Abstract
Sepsis is a severe medical condition characterized by a systemic inflammatory response, often culminating in multiple organ dysfunction and high mortality rates. In recent years, there has been a growing recognition of the pivotal role played by mitochondrial damage in driving the progression of sepsis. Various factors contribute to mitochondrial impairment during sepsis, encompassing mechanisms such as reactive nitrogen/oxygen species generation, mitophagy inhibition, mitochondrial dynamics change, and mitochondrial membrane permeabilization. Damaged mitochondria actively participate in shaping the inflammatory milieu by triggering key signaling pathways, including those mediated by Toll-like receptors, NOD-like receptors, and cyclic GMP-AMP synthase. Consequently, there has been a surge of interest in developing therapeutic strategies targeting mitochondria to mitigate septic pathogenesis. This review aims to delve into the intricate mechanisms underpinning mitochondrial dysfunction during sepsis and its significant impact on immune dysregulation. Moreover, we spotlight promising mitochondria-targeted interventions that have demonstrated therapeutic efficacy in preclinical sepsis models.
Similar content being viewed by others
Introduction
To advance understanding of sepsis and septic shock, the task force convened by the European Society of Intensive Care Medicine and the Society of Critical Care Medicine introduced the "Sepsis-3" definition between 2014 and 2015 [1]. This update redefined sepsis as life-threatening organ dysfunction caused by a dysregulated inflammatory response to infection [1]. Sepsis contributes to 11 million deaths out of the 48.9 million reported cases, accounting for 19.7% of all global deaths in 2017 [2, 3]. The request for novel therapeutic targets in sepsis management assumes paramount importance.
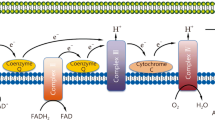
Mitochondria are primary energy generators and participate in cellular processes, such as maintaining redox balance, buffering Ca2+, and initiating apoptosis [4]. A mitochondrion contains two membranes, the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM), forming the matrix and intermembrane space (IMS) [5]. The mitochondrial respiratory chain comprises five complexes localized within the IMM: complex I, II, III, IV, and V. Complexes I–IV receive electrons, creating an electrochemical gradient of proton across the IMM referred to as mitochondrial membrane potential (ΔΨm). This ΔΨm is utilized by the complex V (F1F0 ATP synthase) to drive the production of ATP [6].
Accumulative evidence suggests that mitochondria play crucial roles in the pathogenesis of sepsis [7, 8]. Both laboratory studies and clinical investigations have documented the increase in mitochondrial damage during sepsis [9, 10]. Mitochondrial dysfunction occurs when mitochondria, fail to function properly upon damage. Mitochondria are not only involved in energy production, but also play critical roles in regulating cell metabolism and several cellular processes. The effects of mitochondrial dysfunction are enormous, including energy deficiency and activation of multiple pathways that alter cell function and fate. In the context of sepsis, the damaged mitochondria actively partake in shaping the inflammatory response via various signaling pathways [11]. Advances in mitochondria-targeted therapeutics have demonstrated that improving mitochondrial quality can reduce sepsis-induced inflammation, organ failure, and consequent mortality [12,13,14]. Thus, regulating mitochondrial quality is emerging as a promising strategy for sepsis treatment.
This review seeks to explore the complex mechanisms underlying mitochondrial dysfunction in sepsis and its profound influence on immune dysregulation. Additionally, we highlight promising interventions targeted at mitochondria that have shown therapeutic effectiveness in preclinical sepsis models. By comprehensively understanding the interplay between mitochondrial integrity and immune responses, we strive to pave the way for the development of novel and effective therapeutic approaches in combating sepsis-associated morbidity and mortality.
Mitochondrial stress and damage during sepsis
Sepsis triggers significant stress to mitochondria within immune cells and various tissues, leading to structural distortions, potential loss, and a marked decrease in respiratory activity. This section explores the specifics of the mitochondrial damage and the underlying mechanisms.
Mitochondrial damage
Despite the limited amount, clinical data clearly indicate mitochondrial dysfunction during sepsis. For instance, Japiassu and colleagues demonstrated that peripheral blood mononuclear cells (PBMCs) from septic shock patients have impaired mitochondrial ATP synthase activity [15]. Garrabou et al. observed inhibition of respiratory complexes in the PBMCs from septic patients without shock or multi-organ failure (MOF), suggesting that mitochondrial impairment precede these symptoms [16]. The platelets from septic patients showed lower activity of mitochondrial nicotinamide adenine dinucleotide dehydrogenase (NADH), complex I, I/III, and IV [17]. In addition to blood cells, muscle tissue of septic patients has also been reported with mitochondrial dysfunction and ATP depletion [18, 19]. Severe cardiac impairment has been observed in the non-surviving septic patients, with histologic analysis of heart sections showing mitochondrial cristae derangement (Table 1) [20].
In animal models, cecal ligation and puncture (CLP), endotoxins/bacteria administration, and colon ascendens stent peritonitis (CASP) are commonly utilized to trigger sepsis [21]. Studies using these models support the clinical findings, highlighting the substantial impact of sepsis on mitochondrial homeostasis (Table 1). However, it is essential to acknowledge the presence of conflicting data showing unchanged or even increased mitochondrial activity in some cases, as previously discussed [22, 23]. Variables such as the nature of the septic insult, the severity of sepsis, timing of assessments, and methods of measurement may contribute to the discrepancies [24,25,26].
In addition, the common practice of using isolated cells or mitochondria from septic tissues for complex activity assays may not accurately reflect the in vivo mitochondrial status. This issue arises from the absence of circulating substances, such as cytokines, in vitro, which play pivotal roles in regulating mitochondrial respiration [16]. For instance, Boulos et al. demonstrated that serum from septic patients significantly reduced endothelial cell mitochondrial respiration compared to serum from healthy individuals, revealing the impact of circulating substances on mitochondrial function during sepsis [27].
Furthermore, normalization methods profoundly influence the evaluation of mitochondrial function. In a previous study, Fredriksson et al. measured the complex I activities in intercostal muscle mitochondria from septic patients and observed a 60% decrease when normalized to the dry weight of the muscle. However, no difference was found when normalization was conducted against citrate synthase activity. This was because the citrate synthase activity also declined during sepsis [28], emphasizing the importance of meticulous methodological consideration in such analyses.
Mechanisms of mitochondrial damage
Mitochondrial injury during sepsis is regulated by several factors, including reactive nitrogen species (RNS)/reactive oxygen species (ROS), mitophagy, mitochondrial dynamics, and mitochondrial membrane permeabilization, etc. In this section, we will examine the regulatory roles of these factors in mitochondrial health amidst septic conditions.
RNS/ROS burst
One of the well-exploited triggers of mitochondrial damage is nitric oxide (⋅NO), an RNS that is produced by inducible nitric oxide synthase (iNOS) [40]. ⋅NO could be converted to other reactive nitrogen species such as nitrite (NO2−) and nitrogen dioxide (·NO2) (Fig. 1) [41]. Escames et al. have investigated the role of iNOS in skeletal muscle mitochondria using a CLP mouse model. They observed a reduction in mitochondrial respiratory complex activity accompanied by elevated expression of iNOS during sepsis. In contrast, the iNOS-deficient mice did not exhibit such mitochondrial impairment [40]. A similar observation has been observed in the liver mitochondria of LPS-treated mice [42]. In line with these, inhibition of mitochondrial respiration induced by septic serum could be mitigated by the nitric oxide synthase inhibitor [27]. These data collectively suggest that iNOS play a crucial role in mitochondrial damage during sepsis.
Pathways of RNS and ROS production in sepsis. Excessive nitric oxide is generated by both cytosolic and mitochondrial iNOS during sepsis. The nitric oxide can be converted to other reactive nitrogen species (RNS), such as nitrite and nitrogen dioxide. On the other hand, excessive reactive oxygen species (ROS) are produced by NOXs and mitochondrial electron transport chain (ETC). Nitric oxide and superoxide react to form additional derivatives, notably the highly toxic peroxynitrite. The accumulation of these reactive species inflicts damage to cellular components, including lipids, proteins, and nucleic acids, thereby compromising mitochondrial respiration and integrity
Sepsis also stimulates the formation of ROS. Superoxide radical (O2·−) originates from nicotinamide adenine dinucleotide phosphate oxidases (NOXs) and mitochondria ETC [43]. O2·− is considered unstable and can be converted to other types of ROS (Fig. 1) [44]. On the other hand, declines in the antioxidative system happen during sepsis. For example, sirtuin proteins, which can protect cells from oxidative stress, have been found to decrease after septic insults [45, 46]. Oxidative stress occurs when ROS level overwhelms the antioxidant defense system in cells, causing damage to lipids, proteins, and nucleic acids [47].
Mechanistically, ·NO selectively binds to complex IV, where it competes with O2 for the binuclear CuB/cytochrome a3 center, resulting in a reversible inhibition of the complex IV [48]. While ·NO is relatively unreactive, its reaction with ROS gives rise to a series of more reactive derivatives [49]. Peroxynitrite (ONOO–) is one of the highly toxic derivatives (Fig. 1). Peroxynitrite can cause impairments to mitochondria via oxidation, irreversibly inhibiting mitochondrial complex I, CII, CIV, ATP synthase, and several critical enzymes. Consequently, the RNS/ROS exert a profound inhibitory effect on mitochondrial respiration and contribute to mitochondrial damage during sepsis [50].
Mitophagy
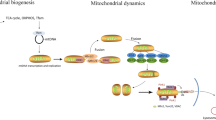
Mitophagy is a specialized form of autophagy that selectively degrades damaged mitochondria (Fig. 2a) [51]. Impaired mitophagy prevents mitochondrial turnover, leading to the accumulation of dysfunctional mitochondria [52]. Growing evidence shows that inhibition of mitophagy occurs in immune cells during the inflammatory response. Yu et al. found that Caspase-1 in macrophages cleaves Parkin to inhibit mitophagy upon inflammasome activation [53]. Similarly, Patoli et al. demonstrated mitophagy inhibition in the LPS/IFN-γ-treated macrophage cells, CLP mouse model, and septic patients, revealing caspases 1/11 dependent PINK1 degradation in the early stage of inflammation [54]. Additionally, inflammation-induced pro-IL-1α can interact with cardiolipin, preventing it from serving as a mitophagy receptor [55]. AMP-activated protein kinase (AMPK) is a regulator of autophagy, which positively regulates mitochondrial clearance by activating unc-51 like autophagy activating kinase 1 (ULK1) [56]. TLR4 signaling has been reported to prevent the activation of AMPK in neutrophil and macrophage [57]. Thus, lack of AMPK activation could be another reason for the accumulation of damaged mitochondria in sepsis.
Pathways of mitophagy and mitochondrial dynamics. a The most extensively studied mitophagy pathway involves PTEN-induced kinase 1 (PINK1) and E3 ubiquitin ligase Parkin. Upon ΔΨm loss, PINK1 is stabilized on the OMM, where it recruits and activates Parkin. Parkin subsequently ubiquitinates mitochondrial surface proteins, signaling the autophagosome to encapsulate the dysfunctional mitochondrion. Adaptor proteins including p62, Optineurin (OPTN), calcium binding and coiled-coil domain-containing protein 2 (NDP52), tax1-binding protein 1 (TAX1BP1), and next to BRCA1 gene 1 protein (NBR1) play a role in linking the ubiquitin chains to microtubule-associated protein 1 light chain 3 (LC3) on phagophores. On the other hand, some mitochondrial receptor proteins (or lipids) such as FUN14 domain containing 1 (FUNDC1), prohibitin (PHB), BCL2 interacting protein3 (BNIP3), Nip3-like protein X (NIX), cardiolipin, and syntaxin 17 (STX17) can recruit phagophore membranes independent of ubiquitin. The autophagosome finally fuses with a lysosome for the degradation and recycling of mitochondria. b Mitochondrial dynamics is regulated by several dynamin-related GTPases, including Mfn1/2, OPA1, Drp1, Fis1, MiD49/51, and Mff. Mfn1/2 facilitate the fusion of the OMM, while OPA1 is responsible for the fusion of the IMM. The fission process is initiated by the endoplasmic reticulum (ER)-mediated pre-constriction of mitochondria. Drp1 is recruited to the pre-constricted sites by receptors (Mff, Fis1, and MiD49/51). After binding to OMM, Drp1 oligomerizes into ring-shaped filaments. Hydrolysis of GTP by Drp1 results in constriction and closure of the ring. Dnm2 is finally recruited to the constricting site to complete the fission. In addition to the ER, lysosome and Golgi-derived vesicles have also been reported to regulate mitochondrial fission
Interestingly, activation of mitophagy also occurs during immune response. Ip and colleagues showed that interleukin 10 (IL-10) promotes mitophagy by inhibiting the mammalian target of rapamycin (mTOR) in macrophages [58]. Moreover, nuclear factor κB (NF-κB) facilitates mitophagy by inducing the expression of autophagic receptor p62. This NF-κB-p62-mitophagy pathway exists as a self-limiting mechanism to prevent excessive inflammation and maintain homeostasis [59]. Sestrin 2 (SESN2) also play a role in the p62-dependent mitophagy [60]. Extended LPS stimulation upregulates the protein level of SESN2, which interacts with p62 to facilitate its aggregation on mitochondria. Besides, SESN2 activates the autophagic machinery by raising the ULK1 protein level [60]. Overall, the accumulation of damaged mitochondria is likely to result from both forward and reverse regulation of mitophagy. How these different mitophagic pathways are spatiotemporally coordinated in sepsis is still elusive. Given the critical role of damaged mitochondria in promoting immune response (which will be discussed in the later section), it is plausible that early-stage inhibition of mitophagy promotes the accumulation of dysfunctional mitochondria and benefit bacterial defense [54], while late-stage activation of mitophagy represents a cellular attempt to mitigate inflammation and restore host homeostasis [59].
Unlike in immune cells, the overall mitophagy in various organs is enhanced during sepsis. The heart samples from LPS-challenged mice have shown decreased mitochondrial number and volume, suggesting autophagic removal of mitochondria [29, 61]. Immunoblot analysis of kidney tissue from LPS or CLP-treated mice has shown a decrease in mitochondrial protein levels (TOM20 and TIM23) compared with control mice, providing substantial evidence that mitophagy is induced during the sepsis-caused acute kidney injury [62]. It was also demonstrated that the mitophagy is mainly mediated by the PINK1/Parkin/OPTN pathway [62]. Reductions of mitochondrial mass were also reported in the liver of CLP or LPS-treated septic mice [63]. These studies collectively suggest that mitophagy is enhanced in various organs during sepsis.
Mitochondrial dynamics
Mitochondria constantly undergo fusion and fission processes (Fig. 2b). Fusion forms interconnected mitochondrial networks, promoting content exchange, which is essential for the integrity of the mitochondrial genome and proteome. Conversely, fission fragments mitochondria, aiding in the elimination of dysfunctional parts [64, 65]. Dysregulation of mitochondrial dynamics is a crucial mechanism that induces mitochondrial stress [66].
During sepsis, excessive mitochondrial fragmentation happens in various tissues, revealing the change in mitochondrial dynamics [62, 67, 68]. To exploit the influence of mitochondrial fission in sepsis, Gonzalez et al. treated rats with the Drp1 inhibitor mdivi-1 before CLP administration. This treatment restored mitochondrial shape and prevented the reduction of complex activities and apoptosis in hepatocytes [68]. Similar beneficial effects of mdivi-1 have been confirmed in other studies [13, 69, 70]. Pharmacological administration of alternative fission inhibitors, such as P110 (inhibit Drp1/Fis1 interaction), also showed protection to mitochondria under septic stress [71, 72]. These findings demonstrate that excessive fission significantly contributes to the impairment of mitochondria during sepsis. Inhibition of this process, therefore, is an effective method to restore mitochondrial function.
Mitochondrial membrane permeabilization
Mitochondrial membrane pores cause mitochondrial swelling, content loss, and ΔΨm dissipation [73]. Three principal mechanisms involved in pore formation during sepsis are mitochondrial outer membrane permeabilization (MOMP), mitochondrial permeability transition (MPT), and more recently characterized gasdermins (GSDMs)-mediated mitochondrial membrane opening (Fig. 3).
Mechanisms of mitochondrial membrane permeabilization in sepsis. Three principal mechanisms are implicated in sepsis-related mitochondrial membrane pore formation. Mitochondrial outer membrane permeabilization (MOMP) is mediated by Bcl-2 family proteins Bax/Bak and regulated tBid. Large BAK/BAX pores allow the protrusion of IMM into the cytosol, therefore, enabling the escape of mitochondrial matrix contents. Mitochondrial permeability transition pore (MPTP) represents another type of mitochondrial pore in sepsis. Despite the opening mechanism of MPTP is still elusive, mitochondrial components such as F1FO (F)-ATP synthase, ANT, and Cyp-D have been confirmed to play pivotal roles in this process. Gasdermins (GSDMs) are well-known to form pores on plasma membrane during immune response. Recent advances have demonstrated that GSDMs target both the inner and outer mitochondrial membranes via their strong binding preference to cardiolipin. ROS promote the externalization of cardiolipin from IMM to OMM, thus, positively regulate GSDMs-mediate pore formation. Overall, mitochondrial membrane permeabilization leads to mitochondrial swelling, content loss, and dissipation of membrane potential during sepsis
MOMP is mediated by Bcl-2 family proteins Bax and Bak, which open the OMM in response to apoptotic signals. MOMP leads to mitochondrial damage and release of intermembrane space molecules like cytochrome C [74]. Large Bax/Bak pores also allow the IMM to protrude out of mitochondria, releasing matrix contents [75, 76]. Cytokines such as INF-γ and TNF-α could be recognized by the cell membrane death receptors, thereby activating caspase-8 during sepsis [77]. The activated caspase-8 cleaves Bid, generating a truncated form of Bid (tBid), which translocates to mitochondria and activates Bax/Bak to induce MOMP and cell death [78,79,80]. Chuang et al. have observed increased tBid in mitochondrial fractions of various tissues from the CLP-treated mouse, while Bid deficiency significantly reduced the downstream cell death [81]. In addition to caspase-8, caspase-1 has also been reported to cleave Bid and induce MOMP during the inflammatory response of macrophage [82].
The occurrence of MPT has been described in several septic models [83,84,85,86]. Pharmacological inhibition of MPT by cyclosporin A (CsA) can significantly attenuate mtDNA release and mitochondrial dysfunction in these models, suggesting MPT’s crucial role in sepsis-induced mitochondrial damage [83,84,85]. Although the exact molecular composition and mechanism of MPT remains a topic of ongoing research, key components are believed to include the F1FO (F)-ATP synthase, adenine nucleotide translocator (ANT), cyclophilin D (Cyp-D), and voltage-dependent anion channel (VDAC) [87]. These components assemble into a supramolecular pore (MPTP) at the interface of the IMM and OMM [88]. Elevated ROS levels can induce MPTP opening through oxidative modifications of MPT constituents or other mitochondrial proteins [89, 90].
GSDMs are key players in immune response, particularly in mediating pyroptosis. During inflammation, GSDMs are cleaved by caspases to release their active N-terminal fragments (GSDMs-N). GSDMs-N translocate to the plasma membrane, forming pores and disrupting the membrane [91]. Recent findings indicate that GSDMs also target mitochondrial membranes, representing a new pathway of mitochondrial permeabilization [92,93,94,95,96,97]. Time-lapse imaging analysis revealed that mitochondrial damage occurs much earlier than the opening of cell membrane, suggesting that GSDMs-N permeabilizes the mitochondria before cell membrane [92, 93]. This phenomenon has been attributed to the strong binding preference of GSDMs-N to mitochondrial cardiolipin [92]. Of note, ROS promote the externalization of cardiolipin from IMM to OMM, explaining the accumulation of GSDMD-N on OMM, as cardiolipin predominantly localizes in the IMM under normal conditions [95]. Overall, GSDM-mediated pore formation represents a novel pathway of mitochondrial permeabilization during immune response.
Damaged mitochondria are potent triggers of immune response and metabolic reprogramming
The innate immune response plays a key role in the pathophysiology of sepsis. Upon activation, pattern recognition receptors (PRRs) initiate signaling cascades, leading to the nuclear translocation of NF-κB, interferon regulatory factor (IRF), and other transcription factors. These transcription factors initiate the production of pro-inflammatory cytokines, chemokines, type I interferons (IFNs), etc. [98]. In the past few decades, damaged mitochondria have been widely demonstrated as a crucial regulator of innate immunity, particularly through the NLRP3, TLR9, and cGAS pathways. Thus, mitochondria damage is not simply a passive outcome under stress conditions, but actively contributes to the inflammatory response that is essential for the defense of pathogens. However, in the context of sepsis, excessive or persistent inflammation can cause a fatal inflammatory imbalance in the body, which ultimately leads to tissue and organ damage [99]. In this section, we will highlight the roles of mitochondria in regulating inflammatory pathways.
NLRP3 inflammasome pathway
NLRP3 inflammasome activation
The NLRP3 inflammasome activates pro-caspase-1, which cleaves pro-inflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-18 (IL-18) to promote their maturation [100]. Current studies, mainly in monocytes/macrophages, have revealed several regulatory roles of mitochondria in NLRP3 inflammasome activation. Mitochondrial ROS (mtROS) have been demonstrated as essential molecules for NLRP3 inflammasome activation. In sepsis models, inhibition of mtROS reduces the NLRP3 inflammasome-mediated cytokine production [101,102,103]. Several studies have provided insights into the mechanisms by which ROS modulate NLRP3 activation. In 2010, Zhou et al. identified thioredoxin (TRX)-interacting protein (TXNIP) as a binding partner of NLRP3. Under basal conditions, TXNIP binds to TRX, being unavailable to NLRP3. ROS promote NLRP3 activation by triggering the dissociation of TXNIP from TRX [104]. In another study, mtROS were confirmed to facilitate NLRP3 inflammasome activation through promoting its deubiquitination [105]. Interestingly, Bauernfeind and colleagues reported that ROS increase the NLRP3 expression instead of activating it, suggesting a translational regulation of NLRP3 by ROS [106].
mtDNA also regulates NLRP3 inflammasome activation. Nakahira et al. demonstrated that preventing MPT-mediated mtDNA release significantly reduces IL-1β secretion in macrophages after LPS + ATP challenge [83]. Interestingly, NLRP3 inflammasome is preferentially activated by oxidized mtDNA (ox-mtDNA), rather than normal mtDNA [107]. Zhong et al. found that TLR4 signalling promotes de novo mtDNA synthesis to sustain the generation of ox-mtDNA, since the newly synthesized mtDNA is not packaged and is highly susceptible to oxidation [108]. Before being released into cytosol, the ox-mtDNA undergoes a cleavage process to become 500–650 bp fragments. Xian et al. recently identified the flap structure-specific endonuclease 1 (FEN1) as the key mediator of this process [109]. AIM2 inflammasome can also be activated by the mtDNA. Unlike NLPR3 inflammasome, AIM2 inflammasome is activated by normal DNA to ox-mtDNA [107, 108].
Spatial association between mitochondria and NLRP3
The spatial association of NLRP3 inflammasome with organelles is critical for its activation. Early studies posited mitochondria or mitochondria-associated membrane (MAM) as the docking sites of NLRP3, while more recent publications have implicated trans-Golgi network (TGN) and microtubule-organizing center (MTOC) [110,111,112,113,114].
Zhou et al. reported that inflammatory stimuli trigger NLRP3 to co-localize with mitochondria and MAM in 2010 [112]. This spatial vicinity may facilitate the sensing of mitochondria-derived inflammatory signals [112, 115]. Microtubules also play a role in the mitochondria-NLRP3 association. Mechanistically, mitochondrial dysfunction during inflammation reduces NAD+ (oxidized form of nicotinamide adenine dinucleotide) generation, which in turn inhibits NAD+-dependent α-tubulin deacetylase sirtuin 2, leading to an increase in α-tubulin acetylation. The acetylated α-tubulin subsequently promoted dynein-dependent transport of mitochondria to NLRP3 [116]. Inflammatory stimuli also trigger the transport of NLRP3 towards mitochondria, which is orchestrated by microtubule-affinity regulating kinase 4 (MARK4). Depletion of MARK4 impairs NLRP3 spatial arrangement and inflammasome activation [111].
Mitochondrial antiviral-signaling protein (MAVS) and cardiolipin mediate the anchorage of NLRP3 on mitochondria [117,118,119,120,121]. MAVS lacking mitochondrial targeting domain cannot recruit NLRP3 or induce NLRP3 oligomerization, suggesting its mitochondrial localization is essential for NLRP3 inflammasome activation [120]. Given the well-established role of MAVS in recognizing virus RNA, it is plausible that microbial RNA plays a role in promoting the MAVS-dependent recruitment of NLRP3 during bacterial infection [121, 122]. In line with this, virus or Escherichia coli-induced activation of NLRP3 inflammasome was confirmed to greatly depend on MAVS [121], whereas LPS + ATP or LPS + nigericin (without microbial RNA) induced NLRP3 inflammasome activation is only partially affected by MAVS [117, 120, 121]. Cardiolipin also interacts with NLRP3 and contributes to the inflammasome activation [118]. A recent study suggested that cardiolipin recruits both NLRP3 and caspase-1, serving as a platform for inflammasome assembly [119].
Several recent publications have highlighted the involvement of Golgi apparatus and MTOC in NLRP3 inflammasome activation [113, 114, 123]. It is likely that NLRP3 protein is first transported to meet mitochondria along the microtubules, after which NLRP3 redistributes to the adjacent Golgi apparatus [115, 124]. Then, the NLRP3 is transported to the MTOC and reorganized into a single speck structure. At the MTOC, NLRP3 engages with NEK7, a centrosome-localized kinase that is essential for NLRP3 inflammasome activation (Fig. 4) [111, 114, 123, 125, 126].
Mitochondria-regulated inflammatory pathways in sepsis. Pattern recognition receptors (PRRs) of immune cells recognize pathogen-associated molecular patterns (PAMPs) from microbes or damage-associated molecular patterns (DAMPs) released by damaged host cells. a Mitochondria orchestrate the activation of the NLRP3 inflammasome. mtROS and ox-mtDNA promote the NLRP3 inflammasome activation after their release from the damaged mitochondria. Additionally, mitochondrial antiviral-signaling protein (MAVS) and cardiolipin mediate the spatial association between mitochondria and NLRP3, which may facilitate a rapid and efficient sensing of the inflammatory signals from the damaged mitochondria by NLRP3. Early studies posited mitochondria or mitochondria-associated membrane (MAM) as the docking sites of NLRP3, while more recent publications have indicated the involvement of Golgi and microtubule-organizing center (MTOC). It could be plausible that NLRP3 proteins are first transported to meet mitochondria, after which NLRP3 redistributes to the adjacent Golgi apparatus as oligomeric cages. Then, the NLRP3 cages are transported to MTOC and reorganized into a single inflammasome speck. Activated NLRP3 inflammasome mediates the activation of pro-caspase-1, which proteolytically processes GSDMs and pro-inflammatory cytokines. The matured cytokines are released from the GSDMs pores to transmit inflammatory signals. b Mitochondria regulate the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway. cGAS is activated upon mtDNA binding, leading to the synthesis of cyclic GMP-AMP (cGAMP). cGAMP then induces a conformational change in STING, leading to its activation at ER. Activated STING translocates to Golgi, where it activates transcription factors interferon regulatory factor 3 (IRF3) and nuclear factor κB (NF-κB) to initiate the production of type I interferons and other pro-inflammatory cytokines that are required for effective immune response against pathogens. c Mitochondria regulate the TLR9 pathway. mtDNA shares similarities with bacterial DNA, thus can be recognized by TLR9. As with other TLRs and cGAS-STING axis, TLR9 pathway activates IRF3 and NF-κB to regulate the inflammatory response
cGAS-STING pathway activation
The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway is another mitochondria-regulated inflammatory pathway. cGAS detects cytosolic double-stranded DNA and initiates an innate immune response [127] (Fig. 4). Both normal and oxidized mtDNA can be recognized by cGAS [128], but ox-mtDNA is more resistant to degradation by the cytosolic nuclease 3' repair exonuclease 1, increasing its likelihood of interaction with cGAS [129].
In the CLP mouse model, Huang et al. have demonstrated that mtDNA can be released via the GSDMD pore to activate cGAS-STING pathway [94]. This pathway not only enhances the inflammatory response but also suppresses lung endothelial cell proliferation, which compromises the recovery capacity of host. Notably, the deletion of cGas gene provided mice with substantial protection from lung injury 72 h post-CLP, suggesting that cGAS-STING pathway is crucial in septic lung inflammation [94].
Studies have shown that circulating cell-free mtDNA levels are elevated in septic patients and animal models, correlating with poor prognosis [130,131,132,133]. Like cytosolic mtDNA, circulating mtDNA activates the cGAS-STING pathway and promotes inflammation during sepsis [133]. In CLP mice, the mtDNA-cGAS-STING signal promotes intestinal inflammation and gut barrier dysfunction, which could be significantly attenuated by removing circulating mtDNA with DNase I [134]. Mechanistically, host nuclear DNA, mtDNA and bacterial DNA can be recognized by cGAS, while to what extent mtDNA contributes to the cGAS-STING pathway activation awaits investigation [135, 136].
TLR9 pathway activation
TLR9 is initially known for sensing unmethylated CpG DNA motifs (consisting of a central cytosine-guanine dinucleotide plus flanking regions) from bacterial and viral DNA during innate immune response [137, 138]. However, a subsequent study suggests that TLR9 also recognizes endogenous mtDNA, which shares similarities with bacterial DNA [139]. This interaction stands for another inflammatory pathway regulated by mitochondria in sepsis (Fig. 4).
Experimental models have shown that intravenous administration of mitochondrial debris can elicit an immune response comparable to that of CLP treatment. However, Tlr9 knockout (KO) or DNase pretreatment of the mitochondrial debris greatly attenuates the inflammatory response, suggesting the crucial role of the mtDNA-TLR9 axis in sepsis [140]. In line with this, TLR9 inhibitor OND-I suppresses caspase 1 activation and IL–1β production in LPS-challenged cardiomyocytes [38]. Moreover, Tlr9 KO reduced renal dysfunction and splenic apoptosis after CLP treatment, and increased survival at 96 h [141]. Interestingly, Plitas and colleagues found that Tlr9 KO mice exhibited better bacterial clearance and greater survival than wide type (WT) mice after CLP treatment. The protective effects were associated with increased recruitment of granulocytes to the peritoneum by dendritic cells (DCs) [142]. These findings suggest that TLR9 might have a major role in the immunopathogenesis of polymicrobial sepsis compared to other TLRs [142].
Metabolic reprogramming
During sepsis, a widespread alteration in the metabolic pattern occurs in virtually all cell types, leading to a shift from oxidative phosphorylation (OXPHOS) to glycolysis—a phenomenon known as “metabolic reprogramming” [143]. The metabolic reprogramming is driven by the downregulation of OXPHOS due to mitochondrial dysfunction and the upregulation of glycolysis through the enhancement of glycolytic pathways [144]. This shift in metabolism profoundly affects immune response and organ function in sepsis.
In the immune context, glycolysis, albeit less efficient than OXPHOS, allows rapid generation of ATP and synthesis of metabolic intermediates necessary for immune cell activation and proliferation [145]. Glycolytic inhibitors, such as 2-deoxyglucose (2-DG), have been shown to reduce systemic inflammation in sepsis, indicating their potential in therapeutic interventions [146, 147]. The metabolic reprogramming is also implicated in the development MOF. An early histopathologic study of patients dying of sepsis has revealed that the actual cell death in most organs is minimal, despite severe clinical signs of MOF [148]. This observation indicates that the organ failure is functional rather than structural [149]. Given that mitochondrial dysfunction results in less efficient ATP production, cells in affected organs face an energy crisis during sepsis. Consequently, metabolic reprogramming and the resultant bioenergetic failure are recognized as critical contributors to MOF in sepsis [150]. A key question regarding MOF in sepsis is whether it represents an adaptive or maladaptive event. Singer et al. have suggested that sepsis-induced MOF might be an adaptation by the host to increase the chances of cell survival against the infection. This response mirrors a state of “hibernation” in which non-essential functions are downregulated [9, 149].
Mitochondria-based therapeutic interventions
Effective treatments are crucial for aiding critically ill patients with sepsis. Previous research has shown that sepsis survivors responded early to the illness with induction of mitochondrial biogenesis and antioxidant defense, indicating the potential of maintaining mitochondrial homeostasis for sepsis therapy [19].
Antioxidants
Given the roles of ROS in triggering mitochondrial damage and inflammation, antioxidants have been extensively studied as therapeutic agents against sepsis. Mitochondria-targeted antioxidants have shown better effects on reducing inflammation compared to the untargeted equivalents [101, 151, 152]. The most frequently used mitochondria-specific antioxidants include MitoQ, MitoVitE, and MitoTempol (Fig. 5) [153].
Mitochondria-based therapeutic interventions. Mitochondria-targeted antioxidants such as MitoQ, MitoVitE, and MitoTempol are selectively targeted to mitochondria by conjugating to a lipophilic cation decyl-triphenylphosphonium (TPP+). The positive charge of cation enables the molecules to accumulate in mitochondrial matrix, therefore, specifically quench the mitochondrial ROS. Non-antioxidants Urolithin A, Mdivi-1, and Cyclosporin A improve mitochondrial quality by promoting mitophagy, inhibiting mitochondrial fission, and blocking MPTP, respectively. Metformin plays multiple roles on mitochondria, including enhancing mitophagy, reducing mtROS, preventing ox-mtDNA production, and inhibiting mitochondrial protein translation. These compounds target different aspects of mitochondria and exhibit great potential in reducing sepsis-induced mitochondrial damage, excessive inflammation, and organ dysfunction
MitoQ
MitoQ is synthesized by conjugating ubiquinone with the lipophilic cation decyl-triphenylphosphonium (TPP+). Although it has not received approval from the U.S. Food and Drug Administration (FDA), MitoQ has been the subject of several clinical trials aiming to explore its potential benefits for treating oxidative pathologies [154]. Lowes et al. demonstrated that MitoQ protects mitochondria during sepsis, and suppresses pro-inflammatory cytokine release, leading to reduced acute liver and renal dysfunction [102]. The beneficial effects have also been confirmed in other organs, including the lung, intestinal barrier, skeletal muscle, and heart, leading to the increased survival rate of septic animals [12, 155,156,157]. Notably, MitoQ treatment 6 h after sepsis induction yielded comparable outcomes in comparison with immediate treatment in the diaphragm muscle. This property makes MitoQ a good candidate for clinical application, considering the typical delay between sepsis onset and treatment initiation [157].
MitoVitE
MitoVitE is a modified form of vitamin E, attached to the TPP+ [158]. Zang et al. examined its effects on cardiac dysfunction in a rat pneumonia-related sepsis model, and confirmed that a single dose of MitoVitE protected cardiac mitochondria, suppressed cytokine burst, and neutrophil infiltration in the myocardium, ultimately improving the cardiac function [101]. Another study using LPS/peptidoglycan (PepG)-induced sepsis rat model revealed that MitoVitE offers similar protection as MitoQ in improving mitochondrial health, mitigating inflammation, and reducing organ dysfunction [159].
MitoTempol
MitoTempol, constructed by combining the piperidine nitroxide (Tempol) with TPP+, is another mitochondria-targeted antioxidant [160]. In a rat model of fecal peritonitis, MitoTempol administration reduced IL-1β level, renal oxidative stress, and improved renal function [161]. Interestingly, while this study showed no beneficial effect on the core body temperature and survival rate [161], another investigation revealed that even a 6 h delayed MitoTempol therapy significantly improved core body temperature and increased the survival rate from 40 to 83% in CLP mice [35]. These contradictory results may be related to MitoTempol dosage. Insufficient dosing may fail to provide benefits, whereas excessive dosing may lead to over-accumulation of this cationic agent, resulting in mitochondrial damage [35]. Thus, fine-tuning the dosage is essential to minimize side effects and maximize therapeutic benefits.
NAD+
NAD+ is known for its functions in redox metabolism, immune response, aging, and DNA repair [162]. NAD+ shortage occurs during sepsis, prompting the exploration of NAD+ supplementation as a potential therapeutic strategy [163, 164]. NAD+ precursors, such as nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR), are emerging as novel candidates for sepsis treatment. These precursors, naturally present in food and widely used as health supplements, have shown potential in preclinical studies by mitigating mitochondrial dysfunction, oxidative stress, inflammatory response, and multiorgan injury associated with sepsis [164,165,166]. The precise regulatory mechanisms of NAD+ in sepsis remain incompletely understood. As a critical co-enzyme, NAD+ plays a role in alleviating oxidative stress, in part through the activation of sirtuins, like Sirt3, a mitochondria-localized sirtuin [164, 165].
Non-antioxidants
Several non-antioxidant agents targeting mitophagy, mitochondrial dynamics, mitochondrial permeabilization, and other pathways have been reported to mitigate sepsis (Fig. 5).
Urolithin A
Urolithin A (UA) is a natural anti-aging compound known for inducing mitophagy [167]. It has attracted significant attention for the role in regulating inflammation [168, 169]. Two clinical trials in elderly individuals have shown that UA is safe, bioavailable, and well-tolerated [170, 171].
UA has been reported to sustain mitochondrial health in cardiomyocytes challenged with LPS in vitro [172]. In mice, UA administration alleviated the LPS-induced cardiac depression [173]. Interestingly, this protection was diminished upon FUNDC1 knockout, indicating UA activates mitophagy in a FUNDC1-dependent manner [173]. UA pre-administration was also found to alleviate pulmonary injury upon LPS challenge, and improve the survival rate [174]. Overall, while research on UA for sepsis treatment is still nascent, the existing evidence suggests significant therapeutic potential. Future research using more appropriate sepsis models would be recommended, as LPS treatment does not recapitulate the complex pathophysiological consequence of human sepsis [175, 176].
Mdivi-1
Mdivi-1 was first identified as an inhibitor of mitochondrial fission via chemical screening in 2008, [177]. Thereafter, numerous articles have been published on Mdivi-1, confirming its potency in inhibiting mitochondrial fission, improving mitochondrial health, and protecting cells from stress under various circumstances [178]. The wide application of Mdivi-1 promotes the development of novel therapeutic strategies for mitochondria-related diseases, including sepsis.
Mdivi-1 was shown to protect liver and brain function in the CLP mice model [179, 180]. Moreover, Zhu et al. showed that Mdivi-1 significantly restored the function of multiple organs in CLP-treated rats, and extended the average survival time from 8.83 h to 60.3 h. In this study, Mdivi-1 was administered 12 h after sepsis, which mirrors a clinically relevant scenario [13]. Other fission inhibitors, like P100 and irisin, have also been reported to mitigate organ injury and reduce mortality after sepsis [71, 72, 181].
Cyclosporin A
CsA is an FDA approved compound that has been utilized as an anti-inflammatory drug to treat ocular surface diseases [182]. Cyclosporin A has been widely used as an immunosuppressant to prevent immune responses against transplanted organs in clinic [183]. CsA potentially inhibits immune response via two pathways. First, CsA inhibits MPTP by interacting with Cyp-D, thus, reducing DAMPs release from mitochondria [184]. Second, CsA forms a complex with cyclophilin A to inhibit calcineurin. This inhibition thereafter impairs the nuclear translocation of nuclear factor of activated T cells (NFAT) and NFκB in immune cells, leading to reduced production of iNOS, inflammatory cytokines and prostaglandins [185].
Over the past two decades, CsA has been reported to attenuate mtDNA release and mitochondrial damage in various septic models [83,84,85, 186]. These studies confirmed the protective effects of CsA on sepsis-induced organ dysfunction and mortality [83,84,85, 186]. CsA and NIM811 (a cyclophilin non-binding derivate of CsA that inhibits MPTP) rather than tacrolimus (a potent immunosuppressive compound which does not inhibit MPTP) provided protection against septic insult, suggesting that the beneficial effects of CsA were predominantly related to the inhibition of MPTP [84, 187]. However, Joshi et al. reported that tacrolimus provided similar cardiac protection as CsA upon LPS treatment [188]. This discrepancy maybe due to the severity of mitochondrial injury; CsA's MPTP inhibitory function becomes critical only when mitochondrial damage is severe.
Metformin
Metformin has been widely used to treat type 2 diabetes, whose clinical experience and trial data have raised almost no safety concerns [189, 190]. Several studies have highlighted the capacity of metformin in combating sepsis. For instance, Liu et al. demonstrated that metformin prevents excessive inflammation and the development of immunosuppression, increasing bacterial clearance in the lungs of septic mice [191]. Metformin also preserves brain, lung, liver, and colon barrier function by suppressing sepsis-induced inflammatory responses in animal models [192,193,194]. In human patients, several retrospective studies have been performed to evaluate the effect of metformin on sepsis. However, some of these studies revealed that metformin users (mainly diabetic patients) had lower in-hospital mortality than nonusers [195,196,197], while others showed that pre-admission of metformin did not change the mortality in septic patients [198, 199]. Two meta-analyses on these published cohort data were, therefore, performed to confirm the contribution of metformin in the mortality in septic adult patients. The results supported the positive effect of metformin in lowering the mortality of sepsis [200, 201]. Recently, three clinical trials (NCT05979038, NCT06181422, NCT05900284) evaluating the efficiency and safety of metformin in sepsis patients are ongoing, which will improve our understanding about the clinical feasibility of metformin in sepsis therapy.
The pharmacological mechanisms of metformin are multifaceted. Metformin regulates inflammation through both AMPK-dependent and independent pathways [202]. Mechanistically, metformin activates AMPK and suppresses the nutrient sensor mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), which promotes autophagy. Autophagic elimination of damaged mitochondria subsequently limits NLRP3 inflammasome activation [203, 204]. AMPK also phosphorylate the PGC-1α to promote mitochondrial biogenesis [205]; or regulate transcription factors to reduce ROS and cytokine production [206, 207]. The activation of AMPK by metformin appears to be dose-dependent: Low-dose metformin drives AMPK activation through the lysosomal pathway, while high-dose metformin takes effect by inhibiting the complex I of respiratory chain, resulting in ATP shortage [208]. Xian et al. have revealed that the metformin-triggered ATP shortage can prevent the production of ox-mtDNA, thus, reducing NLRP3 inflammasome activation [204]. Metformin also diminishes IL-6 secretion, likely via the suppression of JNK and p38 MAPK [204]. Very recently, Marlies Cortés and colleagues uncovered that metformin’s anti-inflammatory effects rely on the expression of ZEB1, which restricts amino acid uptake, thereby downregulating the mTORC1 signaling and mitochondrial protein translation, leading to the inhibition of both acute and chronic inflammation [209].
Conclusion
Sepsis is a significant medical challenge characterized by a systemic inflammatory response. During sepsis, factors such as RNS/ROS, impaired mitophagy, altered mitochondrial dynamics, and membrane permeabilization result in the accumulation of defective mitochondria in immune cells and tissues. These damaged mitochondria promote the immune response via key pathways governed by NLRP3, TLR9, and cGAS. Preclinical studies have identified several agents that target mitochondrial quality control to reduce mitochondrial damage. These agents effectively attenuate inflammation and mortality, offering a novel approach for sepsis treatment.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- AKI:
-
Acute kidney injury
- ALI:
-
Acute lung injury
- AMPK:
-
AMP-activated protein kinase
- ANT:
-
Adenine nucleotide translocator
- BNIP3:
-
BCL2 interacting protein3
- CALCOCO2/NDP52:
-
Calcium binding and coiled-coil domain-containing protein 2
- CASP:
-
Colon ascendens stent peritonitis
- cGAS:
-
Cyclic GMP-AMP synthase
- CLP:
-
Cecal ligation and puncture
- CsA:
-
Cyclosporin A
- Cyp-D:
-
Cyclophilin D
- DAMPs:
-
Damage-associated molecular patterns
- DCs:
-
Dendritic cells
- Drp1:
-
Dynamin-related protein 1
- ER:
-
Endoplasmic reticulum
- ETC:
-
Electron transport chain
- FDA:
-
U.S. food and drug administration
- FEN1:
-
Flap structure-specific endonuclease 1
- Fis1:
-
Fission 1
- FUNDC1:
-
FUN14 domain containing 1
- GSDMs:
-
Gasdermins
- ICUs:
-
Intensive care units
- IFNs:
-
Interferons
- IL:
-
Interleukin
- IMS:
-
Intermembrane space
- IRF:
-
Interferon regulatory factor
- KO:
-
Knockout
- LC3:
-
Microtubule-associated protein 1 light chain 3
- L-OPA1:
-
Long OPA1 isoforms
- LPS:
-
Lipopolysaccharides
- MAM:
-
Mitochondria-associated membrane
- MARK4:
-
Microtubule-affinity regulating kinase 4
- MAVS:
-
Mitochondrial antiviral-signaling protein
- Mff:
-
Mitochondrial fusion factor
- Mfn1/2:
-
Mitofusin 1/2
- MOF:
-
Multi-organ failure
- MOMP:
-
Mitochondrial outer membrane permeabilization
- MPT:
-
Mitochondrial permeability transition
- mtDNA:
-
Mitochondrial DNA
- MTOC:
-
Microtubule-organizing center
- mTOR:
-
Mammalian target of rapamycin
- mtROS:
-
Mitochondrial ROS
- NADH:
-
Nicotinamide adenine dinucleotide dehydrogenase
- NBR1:
-
Next to BRCA1 gene 1 protein
- NFAT:
-
Nuclear factor of activated T cells
- NF-κB:
-
Nuclear factor κB
- NIX:
-
Nip3-like protein X
- NLRs:
-
NOD-like receptors
- NOXs:
-
Nicotinamide adenine dinucleotide phosphate oxidases
- OMM:
-
Outer mitochondrial membrane
- OPA1:
-
Optic atrophy 1
- OPTN:
-
Optineurin
- ox-mtDNA:
-
Oxidized mitochondrial DNA
- PAMPs:
-
Pathogen-associated molecular patterns
- PBMCs:
-
Peripheral blood mononuclear cells
- PepG:
-
Peptidoglycan
- PGC-1 β:
-
Peroxisome-proliferator-activated receptor γ coactivator 1 β
- PHB:
-
Prohibitin
- PINK1:
-
PTEN-induced kinase 1
- PRRs:
-
Pattern recognition receptors
- RNS:
-
Reactive nitrogen species
- SESN2:
-
Sestrin 2
- S-OPA1:
-
Short OPA1 isoforms
- STING:
-
Stimulator of interferon genes
- STX17:
-
Syntaxin 17
- TAX1BP1:
-
Tax1-binding protein 1
- tBid:
-
Truncated form of Bid
- TCA:
-
Tricarboxylic acid
- Tempol:
-
Piperidine nitroxide
- TFAM:
-
Mitochondrial transcription factor A
- TGN:
-
Trans-Golgi network
- TLRs:
-
Toll-like receptors
- TPP+ :
-
Decyl-triphenylphosphonium
- TRX:
-
Thioredoxin
- TXNIP:
-
Thioredoxin-interacting protein
- UA:
-
Urolithin A
- UCPs:
-
Uncoupling proteins
- ULK1:
-
Unc-51 like autophagy activating kinase 1
- VDAC:
-
Voltage-dependent anion channel
- WT:
-
Wide type
- ΔΨm:
-
Mitochondrial membrane potential
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–11.
Srzic I, Nesek Adam V, Tunjic Pejak D. Sepsis definition: what’s new in the treatment guidelines. Acta Clin Croat. 2022;61(Suppl 1):67–72.
Misrani A, Tabassum S, Yang L. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front Aging Neurosci. 2021;13:617588.
Schenkel LC, Bakovic M. Formation and regulation of mitochondrial membranes. Int J Cell Biol. 2014;2014:709828.
Vercellino I, Sazanov LA. The assembly, regulation and function of the mitochondrial respiratory chain. Nat Rev Mol Cell Biol. 2022;23(2):141–61.
Kong C, Song W, Fu T. Systemic inflammatory response syndrome is triggered by mitochondrial damage (review). Mol Med Rep. 2022;25(4):1–8.
Lira Chavez FM, Gartzke LP, van Beuningen FE, Wink SE, Henning RH, Krenning G, Bouma HR. Restoring the infected powerhouse: Mitochondrial quality control in sepsis. Redox Biol. 2023;68:102968.
Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(1):66–72.
Zhang W, Jiang H, Wu G, Huang P, Wang H, An H, Liu S, Zhang W. The pathogenesis and potential therapeutic targets in sepsis. MedComm (2020). 2023;4(6):e418.
Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159–73.
Li R, Ren T, Zeng J. Mitochondrial coenzyme Q protects sepsis-induced acute lung injury by activating PI3K/Akt/GSK-3beta/mTOR pathway in rats. Biomed Res Int. 2019;2019:5240898.
Zhu Y, Kuang L, Wu Y, Deng H, She H, Zhou Y, Zhang J, Liu L, Li T. Protective effects of inhibition of mitochondrial fission on organ function after sepsis. Front Pharmacol. 2021;12:712489.
Tzanavari T, Varela A, Theocharis S, Ninou E, Kapelouzou A, Cokkinos DV, Kontaridis MI, Karalis KP. Metformin protects against infection-induced myocardial dysfunction. Metabolism. 2016;65(10):1447–58.
Japiassu AM, Santiago AP, d’Avila JC, Garcia-Souza LF, Galina A, Castro Faria-Neto HC, Bozza FA, Oliveira MF. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5’-triphosphate synthase activity. Crit Care Med. 2011;39(5):1056–63.
Garrabou G, Moren C, Lopez S, Tobias E, Cardellach F, Miro O, Casademont J. The effects of sepsis on mitochondria. J Infect Dis. 2012;205(3):392–400.
Protti A, Fortunato F, Artoni A, Lecchi A, Motta G, Mistraletti G, Novembrino C, Comi GP, Gattinoni L. Platelet mitochondrial dysfunction in critically ill patients: comparison between sepsis and cardiogenic shock. Crit Care. 2015;19(1):39.
Fredriksson K, Rooyackers O. Mitochondrial function in sepsis: respiratory versus leg muscle. Crit Care Med. 2007;35(9 Suppl):S449-453.
Carre JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182(6):745–51.
Soriano FG, Nogueira AC, Caldini EG, Lins MH, Teixeira AC, Cappi SB, Lotufo PA, Bernik MM, Zsengeller Z, Chen M, et al. Potential role of poly(adenosine 5’-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Crit Care Med. 2006;34(4):1073–9.
Kingsley SM, Bhat BV. Differential paradigms in animal models of sepsis. Curr Infect Dis Rep. 2016;18(9):26.
Singer M, Brealey D. Mitochondrial dysfunction in sepsis. Biochem Soc Symp. 1999;66:149–66.
Corrêa TD, Jakob SM, Takala J. Mitochondrial function in sepsis. Crit Care Horizons. 2015;1:31–41.
Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R491-497.
Gellerich FN, Trumbeckaite S, Hertel K, Zierz S, Müller-Werdan U, Werdan K, Redl H, Schlag G. Impaired energy metabolism in hearts of septic baboons: diminished activities of Complex I and Complex II of the mitochondrial respiratory chain. Shock (Augusta, GA). 1999;11(5):336–41.
Brealey D, Singer M. Mitochondrial dysfunction in sepsis. Curr Infect Dis Rep. 2003;5(5):365–71.
Boulos M, Astiz ME, Barua RS, Osman M. Impaired mitochondrial function induced by serum from septic shock patients is attenuated by inhibition of nitric oxide synthase and poly(ADP-ribose) synthase. Crit Care Med. 2003;31(2):353–8.
Fredriksson K, Hammarqvist F, Strigard K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291(5):E1044-1050.
Li Y, Feng YF, Liu XT, Li YC, Zhu HM, Sun MR, Li P, Liu B, Yang H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021;38:101771.
Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121(10):4003–14.
Choumar A, Tarhuni A, Letteron P, Reyl-Desmars F, Dauhoo N, Damasse J, Vadrot N, Nahon P, Moreau R, Pessayre D, et al. Lipopolysaccharide-induced mitochondrial DNA depletion. Antioxid Redox Signal. 2011;15(11):2837–54.
Hansen ME, Simmons KJ, Tippetts TS, Thatcher MO, Saito RR, Hubbard ST, Trumbull AM, Parker BA, Taylor OJ, Bikman BT. Lipopolysaccharide disrupts mitochondrial physiology in skeletal muscle via disparate effects on sphingolipid metabolism. Shock. 2015;44(6):585–92.
d’Avila JC, Santiago AP, Amancio RT, Galina A, Oliveira MF, Bozza FA. Sepsis induces brain mitochondrial dysfunction. Crit Care Med. 2008;36(6):1925–32.
Kokkinaki D, Hoffman M, Kalliora C, Kyriazis ID, Maning J, Lucchese AM, Shanmughapriya S, Tomar D, Park JY, Wang H, et al. Chemically synthesized Secoisolariciresinol diglucoside (LGM2605) improves mitochondrial function in cardiac myocytes and alleviates septic cardiomyopathy. J Mol Cell Cardiol. 2019;127:232–45.
Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306(7):F734-743.
Vanasco V, Saez T, Magnani ND, Pereyra L, Marchini T, Corach A, Vaccaro MI, Corach D, Evelson P, Alvarez S. Cardiac mitochondrial biogenesis in endotoxemia is not accompanied by mitochondrial function recovery. Free Radic Biol Med. 2014;77:1–9.
Markley MA, Pierro A, Eaton S. Hepatocyte mitochondrial metabolism is inhibited in neonatal rat endotoxaemia: effects of glutamine. Clin Sci. 2002;102(3):337–44.
Yao X, Carlson D, Sun Y, Ma L, Wolf SE, Minei JP, Zang QS. Mitochondrial ROS Induces Cardiac Inflammation via a Pathway through mtDNA Damage in a Pneumonia-Related Sepsis Model. PLoS ONE. 2015;10(10):e0139416.
Crouser ED, Julian MW, Blaho DV, Pfeiffer DR. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med. 2002;30(2):276–84.
Escames G, Lopez LC, Tapias V, Utrilla P, Reiter RJ, Hitos AB, Leon J, Rodriguez MI, Acuna-Castroviejo D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J Pineal Res. 2006;40(1):71–8.
Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97(16):8841–8.
Garcia JA, Ortiz F, Miana J, Doerrier C, Fernandez-Ortiz M, Rusanova I, Escames G, Garcia JJ, Acuna-Castroviejo D. Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J Physiol Biochem. 2017;73(2):235–44.
Lopes-Pires ME, Frade-Guanaes JO, Quinlan GJ. Clotting dysfunction in sepsis: a role for ROS and potential for therapeutic intervention. Antioxidants (Basel). 2021;11(1):88.
Lee YM, He W, Liou YC. The redox language in neurodegenerative diseases: oxidative post-translational modifications by hydrogen peroxide. Cell Death Dis. 2021;12(1):58.
Zhao WY, Zhang L, Sui MX, Zhu YH, Zeng L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci Rep. 2016;6:33201.
Labiner HE, Sas KM, Baur JA, Sims CA. Sirt3 deletion increases inflammation and mortality in polymicrobial sepsis. Surg Infect (Larchmt). 2023;24(9):788–96.
Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:217037.
Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci USA. 2001;98(13):7212–7.
Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658(1–2):44–9.
Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504(1):46–57.
Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14.
Li A, Gao M, Liu B, Qin Y, Chen L, Liu H, Wu H, Gong G. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022;13(5):444.
Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci USA. 2014;111(43):15514–9.
Patoli D, Mignotte F, Deckert V, Dusuel A, Dumont A, Rieu A, Jalil A, Van Dongen K, Bourgeois T, Gautier T, et al. Inhibition of mitophagy drives macrophage activation and antibacterial defense during sepsis. J Clin Invest. 2020;130(11):5858–74.
Dagvadorj J, Mikulska-Ruminska K, Tumurkhuu G, Ratsimandresy RA, Carriere J, Andres AM, Marek-Iannucci S, Song Y, Chen S, Lane M, et al. Recruitment of pro-IL-1alpha to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc Natl Acad Sci USA. 2021;118(1):15.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35.
Tadie JM, Bae HB, Deshane JS, Bell CP, Lazarowski ER, Chaplin DD, Thannickal VJ, Abraham E, Zmijewski JW. Toll-like receptor 4 engagement inhibits adenosine 5’-monophosphate-activated protein kinase activation through a high mobility group box 1 protein-dependent mechanism. Mol Med. 2012;18(1):659–68.
Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356(6337):513–9.
Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, He F, Boassa D, Perkins G, Ali SR, et al. NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164(5):896–910.
Kim MJ, Bae SH, Ryu JC, Kwon Y, Oh JH, Kwon J, Moon JS, Kim K, Miyawaki A, Lee MG, et al. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy. 2016;12(8):1272–91.
Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 2018;138(20):2247–62.
Wang Y, Zhu J, Liu Z, Shu S, Fu Y, Liu Y, Cai J, Tang C, Liu Y, Yin X, et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021;38:101767.
Crouser ED, Julian MW, Huff JE, Struck J, Cook CH. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med. 2006;34(9):2439–46.
Sabouny R, Shutt TE. Reciprocal regulation of mitochondrial fission and fusion. Trends Biochem Sci. 2020;45(7):564–77.
Held NM, Houtkooper RH. Mitochondrial quality control pathways as determinants of metabolic health. BioEssays. 2015;37(8):867–76.
Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62(3):341–60.
Zhang S, Xu Y, Zhu J, Ma J, Niu Q, Wang X. Carbon monoxide attenuates LPS-induced myocardial dysfunction in rats by regulating the mitochondrial dynamic equilibrium. Eur J Pharmacol. 2020;889:173726.
Gonzalez AS, Elguero ME, Finocchietto P, Holod S, Romorini L, Miriuka SG, Peralta JG, Poderoso JJ, Carreras MC. Abnormal mitochondrial fusion-fission balance contributes to the progression of experimental sepsis. Free Radic Res. 2014;48(7):769–83.
Liu R, Wang SC, Li M, Ma XH, Jia XN, Bu Y, Sun L, Yu KJ. An inhibitor of DRP1 (Mdivi-1) alleviates LPS-induced septic AKI by inhibiting NLRP3 inflammasome activation. Biomed Res Int. 2020;2020:2398420.
Deng S, Zhang L, Mo Y, Huang Y, Li W, Peng Q, Huang L, Ai Y. Mdivi-1 attenuates lipopolysaccharide-induced acute lung injury by inhibiting MAPKs, oxidative stress and apoptosis. Pulm Pharmacol Ther. 2020;62:101918.
Haileselassie B, Mukherjee R, Joshi AU, Napier BA, Massis LM, Ostberg NP, Queliconi BB, Monack D, Bernstein D, Mochly-Rosen D. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. J Mol Cell Cardiol. 2019;130:160–9.
Tan Y, Ouyang H, Xiao X, Zhong J, Dong M. Irisin ameliorates septic cardiomyopathy via inhibiting DRP1-related mitochondrial fission and normalizing the JNK-LATS2 signaling pathway. Cell Stress Chaperones. 2019;24(3):595–608.
Norenberg MD, Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50(7–8):983–97.
Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014;21(2):196–205.
McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, Chappaz S, Davidson S, San Chin H, et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359(6378):eaao6047.
Riley JS, Quarato G, Cloix C, Lopez J, O’Prey J, Pearson M, Chapman J, Sesaki H, Carlin LM, Passos JF, et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 2018;37(17):e99238.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501.
Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111(3):331–42.
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144(5):891–901.
Chung CS, Venet F, Chen Y, Jones LN, Wilson DC, Ayala CA, Ayala A. Deficiency of Bid protein reduces sepsis-induced apoptosis and inflammation, while improving septic survival. Shock. 2010;34(2):150–61.
Heilig R, Dilucca M, Boucher D, Chen KW, Hancz D, Demarco B, Shkarina K, Broz P. Caspase-1 cleaves Bid to release mitochondrial SMAC and drive secondary necrosis in the absence of GSDMD. Life Sci Alliance. 2020;3(6):e202000735.
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30.
Larche J, Lancel S, Hassoun SM, Favory R, Decoster B, Marchetti P, Chopin C, Neviere R. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. J Am Coll Cardiol. 2006;48(2):377–85.
Crouser ED, Julian MW, Joshi MS, Bauer JA, Wewers MD, Hart JM, Pfeiffer DR. Cyclosporin A ameliorates mitochondrial ultrastructural injury in the ileum during acute endotoxemia. Crit Care Med. 2002;30(12):2722–8.
Hu Y, Yan JB, Zheng MZ, Song XH, Wang LL, Shen YL, Chen YY. Mitochondrial aldehyde dehydrogenase activity protects against lipopolysaccharide-induced cardiac dysfunction in rats. Mol Med Rep. 2015;11(2):1509–15.
Bernardi P, Gerle C, Halestrap AP, Jonas EA, Karch J, Mnatsakanyan N, Pavlov E, Sheu S-S, Soukas AA. Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023;30(8):1869–85.
Bonora M, Giorgi C, Pinton P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol. 2022;23(4):266–85.
Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–83.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909–50.
Wang S, Moreau F, Chadee K. Gasdermins in innate host defense against entamoeba histolytica and other protozoan parasites. Front Immunol. 2022;13:900553.
Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10(1):1689.
de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 2019;26(1):146–61.
Huang LS, Hong Z, Wu W, Xiong S, Zhong M, Gao X, Rehman J, Malik AB. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity. 2020;52(3):475-486 e475.
Miao R, Jiang C, Chang WY, Zhang H, An J, Ho F, Chen P, Zhang H, Junqueira C, Amgalan D, et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity. 2023;56(11):2523-2541 e2528.
Lin PH, Lin HY, Kuo CC, Yang LT. N-terminal functional domain of Gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J Biomed Sci. 2015;22(1):44.
Kondolf HC, D’Orlando DA, Dubyak GR, Abbott DW. Protein engineering reveals that gasdermin A preferentially targets mitochondrial membranes over the plasma membrane during pyroptosis. J Biol Chem. 2023;299(2):102908.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20.
Nong Y, Wei X, Yu D. Inflammatory mechanisms and intervention strategies for sepsis-induced myocardial dysfunction. Immun Inflamm Dis. 2023;11(5):e860.
Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–29.
Zang QS, Sadek H, Maass DL, Martinez B, Ma L, Kilgore JA, Williams NS, Frantz DE, Wigginton JG, Nwariaku FE, et al. Specific inhibition of mitochondrial oxidative stress suppresses inflammation and improves cardiac function in a rat pneumonia-related sepsis model. Am J Physiol Heart Circ Physiol. 2012;302(9):H1847-1859.
Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45(11):1559–65.
Xia Y, Cao Y, Sun Y, Hong X, Tang Y, Yu J, Hu H, Ma W, Qin K, Bao R. Calycosin alleviates sepsis-induced acute lung injury via the inhibition of mitochondrial ROS-mediated inflammasome activation. Front Pharmacol. 2021;12:690549.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–40.
Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–22.
Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187(2):613–7.
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14.
Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, Wong J, Ding S, Seki E, Schnabl B, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018;560(7717):198–203.
Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, Ying W, Hoffman HM, Shadel GS, Karin M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55(8):1370-1385 e1378.
Nozaki K, Li L, Miao EA. Innate sensors trigger regulated cell death to combat intracellular infection. Annu Rev Immunol. 2022;40:469–98.
Li X, Thome S, Ma X, Amrute-Nayak M, Finigan A, Kitt L, Masters L, James JR, Shi Y, Meng G, et al. MARK4 regulates NLRP3 positioning and inflammasome activation through a microtubule-dependent mechanism. Nat Commun. 2017;8:15986.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5.
Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564(7734):71–6.
Magupalli VG, Negro R, Tian Y, Hauenstein AV, Di Caprio G, Skillern W, Deng Q, Orning P, Alam HB, Maliga Z, et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science. 2020;369(6510):eaas8995.
Pandey A, Shen C, Feng S, Man SM. Cell biology of inflammasome activation. Trends Cell Biol. 2021;31(11):924–39.
Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14(5):454–60.
Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153(2):348–61.
Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–23.
Elliott EI, Miller AN, Banoth B, Iyer SS, Stotland A, Weiss JP, Gottlieb RA, Sutterwala FS, Cassel SL. Cutting edge: mitochondrial assembly of the NLRP3 inflammasome complex is initiated at priming. J Immunol. 2018;200(9):3047–52.
Park S, Juliana C, Hong S, Datta P, Hwang I, Fernandes-Alnemri T, Yu JW, Alnemri ES. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol. 2013;191(8):4358–66.
Franchi L, Eigenbrod T, Munoz-Planillo R, Ozkurede U, Kim YG, Arindam C, Gale M Jr, Silverman RH, Colonna M, Akira S, et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol. 2014;193(8):4214–22.
Ren Z, Ding T, Zuo Z, Xu Z, Deng J, Wei Z. Regulation of MAVS expression and signaling function in the antiviral innate immune response. Front Immunol. 2020;11:1030.
Andreeva L, David L, Rawson S, Shen C, Pasricha T, Pelegrin P, Wu H. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell. 2021;184(26):6299-6312 e6222.
Zhang Z, Meszaros G, He WT, Xu Y, de Fatima MH, Mailly L, Mihlan M, Liu Y, Puig Gamez M, Goginashvili A, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. 2017;214(9):2671–93.
Xiao L, Magupalli VG, Wu H. Cryo-EM structures of the active NLRP3 inflammasome disc. Nature. 2023;613(7944):595–600.
Fu J, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. 2023;41:301–16.
Ablasser A, Chen ZJ. cGAS in action: Expanding roles in immunity and inflammation. Science. 2019;363(6431):eaat8657.
Xian H, Karin M. Oxidized mitochondrial DNA: a protective signal gone awry. Trends Immunol. 2023;44(3):188–200.
Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, Tuting T, Hartmann G, Barchet W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39(3):482–95.
Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med 2013;10(12):e1001577; discussion e1001577.
Wang L, Zhou W, Wang K, He S, Chen Y. Predictive value of circulating plasma mitochondrial DNA for Sepsis in the emergency department: observational study based on the Sepsis-3 definition. BMC Emerg Med. 2020;20(1):25.
Schneck E, Edinger F, Hecker M, Sommer N, Pak O, Weissmann N, Hecker A, Reichert M, Markmann M, Sander M, et al. Blood levels of free-circulating mitochondrial DNA in septic shock and postsurgical systemic inflammation and its influence on coagulation: a secondary analysis of a prospective observational study. J Clin Med. 2020;9(7):2056.
Liu Q, Wu J, Zhang X, Li X, Wu X, Zhao Y, Ren J. Circulating mitochondrial DNA-triggered autophagy dysfunction via STING underlies sepsis-related acute lung injury. Cell Death Dis. 2021;12(7):673.
Hu Q, Ren H, Li G, Wang D, Zhou Q, Wu J, Zheng J, Huang J, Slade DA, Wu X, et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine. 2019;41:497–508.
Xu L, Li M, Yang Y, Zhang C, Xie Z, Tang J, Shi Z, Chen S, Li G, Gu Y, et al. Salmonella induces the cGAS-STING-dependent type I interferon response in murine macrophages by triggering mtDNA release. MBio. 2022;13(3):e0363221.
Zhang RX, Kang R, Tang DL. STING1 in sepsis: mechanisms, functions, and implications. Chin J Traumatol. 2022;25(1):1–10.
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5.
Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198(3):513–20.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7.
Tsuji N, Tsuji T, Ohashi N, Kato A, Fujigaki Y, Yasuda H. Role of Mitochondrial DNA in septic AKI via toll-like receptor 9. J Am Soc Nephrol. 2016;27(7):2009–20.
Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294(5):F1050–8.
Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205(6):1277–83.
Liu J, Zhou G, Wang X, Liu D. Metabolic reprogramming consequences of sepsis: adaptations and contradictions. Cell Mol Life Sci. 2022;79(8):456.
Liu C, Wei W, Huang Y, Fu P, Zhang L, Zhao Y. Metabolic reprogramming in septic acute kidney injury: pathogenesis and therapeutic implications. Metabolism. 2024;158:155974.
Van Wyngene L, Vandewalle J, Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med. 2018;10(8):e8712.
Pan T, Sun S, Chen Y, Tian R, Chen E, Tan R, Wang X, Liu Z, Liu J, Qu H. Immune effects of PI3K/Akt/HIF-1alpha-regulated glycolysis in polymorphonuclear neutrophils during sepsis. Crit Care. 2022;26(1):29.
Tan C, Gu J, Chen H, Li T, Deng H, Liu K, Liu M, Tan S, Xiao Z, Zhang H, et al. Inhibition of aerobic glycolysis promotes neutrophil to influx to the infectious site via CXCR2 in sepsis. Shock. 2020;53(1):114–23.
Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27(7):1230–51.
Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364(9433):545–8.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23.
Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem. 2004;279(36):37575–87.
McCormick B, Lowes DA, Colvin L, Torsney C, Galley HF. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br J Anaesth. 2016;117(5):659–66.
Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592(12):2549–61.
Kalyanaraman B, Cheng G, Hardy M, You M. OXPHOS-targeting drugs in oncology: new perspectives. Expert Opin Ther Targets. 2023;27(10):939–52.
Zhang S, Zhou Q, Li Y, Zhang Y, Wu Y. MitoQ modulates lipopolysaccharide-induced intestinal barrier dysfunction via regulating Nrf2 signaling. Mediat Inflamm. 2020;2020:3276148.
Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2009;297(4):R1095-1102.
Supinski GS, Schroder EA, Wang L, Morris AJ, Callahan LAP. Mitoquinone mesylate (MitoQ) prevents sepsis-induced diaphragm dysfunction. J Appl Physiol (1985). 2021;131(2):778–87.
Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263(3):709–16.
Lowes DA, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth. 2013;110(3):472–80.
Trnka J, Blaikie FH, Smith RA, Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med. 2008;44(7):1406–19.
Arulkumaran N, Pollen SJ, Tidswell R, Gaupp C, Peters VBM, Stanzani G, Snow TAC, Duchen MR, Singer M. Selective mitochondrial antioxidant MitoTEMPO reduces renal dysfunction and systemic inflammation in experimental sepsis in rats. Br J Anaesth. 2021;127(4):577–86.
Navas LE, Carnero A. NAD(+) metabolism, stemness, the immune response, and cancer. Signal Transduct Target Ther. 2021;6(1):2.
Liaudet L, Mabley JG, Soriano FG, Pacher P, Marton A, Hasko G, Szabo C. Inosine reduces systemic inflammation and improves survival in septic shock induced by cecal ligation and puncture. Am J Respir Crit Care Med. 2001;164(7):1213–20.
Cao T, Ni R, Ding W, Ji X, Fan GC, Zhang Z, Peng T. Nicotinamide mononucleotide as a therapeutic agent to alleviate multi-organ failure in sepsis. J Transl Med. 2023;21(1):883.
Hong G, Zheng D, Zhang L, Ni R, Wang G, Fan GC, Lu Z, Peng T. Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic Biol Med. 2018;123:125–37.
Ye M, Zhao Y, Wang Y, Xie R, Tong Y, Sauer JD, Gong S. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat Nanotechnol. 2022;17(8):880–90.
Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA, Williams EG, Jha P, Lo Sasso G, Huzard D, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22(8):879–88.
Yang Y, Hu Q, Kang H, Li J, Zhao X, Zhu L, Tang W, Wan M. Urolithin A protects severe acute pancreatitis-associated acute cardiac injury by regulating mitochondrial fatty acid oxidative metabolism in cardiomyocytes. MedComm (2020). 2023;4(6):e459.
Abdelazeem KNM, Kalo MZ, Beer-Hammer S, Lang F. The gut microbiota metabolite urolithin A inhibits NF-kappaB activation in LPS stimulated BMDMs. Sci Rep. 2021;11(1):7117.
Liu S, D’Amico D, Shankland E, Bhayana S, Garcia JM, Aebischer P, Rinsch C, Singh A, Marcinek DJ. Effect of urolithin a supplementation on muscle endurance and mitochondrial health in older adults: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2144279.
Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, Auwerx J, Singh A, Rinsch C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1(6):595–603.
Zhu P, Chen Y, Wang J, Lin G, Wang R, Que Y, Zhou J, Xu G, Luo J, Du Y. Receptor-interacting protein kinase 3 suppresses mitophagy activation via the yes-associated protein/transcription factor EB pathways in septic cardiomyopathy. Front Cardiovasc Med. 2022;9:856041.
Wang Y, Jasper H, Toan S, Muid D, Chang X, Zhou H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021;45:102049.
Jiao P, Wang Y, Ren G, Chu D, Li Y, Yang Y, Sang T. Urolithin A exerts a protective effect on lipopolysaccharide-induced acute lung injury by regulating HMGB1-mediated MAPK and NF-kappaB signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(8):5765–77.
Libert C, Ayala A, Bauer M, Cavaillon JM, Deutschman C, Frostell C, Knapp S, Kozlov AV, Wang P, Osuchowski MF, et al. Part II: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for types of infections and organ dysfunction endpoints. Shock. 2019;51(1):23–32.
Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020;202(3):361–70.
Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204.
Manczak M, Kandimalla R, Yin X, Reddy PH. Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum Mol Genet. 2019;28(2):177–99.
Zhang Q, Liu Z, Huang X, Heng X, Wu J, Chen Z, Guo X, Fan J, Huang Q. Mdivi-1 alleviates sepsis-induced liver injury by inhibiting sting signaling activation. Shock. 2024;62(1):95–102.
Fu Q, Zhang YB, Shi CX, Jiang M, Lu K, Fu ZH, Ruan JP, Wu J, Gu XP. GSDMD/Drp1 signaling pathway mediates hippocampal synaptic damage and neural oscillation abnormalities in a mouse model of sepsis-associated encephalopathy. J Neuroinflamm. 2024;21(1):96.
Haileselassie B, Joshi AU, Minhas PS, Mukherjee R, Andreasson KI, Mochly-Rosen D. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy. J Neuroinflammation. 2020;17(1):36.
de Paiva CS, Pflugfelder SC, Ng SM, Akpek EK. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst Rev. 2019;9(9):CD010051.
Molnar AO, Fergusson D, Tsampalieros AK, Bennett A, Fergusson N, Ramsay T, Knoll GA. Generic immunosuppression in solid organ transplantation: systematic review and meta-analysis. BMJ. 2015;350:h3163.
Luna-Sanchez M, Bianchi P, Quintana A. Mitochondria-induced immune response as a trigger for neurodegeneration: a pathogen from within. Int J Mol Sci. 2021;22(16):8523.
Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol. 2019;163:472–80.
Xiao Z, Jia B, Zhao X, Bi S, Meng W. Attenuation of lipopolysaccharide-induced acute lung injury by cyclosporine-A via suppression of mitochondrial DNA. Med Sci Monit. 2018;24:7682–8.
Fauvel H, Marchetti P, Obert G, Joulain O, Chopin C, Formstecher P, Neviere R. Protective effects of cyclosporin A from endotoxin-induced myocardial dysfunction and apoptosis in rats. Am J Respir Crit Care Med. 2002;165(4):449–55.
Joshi MS, Julian MW, Huff JE, Bauer JA, Xia Y, Crouser ED. Calcineurin regulates myocardial function during acute endotoxemia. Am J Respir Crit Care Med. 2006;173(9):999–1007.
Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321(19):1926–7.
Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol (Lausanne). 2020;11:191.
Liu Z, Bone N, Jiang S, Park DW, Tadie JM, Deshane J, Rodriguez CA, Pittet JF, Abraham E, Zmijewski JW. AMP-activated protein kinase and glycogen synthase kinase 3beta modulate the severity of sepsis-induced lung injury. Mol Med. 2016;21(1):937–50.
Fan SY, Zhao ZC, Liu XL, Peng YG, Zhu HM, Yan SF, Liu YJ, Xie Q, Jiang Y, Zeng SZ. Metformin mitigates sepsis-induced acute lung injury and inflammation in young mice by suppressing the S100A8/A9-NLRP3-IL-1beta signaling pathway. J Inflamm Res. 2024;17:3785–99.
Tang G, Yang H, Chen J, Shi M, Ge L, Ge X, Zhu G. Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway. Oncotarget. 2017;8(58):97977–89.
Liang H, Song H, Zhang X, Song G, Wang Y, Ding X, Duan X, Li L, Sun T, Kan Q. Metformin attenuated sepsis-related liver injury by modulating gut microbiota. Emerg Microbes Infect. 2022;11(1):815–28.
Doenyas-Barak K, Beberashvili I, Marcus R, Efrati S. Lactic acidosis and severe septic shock in metformin users: a cohort study. Crit Care. 2016;20:10.
Green JP, Berger T, Garg N, Suarez A, Hagar Y, Radeos MS, Panacek EA. Impact of metformin use on the prognostic value of lactate in sepsis. Am J Emerg Med. 2012;30(9):1667–73.
Jochmans S, Alphonsine JE, Chelly J, Vong LVP, Sy O, Rolin N, Ellrodt O, Monchi M, Vinsonneau C. Does metformin exposure before ICU stay have any impact on patients’ outcome? A retrospective cohort study of diabetic patients. Ann Intensive Care. 2017;7(1):116.
van Vught LA, Scicluna BP, Hoogendijk AJ, Wiewel MA, Klein Klouwenberg PM, Cremer OL, Horn J, Nurnberg P, Bonten MM, Schultz MJ, et al. Association of diabetes and diabetes treatment with the host response in critically ill sepsis patients. Crit Care. 2016;20(1):252.
Park J, Hwang SY, Jo IJ, Jeon K, Suh GY, Lee TR, Yoon H, Cha WC, Sim MS, Carriere KC, et al. Impact of metformin use on lactate kinetics in patients with severe sepsis and septic shock. Shock. 2017;47(5):582–7.
Liang H, Ding X, Li L, Wang T, Kan Q, Wang L, Sun T. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care. 2019;23(1):50.
Yang Q, Zheng J, Chen W, Chen X, Wen D, Chen W, Xiong X, Zhang Z. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: a cohort study. Front Med (Lausanne). 2021;8:640785.
Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. 2023;19(8):460–76.
Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, Hoxhaj G, Saghatelian A, Shaw RJ, Manning BD. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2):463–71.
Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench GR, Lewis G, Chen W, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54(7):1463-1477 e1411.
Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104(29):12017–22.
Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem. 2014;289(33):23246–55.
Wu W, Wang S, Liu Q, Shan T, Wang Y. Metformin protects against lps-induced intestinal barrier dysfunction by activating AMPK pathway. Mol Pharm. 2018;15(8):3272–84.
Ma T, Tian X, Zhang B, Li M, Wang Y, Yang C, Wu J, Wei X, Qu Q, Yu Y, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603(7899):159–65.
Cortes M, Brischetto A, Martinez-Campanario MC, Ninfali C, Dominguez V, Fernandez S, Celis R, Esteve-Codina A, Lozano JJ, Sidorova J, et al. Inflammatory macrophages reprogram to immunosuppression by reducing mitochondrial translation. Nat Commun. 2023;14(1):7471.
Acknowledgements
Figures used in this review are made in BioRender.com.
Funding
This work is financially supported by MOE Tier2 and Tier1 (A-8000985 and A-8000412) grants from the Ministry of Education (MOE), Singapore, awarded to Y-C. Liou.
Author information
Authors and Affiliations
Contributions
Dongxue Hu and Harshini Sheeja Prabhakaran contributed to the data collection of the article. Dongxue Hu, Harshini Sheeja Prabhakaran, Weifeng He, and Yih-Cherng Liou contributed to the conception, preparation and organization of this article. Yuan-Yuan Zhang and Gaoxing Luo contributed to the organization and constructive discussions. Weifeng He and Yih-Cherng Liou revised the draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, D., Sheeja Prabhakaran, H., Zhang, YY. et al. Mitochondrial dysfunction in sepsis: mechanisms and therapeutic perspectives. Crit Care 28, 292 (2024). https://doi.org/10.1186/s13054-024-05069-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-05069-w