesenchymal/VIM-positive cells [16,17,18,19, 24, 39, 44, 46], we therefore focused on type 1 p18−/−;Brca1MGKO tumor cells. When type 1 p18−/−;Brca1MGKO tumor cells were examined by immunofluorescent staining and FACS, we found that E2 treatment enhanced the number of Vim-positive cells - converting Vim-positive cells from 57-62% to 80–90% after 72 h (Fig. 3d, f, h). Furthermore, the increase in Vim expression and conversion of cells from Vim-negative to positive by E2 in p18−/−;Brca1MGKO tumor cells was not blocked by 4OHT (Fig. 3g, h), whereas, 4OHT effectively blocked E2-enhanced cell proliferation and RB phosphorylation in ER-positive MCF7 cells, as expected (Additional file 7: Figure S7). These results demonstrated that estrogen activates EMT in a subset of Brca1-deficient tumor cells that have not undergone EMT, and further indicated that estrogen promotes EMT in ER-negative Brca1-deficient tumor cells independent of ER.
We then selected a BRCA1 mutant, ER-negative human breast cancer cell line, SUM149, in which p16 is deleted and VIM is expressed in 10–30% of total cells [35, 47,48,49]. This cell line shares many genetic (p16/p18 and Brca1 loss-of-function mutation) and phenotypical (ER negative with a subset of cells exhibiting EMT features) similarities with p16;Brca1 and p18;Brca1 mutant murine mammary tumor cells. We found that E2 treatment also drastically enhanced the number of Vim-positive cells - from 19% to 50% after 72 h (Fig. 3i). Notably, it has been reported that in the SUM149 cell line, VIM-positive, stem/basal cells are able to efficiently generate both basal and luminal cells, whereas, VIM-negative, luminal cells rarely generate VIM-positive, stem/basal cells under serum-containing culture condition [47]. In addition, we also detected by western blot the increase in VIM, p-FRA1, and SNAIL in SUM149 cells in response to E2 treatment (Additional file 6: Figure S6E), indicating the activation of EMT by estrogen.
In summary, these in vitro results confirmed that estrogen activates EMT in a subset of Brca1-deficient tumor cells with epithelial features, which is independent of ER.
Estrogen stimulates Brca1-deficient tumor cell proliferation
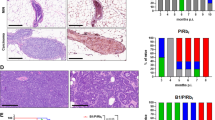
We performed IHC of mammary tumors generated by primary tumor cells derived from three individual p16−/−; Brca1MGKO and four p18−/−;Brca1MGKO tumors. We found that all newly generated tumors contained Ck5 or Ck14 (Ck5/Ck14)-positive cells. We observed that the more Ck5/Ck14-positive cells in primary tumors, the more Ck5/Ck14-positive cells will be detected in the regenerated tumors, though the percentages of Ck5-positive or Ck14-positive cells varied among tumors (Figs. 4, 6 and data not shown) [19, 23, 39]. These results suggest that newly generated p16−/−;Brca1MGKO and p18−/−; Brca1MGKO mammary tumors maintain their basal-like tumor phenotype. We investigated cell proliferation in tumors by Ki67 staining and found that there were more Ki67-positive cells in E2-treated tumors than in placebo-treated tumors. Furthermore, the majority of Ki67 positive cells in E2-treated tumors were also positive for CK14 (Fig. 4a). We then performed similar analysis for BRCA1 mutant PDX tumors and found that E2 treatment also enhanced the number of Ki67-positive cells in tumors, most of which are also CK14 positive (Fig. 4b, and data not shown). These results indicate that estrogen stimulates proliferation of Brca1-deficient basal-like tumor cells in vivo.
Estrogen stimulates proliferation of Brca1-deficient tumor cells. a, b Expression of Ki67 and Ck14 in p18−/−;Brca1MGKO (a) and BRCA1 mutant patient-derived xenograft (b) tumors treated with E2 or placebo was analyzed by immunostaining. The percentages of Ki67+ and/or Ck14+ cells were calculated from 4',6-diamidino-2-phenylindole (DAPI)+ tumor cells and quantitated in four randomly selected fields for each section of a tumor, and the results represent the mean ± SD of three individual tumors per group. c, d p18−/−;Brca1MGKO tumor cells were treated with DMSO or E2 for 24 h. The expression of p-RB was determined by western blot (c) and the bromodeoxyuridine (Brdu) incorporation was determined by fluorescence-activated cell sorting (FACS) (d). The percentages of Brdu-positive cells in (d) are indicated
We cultured cells derived from three individual p18−/−; Brca1MGKO tumors and found that E2 treatment in both type 1 and type 2 tumor cells enhanced the number of cells (Fig. 6b, Additional file 8: Figure S8C), promoted phosphorylation of RB (Fig. 4c), and stimulated incorporation of BrdU in cells (Fig. 4d). These data confirm the role of estrogen in stimulation of proliferation of Brca1-deficient tumor cells.
Estrogen activates the AKT pathway in BRCA1-deficient mammary tumors
During the course of analysis of the role of estrogen in activating EMT, we observed that E2 stimulated expression of p-Akt, p-Gsk3β, and p-4Ebp1, downstream targets of Akt, in p18−/−;Brca1MGKO tumor cells, which was not blocked by 4OHT (Fig. 3e, g). We examined tumors by IHC and western blot analysis, and found that the expression of p-Akt and its targets, p-4Ebp1, p-mTor, and p-Gsk3β, was significantly enhanced in E2-treated tumors when compared with placebo-treated tumors. (Fig. 5a, b). We treated p18−/−;Brca1MGKO and SUM149 tumor cells with or without E2 and found that E2 stimulated expression of p-Akt, p-4Ebp1, p-mTor, and p-Gsk3β in all time points tested from 2 to 144 h (Fig. 3e, g, Fig. 5c, Fig. 6a and Additional file 6: Figure S6A, B, E). Consistent with the data derived from p18−/−;Brca1MGKO tumors, E2 treatment also significantly enhanced the expression of p-AKT, p-4EBP1, p-mTOR, and p-GSK3β in BRCA1 mutant PDX tumors relative to placebo treatment (Fig. 5d, e and Additional file 8: Figure S8A, B). These results indicate that estrogen activates the AKT pathway in BRCA1-deficient mammary tumor cells independent of ER.
Estrogen activates the Akt pathway in Brca1-deficient mammary tumors. a, b Representative p18−/−;Brca1MGKO tumors treated with E2 or placebo were analyzed by immunohistochemical staining (IHC) (a) and western blot (b). H-scores for p-Akt and p-4E-bp1 in a were calculated in four randomly selected fields for each section of a tumor, and the results represent the mean ± SD of three individual tumors per group. c p18−/−;Brca1MGKO tumor cells were treated with DMSO or E2, and the expression of p-Akt, p-mTor, p-Gsk3β and p-4E-bp1 was analyzed by western blot. d BRCA1 mutant patient-derived xenograft (PDX) tumors treated with E2 or placebo were analyzed by IHC. H-scores for p-Akt and p-4E-bp1 were calculated in four randomly selected fields for each section of a tumor, and the results represent the mean ± SD of three individual tumors per group. e Representative BRCA1 mutant PDX tumors treated with E2 or placebo were analyzed by western blot
Pharmaceutical inhibition of Akt suppresses epithelial-mesenchymal transition and cell proliferation preventing Brca1-deficient tumor progression. a p18−/−;Brca1MGKO tumor cells were treated with dimethyl sulfoxide (DMSO) or 5 nM E2 in the presence or absence of different dosage of AZD5363 for 2 h, the expression of p-mTor, p-4E-bp1, p-Gsk3β, p-Fra1 and vimentin (Vim) were analyzed by western blot. b p18−/−; Brca1MGKO tumor cells were treated with DMSO or 5 nM E2 in the presence or absence of different dosage of AZD5363, and the numbers of viable cells were determined on day 1, day 3, and day 5. *p < 0.05 between E2-treated and E2 + AZD5363-treated groups at the time points (Student t test). Data are represented as mean ± SD (n = 4). c p18−/−;Brca1MGKO tumor cells were treated with 5 nM E2 with or without 1 μM AZD5363 for 24 h, and bromodeoxyuridine (Brdu) incorporation was then determined by fluorescence-activated cell sorting (FACS). d-g We transplanted 1 × 106 p18−/−; Brca1MGKO tumor cells into the mammary fat pads of NSG mice along with E2 pellet under the skin, and tumors were allowed to reach ~ 250 mm3 in size. Mice were then treated with AZD5363 at 150 mg/kg body weight or vehicle daily by oral gavage. The tumor size was determined and plotted (d). Data in d are represented as mean ± SD of four tumors in each group. *p < 0.05 between two groups at each time point (Student t test). p18−/−; Brca1MGKO tumors treated with AZD5363 or vehicle for 7 days (d) were analyzed by western blot (e), immunohistochemical staining (f), and immunofluorescent staining (g). Samples in e were derived from eight different tumors developed in eight individual mice. The percentages of Ki67+ and/or Ck14+ cells (g) were quantitated in four randomly selected fields for each section of a tumor, and the results represent the mean ± SD of three individual tumors per group
Inhibition of AKT suppresses EMT and cell proliferation preventing Brca1-deficient tumor progression
Along with activation of the Akt pathway, estrogen promoted EMT and proliferation in Brca1-deficient mammary tumor cells, which prompted us to test whether pharmaceutical inhibition of Akt activity has any effect on Brca1-deficient cell proliferation and tumor progression. We treated p18−/−;Brca1MGKO tumor cells with E2 in the presence or absence of AZD5363, a well-studied preclinical Akt inhibitor [50]. We found that AZD5363 drastically inhibited E2-enhanced expression of p-4Ebp1, p-mTor, and p-Gsk3β, and that of Vim and p-Fra1 (Fig. 6a), suggesting that AZD5363 efficiently suppresses estrogen-enhanced Akt pathway and EMT program in Brca1 deficient tumor cells. Treatment of p18−/−; Brca1MGKO tumor cells including both type 1 and type 2 cells with AZD5363 significantly reduced the E2-enhanced number of cells and incorporation of BrdU (Fig. 6b, c and Additional file 8: Figure S8C), indicating that AZD5363 inhibits estrogen-enhanced proliferation of Brca1-deficient tumor cells.
We then determined if pharmaceutical inhibition of Akt activity suppresses the progression of pre-existing Brca1-deficient tumors. Transplanted p18−/−;Brca1MGKO tumors with E2 supplement were allowed to reach ~ 250 mm3 in size and then mice were treated with vehicle or AZD5363 daily. Three days after treatment, tumors from AZD5363-treated mice began to show a significant size reduction in comparison with the tumors from vehicle-treated animals. After 7-day treatment, tumors from vehicle-treated mice reached ~ 1475 mm3 in size, whereas those from AZD5363 -treated mice only reached ~ 188 mm3, which was comparable with that of the tumor size at the start of treatment (~ 224 mm3) (p = 0.59) (Fig. 6d), indicating that treatment with AZD5363 prevents estrogen-enhanced Brca1-deficient tumor progression.
Further analysis of tumors by IHC and western blot revealed that in tumors from mice with E2 supplement the expression of p-4Ebp1, p-mTor, and p-Gsk3β was clearly reduced by AZD5363 treatment relative to vehicle treatment (Fig. 6e and Additional file 8: Figure S8D), confirming the role of AZD5363 in inhibition of the estrogen-induced Akt pathway in Brca1-deficient tumor progression. Notably, the expression of Vim, Fn and Slug in tumors with E2 was drastically reduced and that of E-cad was enhanced in AZD5363-treated mice relative to control (Fig. 6e, f). These data demonstrate that AZD5363 efficiently suppresses estrogen-activated EMT in Brca1-deficient tumors in vivo. Consistent with the findings derived from p18−/−;Brca1MGKO tumor cells in vitro (Fig. 6b), we also detected that AZD5363 significantly reduced the number of Ki67 and Ck14 double-positive cells stimulated by E2 in p18−/−;Brca1MGKO tumors (Fig. 6g). Together, these results indicate that estrogen promotes EMT and proliferation of Brca1-deficinet tumor cells leading to tumor progression, which is dependent on the activation of the Akt pathway.
Discussion
In this study, we found that deletion of Brca1 enhances the tumor-initiating potential of tumor cells, and that estrogen stimulates proliferation and the tumor-initiating potential of both Brca1-proficient ER-positive and Brca1-deficient ER-negative tumor cells. We discovered that estrogen activates EMT in a subset of Brca1-deficient tumor cells that maintain epithelial features, and enhances the number of CSCs, promoting ER-negative basal-like tumor progression and metastasis. We found that estrogen activates EMT independent of ER in Brca1-deficient but not Brca1-proficient tumor cells. We further discovered that estrogen activates the Akt pathway in Brca1-deficient mammary tumor cells independent of ER, and that pharmaceutical inhibition of Akt activity suppresses EMT and cell proliferation preventing Brca1-deficient tumor progression. To the best of our knowledge, this study reveals for the first time that estrogen promotes BRCA1-deficient tumor initiation and progression by stimulation of cell proliferation and activation of EMT, which are dependent on AKT activation and independent of ER.
It has long been known that estrogen is required for normal mammary development and that it promotes mammary tumorigenesis and progression [1, 2]. Not until recently has the role of estrogen in stimulation of normal and cancerous mammary stem cells been investigated. Though mammary stem cells lack the ER [43], they are highly responsive to estrogen [51]. Estrogen expands the number and promotes the function of mammary stem cells, likely mediated through paracrine signaling from RANK ligand produced in ER-positive luminal epithelial cells [51]. More recently, it has been reported that estrogen promotes mammary stem cells by paracrine signaling from IGF and WNT provided in stromal cells, in which Gli2 induces expression of ER and coordinates the induction of stem cell support factors including insulin-like growth factor (IGF) and WNT [52]. Interestingly, estrogen has been found to promote ER-negative breast CSCs derived from ER-positive cell lines through paracrine FGF-Tbx3, EGFR, and Notch signaling provided in ER-positive cells [5, 7, 8]. Notably, paracrine factors produced in response to estrogen in ER-positive cancer cells also expands CSCs derived from ER-negative breast cancer cell lines [7]. However, all of these findings on the role of estrogen in promoting breast CSCs were obtained from cell lines in vitro, and were dependent on the paracrine stem cell supporting and promoting factors that were provided by ER-positive cells. It remains elusive whether estrogen directly regulates breast CSCs in ER-negative breast cancers. We have previously reported that heterozygous germline deletion or mammary epithelial specific deletion of Brca1 in mice promotes CSCs and induces development of ER-negative mammary tumors [19, 23, 39]. We report in this paper that estrogen enhances the number of breast CSCs in Brca1-deficient, ER-negative mammary tumors independent of ER. Our results indicate that BRCA1 deficiency caused by somatic mutation or promoter methylation of BRCA1 in sporadic ER-negative breast cancers sensitizes tumor cells to endogenous estrogen activating EMT, promoting CSCs and, therefore, inducing tumor progression. Our finding supports the development of strategies to inhibit estrogen synthesis for treatment of BRCA1-deficient BLBCs.
EMT plays a critical role in generating CSCs in tumor development and progression [12, 13]. The role of estrogen in regulation of EMT in breast cancer is controversial. On one hand, it is extensively studied and reported that estrogen activates ERα signaling, maintaining epithelial phenotype and suppressing EMT (as reviewed [53]), but on the other hand, estrogen has been found to activate EMT in ER-positive breast and ovarian cancer cells, promoting their stemness and invasiveness [5,6,7, 54]. Again, all the findings on promotion of EMT by estrogen were obtained from cancer cell lines and are dependent on activation of ER signaling. However, it remains obscure whether estrogen regulates EMT in ER-negative cancer cells and alters their properties in tumor initiation and progression. We showed that estrogen does not activate EMT in ER-positive and Brca1-proficient tumor cells in vitro and tumorigenesis in vivo. These data warrant further investigation of the role of estrogen/ER signaling in regulation of EMT in tumorigenesis. Importantly, we demonstrated that estrogen activates EMT in a subset of ER-negative and Brca1-deficient mammary tumor cells, promoting their properties in tumor initiation and progression. These results provide the first genetic evidence demonstrating that estrogen activates EMT in ER-negative breast cancer cells independent of paracrine factors produced by ER-positive cells.
The PI3K/AKT pathway stimulates tumor development and progression through multiple mechanisms including promotion of cell proliferation, survival, and EMT [25, 26]. Estrogen activates the PI3K/AKT pathway in ER-negative breast cancer cells, and promotes survival of Brca1-deficient tumor cells, which stimulate tumor growth [30]. Estrogen also stimulates proliferation of Brca1-mutant cells through activation of remaining ERα, which is gradually diminished during tumor progression in Brca1Δ11/Δ11p53+/− mice [55]. Depletion or inhibition of the PI3K/Akt pathway reduces proliferation of BRCA1-deficient MEFs suppressing growth of tumors generated by transplantation of BRCA1-deficient MEFs [28]. However, it remains elusive whether estrogen stimulates proliferation of ER-negative BRCA1-deficient tumor cells in vivo through activation of the Akt pathway. Further, due to the lack of a proper model system, i.e. ER-negative BRCA1-deficient tumor cells that can be induced by estrogen to EMT in vitro and in vivo, it is unknown if estrogen-activated EMT in ER negative BRCA1-deficient tumor cells is dependent on AKT activation. In the present study, we demonstrated that estrogen activates the Akt pathway not only stimulating proliferation but also promoting EMT in ER-negative, BRCA1-deficient tumor cells, which further enhances tumor progression.
In this study, we investigated the effect of estrogen on ER-negative BRCA1-deficient tumor progression by transplantation of tumor cells into the MFPs of NSG mice that did not receive ovariectomy. Host estrogen in recipient mice may play a role in ER-negative BRCA1-deficient tumor growth and progression. Transplantation of ER-positive p18−/− tumor cells into NSG mice did not produce tumors, whereas, with exogenous E2 supplement, p18−/− tumor cells generated ER-positive tumors in NSG mice, suggesting that host estrogen is not sufficient to induce p18 deficient, Brca1-proficient tumor initiation. Notably, transplantation of either p18−/−; Brca1MGKO or p16−/−;Brca1MGKO tumor cells into NSG mice generated tumors efficiently, which were further promoted by exogenous E2 administration. Taking into consideration our finding that estrogen promotes EMT, proliferation, sphere-forming potential, and Akt activation in Brca1-deficient tumor cells in vitro, these results suggest that host estrogen likely promotes Brca1-deficient tumor initiation, which support our conclusion. Importantly, it has been demonstrated that the E2 pellet (0.72 mg pellet) we used produces between 18 and 40 times higher concentrations of serum E2 than the physiological range in intact mice [56], suggesting that the effect of host estrogen on tumor growth in recipient mice is minor on supplementation with the E2 pellet. Furthermore, as estrogen is produced in the ovaries, adrenal glands, and fat tissues, an ovariectomy reduces only about 1/3 of the serum E2 concentration in female mice when compared with the serum E2 concentration in mice with intact ovaries [57]. Whether the reduced host estrogen due to ovariectomy in recipient mice impacts Brca1-deficient tumorigenesis remains elusive.
Conclusions
Our finding, for the first time, demonstrates that estrogen promotes BRCA1-deficient tumor initiation and progression by stimulation of cell proliferation and activation of EMT, which are dependent on AKT activation and independent of ER. This study not only reveals the molecular mechanisms underlying the role of estrogen in promoting development and progression of ER-negative basal-like breast cancers but also identifies a druggable AKT pathway that is activated by estrogen in BRCA1-deficient breast cancers. This investigation will support to develop strategies to inhibit estrogen synthesis and to target AKT for treatment of BRCA1-deficient basal-like breast cancers.
Abbreviations
- BLBCs:
-
Basal-like breast cancers
- Brca1 MGKO :
-
Brca1f/f;MMTV-Cre or Brca1f/−;MMTV-Cre)
- BrdU:
-
Bromodeoxyuridine
- CSCs:
-
Cancer stem cells
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- E2:
-
17β-estradiol
- E-cad:
-
E-cadherin
- EMT:
-
Epithelial-mesenchymal transition
- EMT-TFs:
-
Epithelial-mesenchymal transition-inducing transcription factors
- ER:
-
Estrogen receptor
- FACS:
-
Fluorescence-activated cell sorting
- FBS:
-
Fetal bovine serum
- Fn:
-
Fibronectin
- H.E.:
-
Hematoxylin and eosin
- IACUC:
-
Institutional Animal Care and Use Committee
- IHC:
-
Immunohistochemical analysis
- INK4:
-
Inhibitors of CDK4/6
- MECs:
-
Mammary epithelial cells
- MEFs:
-
Mouse embryonic fibroblasts
- MFPs:
-
Mammary fat pads
- p16:
-
p16Ink4a
- p18:
-
p18Ink4c
- PDX:
-
Patient-derived xenografts
- PI3K:
-
Phosphatidylinositol-3-kinase
- Vim:
-
Vimentin
References
Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–70.
Dall GV, Britt KL. Estrogen effects on the mammary gland in early and late life and breast cancer risk. Front Oncol. 2017;7:110.
Swain SM. Tamoxifen for patients with estrogen receptor-negative breast cancer. J Clin Oncol. 2001;19:93S–7S.
Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–7.
Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G, Wei J. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Mol Cancer. 2014;13:137.
Planas-Silva MD, Waltz PK. Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2007;104:11–21.
Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, Kuperwasser C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci U S A. 2010;107:21737–42.
Harrison H, Simoes BM, Rogerson L, Howell SJ, Landberg G, Clarke RB. Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and notch signalling. Breast Cancer Res. 2013;15:R21.
Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, Fulton LL, Dooling DJ, Ding L, Mardis ER, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;487:330–7.
Fedele M, Cerchia L, Chiappetta G. The epithelial-to-mesenchymal transition in breast cancer: focus on basal-like carcinomas. Cancers (Basel). 2017;9(10);134.
Wicha MS. Cancer stem cell heterogeneity in hereditary breast cancer. Breast Cancer Res. 2008;10:105.
Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 2015;25:675–86.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–26.
Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–71.
Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–13.
Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, Sedic M, Gilmore H, Tung N, Naber SP, Schnitt S, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell. 2011;8:149–63.
Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A, Ashworth A, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7:403–17.
Bai F, Smith MD, Chan HL, Pei XH. Germline mutation of Brca1 alters the fate of mammary luminal cells and causes luminal-to-basal mammary tumor transformation. Oncogene. 2013;32:2715–25.
De Summa S, Pinto R, Sambiasi D, Petriella D, Paradiso V, Paradiso A, Tommasi S. BRCAness: a deeper insight into basal-like breast tumors. Ann Oncol. 2013;24(Suppl 8):viii13–21.
Zhu X, Shan L, Wang F, Wang J, Wang F, Shen G, Liu X, Wang B, Yuan Y, Ying J, Yang H. Hypermethylation of BRCA1 gene: implication for prognostic biomarker and therapeutic target in sporadic primary triple-negative breast cancer. Breast Cancer Res Treat. 2015;150:479–86.
Liu X, Holstege H, van der Gulden H, Treur-Mulder M, Zevenhoven J, Velds A, Kerkhoven RM, van Vliet MH, Wessels LF, Peterse JL, et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci U S A. 2007;104:12111–6.
Bai F, Chan HL, Scott A, Smith MD, Fan C, Herschkowitz JI, Perou CM, Livingstone AS, Robbins DJ, Capobianco AJ, Pei XH. BRCA1 suppresses epithelial-to-mesenchymal transition and stem cell dedifferentiation during mammary and tumor development. Cancer Res. 2014;74:6161–72.
Rodriguez-Pinilla SM, Sarrio D, Honrado E, Moreno-Bueno G, Hardisson D, Calero F, Benitez J, Palacios J. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J Clin Pathol. 2007;60:1006–12.
Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes Migr. 2015;9:317–24.
Yang SX, Polley E, Lipkowitz S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev. 2016;45:87–96.
Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050–60.
Xiang T, Jia Y, Sherris D, Li S, Wang H, Lu D, Yang Q. Targeting the Akt/mTOR pathway in Brca1-deficient cancers. Oncogene. 2011;30:2443–50.
Xiang T, Ohashi A, Huang Y, Pandita TK, Ludwig T, Powell SN, Yang Q. Negative regulation of AKT activation by BRCA1. Cancer Res. 2008;68:10040–4.
Gorrini C, Gang BP, Bassi C, Wakeham A, Baniasadi SP, Hao Z, Li WY, Cescon DW, Li YT, Molyneux S, et al. Estrogen controls the survival of BRCA1-deficient cells via a PI3K-NRF2-regulated pathway. Proc Natl Acad Sci U S A. 2014;111:4472–7.
Renoir JM, Marsaud V, Lazennec G. Estrogen receptor signaling as a target for novel breast cancer therapeutics. Biochem Pharmacol. 2013;85:449–65.
Pei XH, Xiong Y. Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene. 2005;24:2787–95.
Jonsson G, Staaf J, Vallon-Christersson J, Ringner M, Gruvberger-Saal SK, Saal LH, Holm K, Hegardt C, Arason A, Fagerholm R, et al. The retinoblastoma gene undergoes rearrangements in BRCA1-deficient basal-like breast cancer. Cancer Res. 2012;72:4028–36.
Stefansson OA, Jonasson JG, Olafsdottir K, Hilmarsdottir H, Olafsdottir G, Esteller M, Johannsson OT, Eyfjord JE. CpG island hypermethylation of BRCA1 and loss of pRb as co-occurring events in basal/triple-negative breast cancer. Epigenetics. 2011;6:638–49.
Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal MJ, et al. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat. 2010;121:53–64.
Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, Stebbings LA, Leroy C, Edkins S, Mudie LJ, et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462:1005–10.
Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai SY, Ho IC, Perou CM, Xiong Y. CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 2009;15:389–401.
Jiang Z, Deng T, Jones R, Li H, Herschkowitz JI, Liu JC, Weigman VJ, Tsao MS, Lane TF, Perou CM, Zacksenhaus E. Rb deletion in mouse mammary progenitors induces luminal-B or basal-like/EMT tumor subtypes depending on p53 status. J Clin Invest. 2010;120:3296–309.
Scott A, Bai F, Chan HL, Liu S, Ma J, Slingerland JM, Robbins DJ, Capobianco AJ, Pei XH. p16INK4a suppresses BRCA1-deficient mammary tumorigenesis. Oncotarget. 2016;7:84496–507.
Sedic M, Skibinski A, Brown N, Gallardo M, Mulligan P, Martinez P, Keller PJ, Glover E, Richardson AL, Cowan J, et al. Haploinsufficiency for BRCA1 leads to cell-type-specific genomic instability and premature senescence. Nat Commun. 2015;6:7505.
Goulding H, Pinder S, Cannon P, Pearson D, Nicholson R, Snead D, Bell J, Elston CW, Robertson JF, Blamey RW, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol. 1995;26:291–4.
Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28:1143–58.
Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–4.
Hassanein M, Huiart L, Bourdon V, Rabayrol L, Geneix J, Nogues C, Peyrat JP, Gesta P, Meynard P, Dreyfus H, et al. Prediction of BRCA1 germ-line mutation status in patients with breast cancer using histoprognosis grade, MS110, Lys27H3, vimentin, and KI67. Pathobiology. 2013;80:219–27.
Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci U S A. 2008;105:14867–72.
Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, Tutt AN. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–32.
Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–44.
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68.
Jordan NV, Prat A, Abell AN, Zawistowski JS, Sciaky N, Karginova OA, Zhou B, Golitz BT, Perou CM, Johnson GL. SWI/SNF chromatin-remodeling factor Smarcd3/Baf60c controls epithelial-mesenchymal transition by inducing Wnt5a signaling. Mol Cell Biol. 2013;33:3011–25.
Hyman DM, Smyth LM, Donoghue MTA, Westin SN, Bedard PL, Dean EJ, Bando H, El-Khoueiry AB, Perez-Fidalgo JA, Mita A, et al. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol. 2017;35:2251–9.
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802.
Zhao C, Cai S, Shin K, Lim A, Kalisky T, Lu WJ, Clarke MF, Beachy PA. Stromal Gli2 activity coordinates a niche signaling program for mammary epithelial stem cells. Science. 2017;356:eaal3485.
Guttilla IK, Adams BD, White BA. ERalpha, microRNAs, and the epithelial-mesenchymal transition in breast cancer. Trends Endocrinol Metab. 2012;23:73–82.
Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol Endocrinol. 2008;22:2085–98.
Li W, Xiao C, Vonderhaar BK, Deng CX. A role of estrogen/ERalpha signaling in BRCA1-associated tissue-specific tumor formation. Oncogene. 2007;26:7204–12.
Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17beta-estradiol administration to mice. Gen Comp Endocrinol. 2012;175:188–93.
Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–7.
Acknowledgements
We thank Drs. Beverly Koller, Chuxia Deng, Norman Sharpless, and Lothar Hennighausen for Brca1 mutant, p16 null, and MMTV-cre mice; Yue Xiong for discussion; Emely Pimentel for technical support; Jun-Zhu (Jenny) Pei for proofreading; the FACS core facility at University of Miami for cell sorting; and the DVR core facility for animal husbandry. Chuying Wang thanks Xi’an Jiaotong University for financial support.
Funding
This study was supported by DOD Idea Expansion Award (W81XWH-13-1-0282), the Woman’s Cancer Association Grant, the Bankhead-Coley Cancer Research grant (TBC07), and research funds from the University of Miami Department of Surgery and Sylvester Comprehensive Cancer Center to Xin-Hai Pei.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author information
Authors and Affiliations
Contributions
CW, FB, and XHP designed the research; CW, FB, and LZ performed the research; AS generated and characterized p16;Brca1 mice; CW, FB, and XHP analyzed the data; EL provided administrative, technical, and material support; CW, FB, and XHP wrote the paper; XHP provided financial support and supervised the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The Institutional Animal Care and Use Committee at the University of Miami approved all animal procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Characterization of mammary tumors developed in mutant mice in Balb/cB6 mixed background. Representative mammary tumors spontaneously developed in p18-/-;Brca1MGKO, p16-/-;Brca1MGKO and p18-/- mice were immunostained with the antibodies indicated. The representative cells are enlarged in the insets.
Additional file 2:
Figure S2. p18-/-;Brca1MGKO and p16-/-;Brca1MGKO tumor cells generate reproducible ER-negative Brca1-deficient mammary tumors. (A) A primary mammary tumor (donor tumor A) developed in a 15-month-old p16-/-;Brca1MGKO mouse was analyzed by FACS. As a control, tumor-free mammary glands from age-matched p16-/- mice were analyzed. Note a predominant CD24?+?CD29high population in the tumor. (B, C) We transplanted 6?×?104 FACS-sorted Lin- cells from a p16-/-;Brca1MGKO tumor (donor tumor B) into MFPs of four NSG mice. Representative tumors generated were analyzed by IHC (B) and western blot (C). (D, E) Representative p18-/- and p18-/-;Brca1MGKO tumor cells were cultured and analyzed. MCF7 cells were used as a positive control of ERa expression. (F, G) We transplanted 1?×?106 cultured p18-/-;Brca1MGKO tumor (donor tumor A) cells into MFPs of four NSG mice. Representative tumors generated were analyzed by IHC (F) and western blot (G). p18-/- tumors were used as control in (C) and (G).
Additional file 3:
Figure S3. Estrogen promotes Brca1-proficient and deficient mammary tumor initiation (A) Western blot analysis of mammary tumors regenerated by p18-/- or p18-/-;Brca1MGKO tumor cells with E2 supplement. (B) Representative gross pictures of p18-/- and p16-/-;Brca1MGKO tumors generated by transplantation. We transplanted 1 x 107 p18-/- or 6 x 104 p16-/-;Brca1MGKO tumor cells into MFPs of NSG mice with or without E2 supplement. Gross pictures were taken 6-7 weeks post-transplantation. (C) Representative H.E. staining of primary p18-/- tumors and tumors generated by p18-/- tumor cells with E2 supplement. Note the well-differentiated cells with glandular structure in both primary and regenerated tumors. (D) Representative H.E. staining of p16-/-;Brca1MGKO tumors generated in the presence or absence of E2 supplement. Note the poorly differentiated cells with increased fibroblast-like cells in the tumors with E2 treatment. Spindle cells (black arrows), cells with high nuclear-cytoplasm ratio (green arrows), mitotic cells (red arrows), and necrosis (yellow arrows) are indicated.
Additional file 4:
Figure S4. Estrogen promotes lung metastasis of Brca1-deficient mammary tumors. (A, B) Metastatic p18-/-;Brca1MGKO tumor cells were inoculated into the MFPs of NSG mice with either E2 or placebo supplement. When newly generated tumors reached maximum size allowed by the IACUC in 3–6 weeks, or the mice became moribund, lungs were dissected for analysis. Representative gross pictures (A) and H.E. staining (B) of lungs are shown.
Additional file 5:
Figure S5. IHC analysis of ERa and EMT markers for tumors with or without E2 treatment. (A-C) Representative p18-/-;Brca1MGKO and p18-/- mammary tumors treated with E2 or placebo were immunostained with the antibodies indicated. Note the negative ERa staining in E2-treated p18-/-;Brca1MGKO tumors (B) and positive ERa staining in E2-treated p18-/- tumors (C).
Additional file 6:
Figure S6. Estrogen promotes EMT in Brca1-deficient tumor cells (A, B) p18-/-;Brca1MGKO type 1 (A) and p18-/- tumor cells (B) were treated with DMSO or E2 for the indicated time and analyzed by western blot. (C, D) p18-/-;Brca1MGKO type 2 tumor cells were treated with DMSO or 50 nM E2 for 2 h or 72 h, and then analyzed by FACS (C) and western blot (D). (E) SUM149 cells were treated with DMSO or 50 nM E2 for 72 h and analyzed by western blot.
Additional file 7:
Figure S7. Estrogen stimulates ER-positive cell proliferation that is blocked by 4OHT. MCF-7 cells were treated with DMSO and 5 nM E2 with or without 5 µM 4OHT. The number of viable cells was determined on day 1, day 3, and day 5 (A). Cells treated for 72 h were collected and analyzed by western blot (B); *p?<?0.05 between E2-treated and E2?+?4OHT-treated groups at the time points (Student t test). Data are represented as mean?±?SD (n?=?4).
Additional file 8:
Figure S8. E2 activates the AKT pathway in BRCA1 mutant PDX tumors, and inhibition of Akt suppresses proliferation of Brca1-deficient tumor cells. (A, B) Representative BRCA1 mutant PDX tumors treated with E2 or placebo were immunostained with the antibodies indicated. (C) p18-/-; Brca1MGKO type 2 tumor cells were treated with DMSO or 5 nM E2 in the presence of different dosage of AZD5363. The number of viable cells were determined on day 1, day 3, and day 5; *p?<?0.05 between E2-treated and E2?+?AZD5363-treated groups at the time points (Student t test). Data are represented as mean?±?SD (n?=?4). (D) Representative p18-/-; Brca1MGKO tumors treated with AZD5363 or vehicle for 7 days were analyzed by IHC. (PDF 3678 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, C., Bai, F., Zhang, Lh. et al. Estrogen promotes estrogen receptor negative BRCA1-deficient tumor initiation and progression. Breast Cancer Res 20, 74 (2018). https://doi.org/10.1186/s13058-018-0996-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-018-0996-9