Abstract
Introduction
Prognostication of outcome in severe stroke patients necessitating invasive mechanical ventilation poses significant challenges. The objective of this study was to assess the prognostic significance and prevalence of early electroencephalogram (EEG) abnormalities in adult stroke patients receiving mechanical ventilation.
Methods
This study is a pre-planned ancillary investigation within the prospective multicenter SPICE cohort study (2017–2019), conducted in 33 intensive care units (ICUs) in the Paris area, France. We included adult stroke patients requiring invasive mechanical ventilation, who underwent at least one intermittent EEG examination during their ICU stay. The primary endpoint was the functional neurological outcome at one year, determined using the modified Rankin scale (mRS), and dichotomized as unfavorable (mRS 4–6, indicating severe disability or death) or favorable (mRS 0–3). Multivariable regression analyses were employed to identify EEG abnormalities associated with functional outcomes.
Results
Of the 364 patients enrolled in the SPICE study, 153 patients (49 ischemic strokes, 52 intracranial hemorrhages, and 52 subarachnoid hemorrhages) underwent at least one EEG at a median time of 4 (interquartile range 2–7) days post-stroke. Rates of diffuse slowing (70% vs. 63%, p = 0.37), focal slowing (38% vs. 32%, p = 0.15), periodic discharges (2.3% vs. 3.7%, p = 0.9), and electrographic seizures (4.5% vs. 3.7%, p = 0.4) were comparable between patients with unfavorable and favorable outcomes. Following adjustment for potential confounders, an unreactive EEG background to auditory and pain stimulations (OR 6.02, 95% CI 2.27–15.99) was independently associated with unfavorable outcomes. An unreactive EEG predicted unfavorable outcome with a specificity of 48% (95% CI 40–56), sensitivity of 79% (95% CI 72–85), and positive predictive value (PPV) of 74% (95% CI 67–81). Conversely, a benign EEG (defined as continuous and reactive background activity without seizure, periodic discharges, triphasic waves, or burst suppression) predicted favorable outcome with a specificity of 89% (95% CI 84–94), and a sensitivity of 37% (95% CI 30–45).
Conclusion
The absence of EEG reactivity independently predicts unfavorable outcomes at one year in severe stroke patients requiring mechanical ventilation in the ICU, although its prognostic value remains limited. Conversely, a benign EEG pattern was associated with a favorable outcome.
Similar content being viewed by others
Introduction
Stroke is one of the most common acute neurological disorders, and is associated with a high rate of long-term disability and mortality [1]. Severe stroke patients may require invasive mechanical ventilation (MV) in an intensive care unit (ICU) mainly because of impairment of consciousness, seizures, neurosurgical and neuroradiological procedures, and/or respiratory complications [2]. Outcome of patients with severe stroke requiring MV remains poor, with mortality rates of ± 50% and good functional outcome at one year in 33% of cases [3]. Although well described in patients with hypoxic ischemic brain injury (HIBI) after cardiac arrest (CA) and traumatic brain injury (TBI), early prognostication markers remain poorly studied in severe stroke patients [4, 5]. Common predictors mainly include clinical severity scales such as the Glasgow Coma Scale (GCS) and National Institutes of Health Stroke Scale (NIHSS), and neuroimaging features at stroke onset [6, 7].
Recently, there has been a growing interest in electroencephalogram (EEG) monitoring in brain injured patients in the ICU [8]. EEG is characterized by a high temporal resolution and could thus detect secondary brain injury in real time, leading to important changes in management, including brain imaging and therapeutic interventions (i.e., neuroradiological or neurosurgical treatment). This tool has also been recognized as a robust prognostic marker after CA, both for unfavorable neurological outcome prediction when “highly malignant patterns” are present (i.e., suppression with or without periodic discharges and burst suppression), and for favorable outcome prediction in cases of “benign EEG” features (i.e.,no malignant or highly malignant patterns) [9, 10]. However, studies focusing on the prognostic value of EEG patterns in severe stroke requiring MV remains scarce [6, 11].
In the present study, we aimed to investigate the prevalence and the prognostic value of early EEG abnormalities in adult stroke patients requiring MV and ICU admission.
Methods
Population
SPICE EEG is a pre-planned, ancillary study of the prospective multicenter longitudinal cohort “SPICE” study, which assessed functional outcomes at one year after stroke requiring MV in the ICU [3]. The study protocol was published previously [2], approved by the Comité de Protection des Personnes Sud Méditerranée 1 (ID RCB 2017-A02452-51) and registered in Clinical Trials (NCT03335995). The SPICE study enrolled adult patients with any type of stroke (i.e., ischemic stroke, intra cerebral hemorrhage, or subarachnoid hemorrhage, excluding those of traumatic origin) and requiring invasive MV in the ICU; Thus, all patients fulfilling the following criteria were eligible for inclusion in SPICE EEG: (1) aged 18 years or more; (2) an acute stroke (i.e., ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage) diagnosed on neuroimaging; (3) an ICU admission within seven days before or after stroke onset; (4) the need for invasive MV for at least 24 h; (5) and at least one EEG recording during the ICU stay. Patients were excluded if they met any of the following criteria: (1) stroke of traumatic origin; (2) refusal to participate; (3) privation of liberty by administrative or judicial decision. Patients were recruited at ICU admission from 33 ICUs located in 26 sites (13 university and 13 general hospitals), in the Greater Paris area, France. For each included patient, written information was given by the local investigator and consent was obtained from the patient herself/himself or her/his next-of-kin. If patients regained capacity during ICU stay or at one of the follow-up visits, they were asked to provide informed consent for the use of acute data and follow-up. Data were collected in an electronic case report form (eCRF) managed by ICUREsearch (ICUREsearch, Paris, France), as previously described [2]. The study was conducted according to the STROBE guidelines [12].
Data collection
Data prospectively collected by local investigators on eCRF during ICU stay included epidemiological data (age and gender), data on initial stroke management (stroke subtype, GCS and NIHSS score at diagnosis), ICU admission characteristics (main reason for ICU admission and SOFA scores with non-neurological SOFA), and ICU outcomes (clinical seizure and duration of ICU stay). During ICU stay, data on vital status, antiseizures medications use, causes of death and decisions to withdraw life sustaining therapies (WLST) were prospectively collected.
EEG assessment
Intermittent digital EEG with video was recorded for at least 20 to 30 min by a technician using 12–19 scalp electrodes according to center practices [13], positioned according to the standard 10–20 system placement. We collected the first EEG performed after stroke. EEG was reformatted to both bipolar and off-head referential montages, with band-pass filter of 0.53 Hz and 70 Hz and amplification set at 100 µV/cm. According to French recommendations [13], repetitive bilateral auditory (hand claps, patient’s name call) and nociceptive (pressure on the nail) stimulations were systematically performed. EEG indications were left to the discretion of treating physicians. According to recommendations, EEG is usually performed for detection of non-convulsive seizures/status epilepticus, brain death diagnosis or prognostication of outcome in comatose patients [13, 14]. For the current study, the EEG was routinely interpreted by local expert neurophysiologists (NK, EPR, VB, JPL, LN, and EA). EEG findings were prospectively collected from the daily report of neurophysiologists in the medical record of each included patient. For each patient, the following EEG features were assessed and prospectively collected in the eCRF (Additional file 1: Table 1): background continuity and symmetry, background reactivity to auditory and/or nociceptive stimulation, presence of generalized or localized periodic discharges, presence of triphasic waves and electrographic seizures. EEG reactivity to stimulation was defined as a reproducible modification of EEG background, either an increase or a decrease in amplitude or frequency according to the ACNS terminology [15].
We used a simplified version of the Westhall et al. classification (developed in CA patients [16]), describing EEG patterns as “highly malignant”, “malignant” or “benign”. This classification was made by two neurointensivists with EEG expertise (SB and RS), who were blinded to other clinical data. A highly malignant pattern was defined as suppression, suppression with periodic discharges, or burst-suppression. A malignant pattern was defined as periodic or rhythmic patterns (including electrographic seizures), and nonreactive background. [16]. Finally, a benign EEG was defined in the absence of malignant or highly malignant features, i.e., when all of the 5 following criteria were present: (1) reactivity to auditory and pain stimulations, (2) absence of electrographic seizures, (3) no periodic discharges and (4) no bust suppression patterns, (5) no triphasic waves.
ICU management
Neurological management was left to the discretion of investigators in the different participating centers. Sedative drug (i.e., propofol and/or midazolam) prescriptions were left to the discretion of investigators and used in accordance with current recommendations [17]. Standard of care for general supportive measures included head elevation (≥ 30°), oxygenation targets > 94%, correction of hypotension, correction of hyperthermia (temperature > 38 °C), correction of hypoglycemia (< 60 mg/dL), and deep vein thrombosis prophylaxis, in accordance with stroke guidelines [18, 19]. Clinical status epilepticus was defined as a seizure whose motor manifestations extend beyond five minutes or by seizure repetition (≥ 2) during short intervals, without interictal consciousness [20]. Prophylactic antiseizure medications (ASMs) were not systematically prescribed after stroke, in accordance with guidelines [19]. In cases of clinical or electrographic seizure, the choice of ASMs was left to the discretion of the physician.
Outcomes
Outcomes at one year after ICU admission (vital status, modified Rankin scale mRS) were assessed via telephone interviews by an independent research assistant trained for neurological evaluation and scoring on the mRS, and blinded to clinical data at admission and EEG results [21]. When patients were unable to be evaluated directly, functional evaluation was performed with help of family members or professional caregivers, as appropriate. The primary endpoint was functional outcome at one year on the mRS, categorized as unfavorable (mRS 4–6, indicating severe disability or death) or favorable (mRS 0–3). Causes of death were categorized by investigators into two groups, i.e. neurologic (brain death or withdrawal of life sustaining therapies (WLST)) or systemic causes (cardiovascular events, shock or multiorgan failure).
Statistical analysis
We reported continuous variables as medians (interquartile range (IQR)) and categorical variables as frequencies (percentage). For between-group comparisons, we used the Wilcoxon rank-sum test for continuous variables and either Pearson’s chi-square test or Fisher’s exact test, as appropriate for categorical variables. Clinically relevant non-collinear variables associated with the primary endpoint (p value < 0.10) in univariable analysis were entered into the multivariable model. As ‘reactivity to auditory stimulation' and ‘reactivity to painful stimulation' variables were collinear with 'benign EEG', only 'unreactive EEG' variables were included in the multivariable model. The multivariable analysis was adjusted for three a priori defined clinical confounders (i.e., stroke sub-type, sedation at time of EEG recording and delay between stroke and EEG). Log-linearity of continuous variables included in the final model was tested. The log-linearity of quantitative variables included in the multivariate analysis was verified using an additive model with a univariate smoothing spline. In the event of non-log-linearity, quantitative variables were categorized based on their distribution (quartiles and median). Missing values of independent variables were imputed to the median or mode for continuous and categorical variables, respectively. For all EEG variables independently associated with outcome, we calculated sensitivities, specificities, positive predictive values and negative predictive values, with their 95% confidence intervals. All tests were two-sided, and a p-value < 0.05 was considered significant. All statistical analyses were performed using SAS 9.4.
Results
Patients’ characteristics
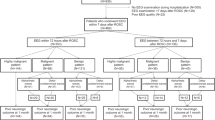
Between March 7, 2017, and December 26, 2019, 364 patients were enrolled in SPICE, among which 153 patients (42%) had had at least one EEG recording during the ICU stay (Additional file 1: Fig. 1) and were included in SPICE EEG. Compared to patients without EEG, patients included in SPICE EEG presented more frequent clinical seizure events at ICU admission (6.5 vs 0.9%), more frequent anti-epileptic drug prescriptions during ICU stay (47.7 vs 10.2%) and a higher NIHSS score (20 (IQR 8–28) vs 16 (IQR 6.5–23), p = 0.015) (Additional file 1: Table 2).
Demographic characteristics of the 153 patients included in SPICE EEG are presented in Table 1. Patients had a median age of 62 years (IQR 50–73) and 78/153 (51%) were females. Patients had severe strokes, as reflected by a median GCS score of 10 (IQR 6–14) and a median score on the NIHSS of 20 (IQR 8–28) at diagnosis. Stroke subtypes consisted of 49 ischemic strokes, 52 intracranial hemorrhages, and 52 subarachnoid hemorrhages, including 7 with multiple stroke type. The main indication for initiation of MV was altered mental status (n = 107, 69.9%) and 10 patients (n = 10, 6.5%) presented clinical seizures or status epilepticus at the time of ICU admission. 72/151 (47.7%) patients were treated with ASMs during ICU stay. Compared to favorable outcome patients, patients with unfavorable outcome were older (65.5 vs 56 years, p = 0.006), had a lower GCS score (9 vs 11, p = 0.02), a higher NIHSS score (NIHSS > 16 in 56/80 (70%) vs 18/39 (42.6%), p = 0.012), and a higher non-neurological SOFA score (4 vs 2, p = 0.01) score at ICU admission. Finally, patients with an unfavorable outcome were less frequently treated with ASMs (43/107 patients, 40.2%) compared to patients with a favorable outcome (29/44 patients, 65.9%, p = 0.004).
The median time between stroke and EEG recording was 4 (IQR 2–7) days (Table 1) and 96% of EEGs were performed during ongoing invasive MV. Diffuse and focal slowing were the most frequent abnormalities, observed in 97/150 (64.7%) and 51/151 (33.8%) patients, respectively. In contrast, triphasic waves (13/137, 8.7%), electrographic seizures (6/150, 4%), periodic discharges (5/136, 3.3%) and burst suppression (6/147, 4%) were rarely observed. An unreactive EEG background to painful and auditory stimuli was observed in 90/138 patients (58.8%), and a benign pattern was highlighted in 28/138 patients (18.4%).
Outcomes
At one year, 108/153 patients (70%) had an unfavorable outcome, including 81 deaths. Causes of death were related to neurologic causes in 55/81 (69.6%) patients, to systemic causes in 22/81 (27.8%), and unknown in 2/81 (2.5%). An unreactive EEG background to painful and auditory stimuli was more frequently observed in unfavorable outcome patients, as compared to favorable outcome patients (65.7% vs 42.2% respectively, p = 0.003) (Fig. 1). Conversely, a benign EEG was more frequently observed among patients with favorable outcome (37.2% vs 11.4%, p < 0.001) (Fig. 2). Diffuse background slowing (69.8% vs 62.6%, p = 0.38), focal background slowing (38.6% vs 31.8%, p = 0.16), periodic discharges (2.3% vs 3.7%, p = 0.9), electrographic seizures (4.5% vs 3.7%, p = 0.4) or burst suppression (4.5% vs 3.7%, p = 1) were not associated with the outcome at one year (Table 1).
One-year outcome associated with a benign EEG pattern. The modified Rankin scale (mRS) score is presented in shades of blue. A benign EEG is defined when all the 5 following criteria are present: (1) reactivity to auditory and pain stimulations, (2) absence of seizures, (3) no periodic discharges and (4) no burst suppression patterns, (5) no triphasic waves. *n = 148 due to 5 missing data
Univariable logistic regression analysis
Univariate logistic regression identified that age (OR 1.03, 95%CI 1–1.06), GCS score < 8 at admission (OR 3.01, 95%CI 1.41–6.41), non-neurological SOFA score (OR 1.19, 95%CI 1.03–1.37), background EEG unreactive to auditory stimulation (OR 4.8, 95%CI 1.84–12.53), EEG unreactive to painful stimulation (OR 4.76 95%CI 1.89–11.96) and EEG unreactive to both auditory and painful stimulations (OR 4.74 95%CI 2.02–11.14) were associated with unfavorable outcome (Additional file 1: Table 3). In contrast, a benign EEG was associated with favorable outcome (OR 0.17, 95%CI 0.06–0.45).
Multivariable logistic regression analysis
Multivariable analysis adjusted for potential clinical confounders (i.e., age, non-neurological SOFA, GCS < 8 at admission, stroke sub-type and sedation) identified that age (OR 1.05, 95%CI 1.01–1.09), non-neurological SOFA score (OR 1.21, 95%CI 1.02–1.42), GCS < 8 at admission (OR 3.74, 95%CI 1.44–9.72), intracerebral hemorrhage (OR 4.53, 95%CI 1.41–14.57) and an EEG unreactive to auditory and painful stimulations (OR 6.02, 95%CI 2.27–15.99) remained independently associated with unfavorable outcome at one year (Table 2). Conversely, diffuse, or focal slowing background and sedation during EEG recording were not associated with the neurological outcome.
Performance metrics of EEG reactivity
EEG unreactive to auditory and painful stimulations predicted unfavorable outcome with a specificity of 0.48 (95% CI 0.40–0.56), a sensitivity of 0.79 (95% CI 0.72–0.85), a positive predictive value (PPV) of 0.74 (95% CI 0.67–0.81) and a negative predictive value (NPV) of 0.55 (95% CI 0.47–0.63). Conversely, a benign EEG predicted favorable outcome with a specificity of 0.89 (95% CI 0.84–0.94), a sensitivity of 0.37 (95% CI 0.30–0.45), a PPV of 0.57 (95% CI 0.49–0.65) and a NPV of 0.78 (95% CI 0.71–0.84) (Table 3).
The prognostic value of variables associated with poor neurological outcome in the original SPICE study (GCS < 8, NIHSS > 16, age ≥ 70 years and Charlson comorbidity index ≥ 2) are presented in the Additional file 1: Table 4. Compared to unreactive EEG, all of these variables presented lower specificities and lower PPV for poor outcome prediction.
Effects of sedation on EEG recordings
A total 53/151 (35.1%) patients were sedated during EEG recording (Table 1). To assess the potential effect of sedation on EEG, we compared the demographic characteristics and EEG findings in patients with and without sedative drug infusion during EEG recording (Additional file 1: Table 5). Clinical seizure at ICU admission and burst suppression were more frequently observed in sedated patients, as compared to non-sedated patients (15.1% vs 2%, p = 0.04, and 9.4% vs 1%, p = 0.02, respectively). Conversely, electrographic seizures (6.1 vs 0%, p = 0.05) and poor outcome at one year (76 vs 60.4%, p = 0.04) were more frequent in the non-sedated group. We observed no significant difference regarding unreactive (58% vs 60.4%, p = 0.79) or benign pattern (18.8% vs 19.2%, p = 0.94) rates between non-sedated and sedated patients.
Discussion
In this prospective multicenter study conducted in severe stroke patients that required invasive MV, we found that an early background EEG unreactive to painful and auditory stimuli was the only EEG parameter independently associated with unfavorable outcome at one year. This association persisted after adjustment for common clinical confounders, including age, stroke subtype, neurological severity at stroke onset, non-neurological organ failure, and sedation infusion during EEG recording. In contrast, a benign EEG (i.e., reactive and continuous without seizures, periodic discharges, triphasic waves, and burst suppression) was independently associated with favorable outcome at one year.
These results suggest that EEG reactivity is a powerful marker of prognostication in this population of severe stroke patients, compared to other early resting state EEG abnormalities. These results are in line with previous studies conducted in less severe stroke unit patients. In a study conducted in massive cerebral hemispheric infarction patients, a dominant alpha unreactive background was associated with an unfavorable outcome [22]. EEG reactivity reflects dynamic brain responses to external stimulations which requires functional integrity of different neuro-anatomical structures, from the brainstem to the subcortical and cortical areas [23]. In animal models, preserved EEG reactivity is associated with structural and functional integrity of both the cortico-thalamic and the thalamus-brainstem loops, which are key areas involved in consciousness [24]. Moreover, our results suggested that sedation seems to have a limited effect on EEG reactivity. Sedative drugs could modify the EEG spectrum, decreasing frequency and amplitude background. Nevertheless, sedation infusion during EEG recording in CA patients did not modify the prognostic value of unreactive EEG [25]. More and more studies also suggest that light-to-moderate sedation in CA patients does probably not significantly impair the prognostic accuracy of the EEG [26,27,28,29]. These results could be explained by a major and recent modification of sedation practices in the ICU, using light to moderate doses, with short acting drugs and a daily interruption of sedation in critically ill patients [30,31,32]. Nevertheless, these results should be confirmed in a larger cohort of severe strokes of different subtypes.
Although EEG was an independent marker of poor outcome in this population, its prognostic value for unfavorable outcome prediction remained limited. In contrast, a benign EEG accurately predicted favorable outcome. These results were not unexpected, because the prognostic value of EEG reactivity remains also limited in other types of brain injury. Among comatose patients after CA, an unreactive background predicts unfavorable outcome with false positive rates ranging from 0 to 50% [25, 29]. In contrast, a reactive EEG predicts favorable outcome with a PPV between 57 and 85% according to various studies [33]. EEG reactivity could also be useful in traumatic brain injury and more generally among patients with severe acute encephalopathy, but its prognostic performance remains even lower in these populations than in CA patients [5, 34,35,36]. In short, our results suggested that a benign EEG could reflect limited or reversible brain injury in this population of MV patients with stroke and could thus encourage intensivists to continue aggressive life support. Conversely, an unreactive background likely reflects severe brain injury. Despite this, EEG reactivity should not be used alone for decisions of WLST, due to its low specificity. Ideally, this dynamic neurophysiological marker should be integrated in a multimodal approach together with other robust validated clinical and imaging prognostic tools or biomarkers of brain injury (Neurone-Specific-Enolase or S100b proteins, for example).
Our results also highlighted that diffuse or focal slowing represented the most frequent spot EEG abnormalities, whereas triphasic waves, periodic discharges, and burst suppression were rarely observed during EEG recording. Because EEG patterns may significantly evolve over time, we cannot exclude that these abnormalities were not observed later or earlier during ICU stay. Furthermore, we found no significant association between any of these EEG patterns and one-year outcome. These results are concordant with a previous study in this population which found a significant association between interictal epileptiform discharges and neurological outcome, but no prognostic value of periodic patterns [6]. However, some other studies highlighted that slow background, asymmetry and periodic discharges were independently associated with unfavorable outcome in non-ICU stroke patients [37,38,39,40]. These discrepancies could be explained by different factors. First, most of these studies were conducted in stroke unit patients while we only included the most severe stroke cases that required MV at the acute phase. Second, these studies were mainly conducted in ischemic stroke patients [6, 22, 37, 41], whereas SPICE included ischemic and hemorrhagic stroke but also subarachnoid hemorrhage [3]. Third, slowing background, periodic discharges and/or triphasic waves are common in ICU patients with encephalopathies of septic, metabolic or toxic origin [34, 42, 43]. Therefore, one could suggest that these EEG abnormalities observed in our population are multifactorial and non-specific to stroke severity itself. Fourth, periodic discharges are often associated with seizures, which in turn contributes to secondary brain injury and worse outcomes. However, these patterns were relatively infrequent in our study [38]. Moreover, the absence of impact of such EEG patterns on outcome could simply be due to a lack of power. Our study also identified that 6.5% of patients had electrographic seizures during EEG. Interestingly, most seizures occurred during the first seven days after stroke onset, meeting the definition of acute symptomatic seizure [44]. These electrographic seizures could be related to the ischemic brain damage itself or be secondary to intracranial hypertension in the most severe patients [45]. We cannot exclude that this low prevalence was related to the use of intermittent EEG recording, and that this prevalence could have been higher in patients monitored with continuous EEG[46]. Nevertheless, this result is relatively concordant with those of others studies, mainly conducted in non-ICU ischemic stroke patients [6, 47,48,49].
Our study has several strengths, including its “real life” situation, with a large multicenter recruitment of ICU patients, and prospective collected data. We a priori defined outcomes at one year, which were measured by an independent trained research assistant. EEGs were prospectively analyzed and collected based on the critical care ACNS terminology, limiting the risk of heterogeneity [50]. Finally, we performed all analyses stratified on centers and adjusted for common confounding factors, including sedation at time of EEG recording.
This study also has limitations. First, we did not re-analyze EEG recordings for study purposes. Second, we assessed EEG reactivity with a visual analysis. Because EEG reactivity assessment is subjective with a moderate inter-rater agreement between neurophysiologists [51], automated quantitative approaches have been developed [52]. Nevertheless, these technics are not used in routine practice. Third, there could be significant variability in EEG reactivity assessment among centers, especially regarding the type of stimuli used [23]. In our real-life study, the reactivity testing protocol was not standardized across different centers, although participants were asked to follow French recommendations regarding the use of EEG in the ICU [13]. Fourth, physicians in charge of included patients were not blinded to EEG results. Consequently, we cannot exclude that this may have resulted in self-fulfilling prophecy for WLST decisions during ICU stay. Nevertheless, EEG reactivity is not included in the guidelines algorithm of neuroprognostication for unfavorable outcome prediction, limiting the risk of self-fulfilling prophecy. Although we did not collect specific indications of EEG studies, we speculate that EEG recording was performed for persistent altered mental status, seizure suspicion and/or prognostication. Moreover, we only collected the first EEG performed after stroke, and did not use continuous EEG, because this is not the practice in the majority of French ICU centers. Finally, we had no data regarding doses and types of sedative drugs during EEG recordings, and we cannot exclude that reactivity might have been affected by concomitant sedation. However, only 35% of patients were sedated during EEG and we found no difference of EEG reactivity between sedated and non-sedated patients.
Conclusion
In this multicenter prospective cohort of severe stroke patients that required invasive ventilation in ICU, the absence of EEG reactivity was independently associated with unfavorable outcome at one year. However, its value for prediction of unfavorable outcome remained limited. Conversely, a benign EEG was associated with favorable outcome at one year. These results confirm that EEG could be useful in severe stroke patients, not only for detection of electrographic seizure but also for assessment of brain injury severity and prognostication, integrated in a multimodal approach.
Availability of data and materials
Data could be available if request to corresponding author, to a reasonable extent.
Abbreviations
- ACNS:
-
American Clinical Neurophysiology Society
- ASMs:
-
Antiseizure medications
- CA:
-
Cardiac Arrest
- CI:
-
Confidence Interval
- eCRF:
-
Electronic case report form
- EEG:
-
Electroencephalogram
- ESM:
-
Electronic Supplementary Material
- GCS:
-
Glasgow Coma Scale
- HIBI:
-
Hypoxic Ischemic Brain Injury
- ICU:
-
Intensive Care Unit
- IQR:
-
Inter Quartile Range
- MV:
-
Mechanical Ventilation
- mRS:
-
Modified Rankin Scale
- NIHSS:
-
National Institutes of Health Stroke Scale
- OR:
-
Odds Ratio
- SOFA:
-
Sequential Organ Failure Assessment
- TBI:
-
Traumatic Brain Injury
- WLST:
-
Withdrawal of Life Sustaining Therapies
References
GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US From 1990–2017: a global Burden of disease study. JAMA Neurol. 2021;78:165–76. https://doi.org/10.1001/jamaneurol.2020.4152.
Sonneville R, Mazighi M, Bresson D, et al. Outcomes of acute stroke patients requiring mechanical ventilation: study protocol for the SPICE multicenter prospective observational study. Neurocrit Care. 2020;32:624–9. https://doi.org/10.1007/s12028-019-00907-0.
Sonneville R, Mazighi M, Collet M, et al. One-year outcomes in patients with acute stroke requiring mechanical ventilation. Stroke. 2023. https://doi.org/10.1161/STROKEAHA.123.042910.
Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47:369–421. https://doi.org/10.1007/s00134-021-06368-4.
Gao G, Wu X, Feng J, et al. Clinical characteristics and outcomes in patients with traumatic brain injury in China: a prospective, multicentre, longitudinal, observational study. Lancet Neurol. 2020;19:670–7. https://doi.org/10.1016/S1474-4422(20)30182-4.
Lima FO, Ricardo JAG, Coan AC, et al. Electroencephalography patterns and prognosis in acute ischemic stroke. Cerebrovasc Dis Basel Switz. 2017;44:128–34. https://doi.org/10.1159/000477674.
Calvet D, Bracard S, Mas J-L, French Society of Intensive Care. Treatment of arterial and venous brain ischemia. Experts’ recommendations: stroke management in the intensive care unit. Rev Neurol (Paris). 2012;168:512–21. https://doi.org/10.1016/j.neurol.2012.01.587.
Alkhachroum A, Appavu B, Egawa S, et al. Electroencephalogram in the intensive care unit: a focused look at acute brain injury. Intensive Care Med. 2022. https://doi.org/10.1007/s00134-022-06854-3.
Rapp PE, Keyser DO, Albano A, et al. Traumatic brain injury detection using electrophysiological methods. Front Hum Neurosci. 2015;9:11.
Benghanem S, Pruvost-Robieux E, Bouchereau E, et al. Prognostication after cardiac arrest: how EEG and evoked potentials may improve the challenge. Ann Intensive Care. 2022;12:111. https://doi.org/10.1186/s13613-022-01083-9.
Diedler J, Sykora M, Jüttler E, et al. EEG power spectrum to predict prognosis after hemicraniectomy for space-occupying middle cerebral artery infarction. Cerebrovasc Dis Basel Switz. 2010;29:162–9. https://doi.org/10.1159/000262313.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet Lond Engl. 2007;370:1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X.
André-Obadia N, Zyss J, Gavaret M, et al. Recommendations for the use of electroencephalography and evoked potentials in comatose patients. Neurophysiol Clin. 2018;48:143–69. https://doi.org/10.1016/j.neucli.2018.05.038.
Claassen J, Taccone FS, Horn P, et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337–51. https://doi.org/10.1007/s00134-013-2938-4.
Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2013;30:1–27. https://doi.org/10.1097/WNP.0b013e3182784729.
Westhall E, Rossetti AO, van Rootselaar A-F, et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology. 2016;86:1482–90. https://doi.org/10.1212/WNL.0000000000002462.
Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–73. https://doi.org/10.1097/CCM.0000000000003299.
Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60. https://doi.org/10.1161/STR.0000000000000069.
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418. https://doi.org/10.1161/STR.0000000000000211.
Outin H, Lefort H, Peigne V, French Group for Status Epilepticus Guidelines. Guidelines for the management of status epilepticus. Eur J Emerg Med Off J Eur Soc Emerg Med. 2021;28:420–2. https://doi.org/10.1097/MEJ.0000000000000857.
Bruno A, Akinwuntan AE, Lin C, et al. Simplified modified rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke. 2011;42:2276–9. https://doi.org/10.1161/STROKEAHA.111.613273.
Su YY, Wang M, Chen WB, et al. Early prediction of poor outcome in severe hemispheric stroke by EEG patterns and gradings. Neurol Res. 2013;35:512–6. https://doi.org/10.1179/1743132813Y.0000000205.
Azabou E, Navarro V, Kubis N, et al. Value and mechanisms of EEG reactivity in the prognosis of patients with impaired consciousness: a systematic review. Crit Care Lond Engl. 2018;22:184. https://doi.org/10.1186/s13054-018-2104-z.
Altwegg-Boussac T, Schramm AE, Ballestero J, et al. Cortical neurons and networks are dormant but fully responsive during isoelectric brain state. Brain J Neurol. 2017;140:2381–98. https://doi.org/10.1093/brain/awx175.
Benghanem S, Paul M, Charpentier J, et al. Value of EEG reactivity for prediction of neurologic outcome after cardiac arrest: Insights from the Parisian registry. Resuscitation. 2019;142:168–74. https://doi.org/10.1016/j.resuscitation.2019.06.009.
Drohan CM, Cardi AI, Rittenberger JC, et al. Effect of sedation on quantitative electroencephalography after cardiac arrest. Resuscitation. 2018;124:132–7. https://doi.org/10.1016/j.resuscitation.2017.11.068.
Sivaraju A, Gilmore EJ, Wira CR, et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015;41:1264–72. https://doi.org/10.1007/s00134-015-3834-x.
Huotari A-M, Koskinen M, Suominen K, et al. Evoked EEG patterns during burst suppression with propofol. Br J Anaesth. 2004;92:18–24. https://doi.org/10.1093/bja/aeh022.
Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46:1803–51. https://doi.org/10.1007/s00134-020-06198-w.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. https://doi.org/10.1056/NEJM200005183422002.
Olsen HT, Nedergaard HK, Strøm T, et al. Nonsedation or light sedation in critically Ill, mechanically ventilated patients. N Engl J Med. 2020;382:1103–11. https://doi.org/10.1056/NEJMoa1906759.
Shehabi Y, Howe BD, Bellomo R, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380:2506–17. https://doi.org/10.1056/NEJMoa1904710.
Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of good neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2022;48:389–413. https://doi.org/10.1007/s00134-022-06618-z.
Kaplan PW, Rossetti AO. EEG patterns and imaging correlations in encephalopathy: encephalopathy part II. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2011;28:233–51. https://doi.org/10.1097/WNP.0b013e31821c33a0.
Jeantin L, Dupuis C, Vellieux G, et al. Electroencephalography for prognostication of outcome in adults with severe herpes simplex encephalitis. Ann Intensive Care. 2023;13:10. https://doi.org/10.1186/s13613-023-01110-3.
Ferlini L, Maenhout C, Crippa IA, et al. The association between the presence and burden of periodic discharges and outcome in septic patients: an observational prospective study. Crit Care Lond Engl. 2023;27:179. https://doi.org/10.1186/s13054-023-04475-w.
Bentes C, Peralta AR, Martins H, et al. Seizures, electroencephalographic abnormalities, and outcome of ischemic stroke patients. Epilepsia Open. 2017;2:441–52. https://doi.org/10.1002/epi4.12075.
Claassen J, Jetté N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65. https://doi.org/10.1212/01.wnl.0000281664.02615.6c.
Osman G, Rahangdale R, Britton JW, et al. Bilateral independent periodic discharges are associated with electrographic seizures and poor outcome: a case-control study. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2018;129:2284–9. https://doi.org/10.1016/j.clinph.2018.07.025.
Gelisse P, Crespel A, Genton P, Jallon P, Kaplan PW. Lateralized periodic discharges: which patterns are interictal, ictal, or peri-ictal? Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2021. https://doi.org/10.1016/j.clinph.2021.04.003.
Bentes C, Peralta AR, Viana P, et al. Quantitative EEG and functional outcome following acute ischemic stroke. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2018;129:1680–7. https://doi.org/10.1016/j.clinph.2018.05.021.
Mazeraud A, Righy C, Bouchereau E, et al. Septic-associated encephalopathy: a comprehensive review. Neurother J Am Soc Exp Neurother. 2020;17:392–403. https://doi.org/10.1007/s13311-020-00862-1.
Sonneville R, Benghanem S, Jeantin L, et al. The spectrum of sepsis-associated encephalopathy: a clinical perspective. Crit Care Lond Engl. 2023;27:386. https://doi.org/10.1186/s13054-023-04655-8.
Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–5. https://doi.org/10.1111/j.1528-1167.2009.02285.x.
Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Ann Neurol. 2013;74:53–64. https://doi.org/10.1002/ana.23859.
Rossetti AO, Schindler K, Sutter R, et al. Continuous vs routine electroencephalogram in critically ill adults with altered consciousness and no recent seizure: a multicenter randomized clinical trial. JAMA Neurol. 2020;77:1225–32. https://doi.org/10.1001/jamaneurol.2020.2264.
Bentes C, Martins H, Peralta AR, et al. Early EEG predicts poststroke epilepsy. Epilepsia Open. 2018;3:203–12. https://doi.org/10.1002/epi4.12103.
Sutcliffe L, Lumley H, Shaw L, et al. Surface electroencephalography (EEG) during the acute phase of stroke to assist with diagnosis and prediction of prognosis: a scoping review. BMC Emerg Med. 2022;22:29. https://doi.org/10.1186/s12873-022-00585-w.
Zöllner JP, Misselwitz B, Kaps M, et al. National Institutes of Health Stroke Scale (NIHSS) on admission predicts acute symptomatic seizure risk in ischemic stroke: a population-based study involving 135,117 cases. Sci Rep. 2020;10:3779. https://doi.org/10.1038/s41598-020-60628-9.
Hirsch LJ, Fong MWK, Leitinger M, et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2021;38:1–29. https://doi.org/10.1097/WNP.0000000000000806.
Admiraal MM, Horn J, Hofmeijer J, et al. EEG reactivity testing for prediction of good outcome in patients after cardiac arrest. Neurology. 2020;95:e653–61. https://doi.org/10.1212/WNL.0000000000009991.
Bouchereau E, Marchi A, Hermann B, et al. Quantitative analysis of early-stage EEG reactivity predicts awakening and recovery of consciousness in patients with severe brain injury. Br J Anaesth. 2022;S0007–0912(22):00506–12. https://doi.org/10.1016/j.bja.2022.09.005.
Acknowledgements
SPICE investigators (collaborators): Tiare Ader, Eric Barré, Hélène Bout, Perrine Boursin, Eric Bodiguel, Damien Bresson, Omar Ben Hadj Salem, Alain Combes, Anne Chrisment, Magalie Collet, Jacque Duranteau, Sophie Crozier, Daniel da Silva, Amexandre Demoule, Maxens Decavele, Eric Delpierre, Jean Luc Diehl, Martin Dres, Frédéric Faugeras, Marie-Céline Fournier, Tobias Gauss, Coralie Gernez, Guillaume Geri, Dominique Hurel, Matthieu Jamme, Laurence Josse, Igor Jurcisin, Lionel Kerhuel, Catherine Lamy, Fariza Lamara, Aymeric Lancelot, Bertrand Lapergue, Christophe Lenclud, Mathilde Lermuzeaux, Eric Magalhaes, Eric Mariotte, Isabelle Malissin, Alain Maldjian, Nathalie Marin, Jérôme Martin, Thibault Martinez, Armand Mekontso Dessap, Mehran Monchi, Giulia Naim, Hervé Outin,David Osman, Gregory Papin, Pierre Pasquier, Claire Pichereau, Matthieu Pissot, Keyvan Razazi, Danielle Reuter, Christian Richard, Stephane Ruckly, Damien Roux, Caroline Schimpf, Quentin Staiquly, Jérôme Servan, Sebastien Tanaka, Laurie-Anne Thion, Karim Toumert, Widad Traki, Marc Tran, Philippe Vassel, Bernard Vigué, Daniel Zafimahazo ; Jonathan Zarka
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
SB performed anaysis and wrote the manuscript. All authors contributed to the data collection. AL performed statistical analysis anf prepared the figures. All authors have thoroughly reviewed and approved the manuscript. RS and MM supervised the study and contributed equally to the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Data collection was approved by the Comité de Protection des Personnes Sud Méditerranée 1 (ID RCB 2017-A02452-51), written information was given by the local investigator and consent was obtained from the patient herself/himself or her/his next-of-kin; The study was registered in Clinical Trials (NCT03335995, first Submitted date: 2017-10-19).
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table 1. EEG data collected in the electronic case report form (eCRF) of the SPICE study. Table 2. Patient characteristics according to EEG recoding. Table 3. Univariable logistic regression for prediction of unfavorable outcome at one year. Table 4. Prognostic values of GCS<8, NIHSS>16, Age ≥ 70 years and Charlson comorbidity index ≥2 for poor outcome prediction. Table 5. Demographic characteristics and EEG findings according to sedation during EEG recording. Figure 1. Flow chart.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Benghanem, S., Kubis, N., Gayat, E. et al. Prognostic value of early EEG abnormalities in severe stroke patients requiring mechanical ventilation: a pre-planned analysis of the SPICE prospective multicenter study. Crit Care 28, 173 (2024). https://doi.org/10.1186/s13054-024-04957-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-04957-5