Abstract
Introduction
The impact of therapeutic plasma exchange (TPE) on short-term mortality in adult patients with sepsis-induced organ dysfunction remains uncertain. The objective of the study is to assess the effect of adjunct TPE in this setting through a comprehensive literature review.
Methods
The National Library of Medicine’s Medline, Ovid (Embase), the Cochrane Library database and clinicaltrial.gov from January 01, 1966, until October 01, 2022, were searched for terms: therapeutic plasma exchange, plasmapheresis, sepsis, and septic shock. We reviewed, selected and extracted data from relevant randomized clinical trials (RCTs) and matched cohort studies (MCSs) comparing short-term mortality in critically ill adult septic patients treated with standard therapy versus those receiving adjunct TPE. Risk of bias was assessed in the RCTs using Cochrane Collaboration tool and in MCSs using ROBINS-I tool. Summary statistics, risk ratios (RRs), and confidence intervals (CIs) were calculated using random effects model.
Results
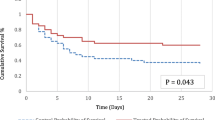
This systematic review included 937 adult critically ill septic patients from five RCTs (n = 367) and fifteen MCSs (n = 570). Of these total, 543 received treatment with TPE in addition to standard care. The meta-analysis includes all five RCTs and only six MCSs (n = 627). The adjunct TPE treatment (n = 300) showed a significant reduction in short-term mortality (RR 0.59, 95% CI 0.47–0.74, I2 3%) compared to standard therapy alone (n = 327). The systematic review of all 20 trials revealed that adding TPE to the standard therapy of critically ill septic patients resulted in faster clinical and/or laboratory recovery.
Conclusions
Our comprehensive and up-to-date review demonstrates that adjunct TPE may provide potential survival benefits when compared to standard care for critically ill adult patients with sepsis-induced organ dysfunction. While results of this meta-analysis are encouraging, large well-designed randomized trials are required to identify the optimal patient population and TPE procedure characteristics prior to widespread adoption into practice.
Similar content being viewed by others
Introduction
Sepsis is defined by the Third International Consensus as “life-threatening organ dysfunction caused by a dysregulated host response to infection” [1], and is comprised of a complex, intertwined interaction of inflammation, endothelial dysfunction, capillary leak, and a spectrum of pathologic coagulation [2]. Various treatments [3] have been previously investigated, targeting specific components of this pathological host response, but apart from rapid administration of antibiotics [4], results have been inconsistent and largely disappointing. TPE has long been hypothesized as a possible treatment [5] through simultaneous actions on multiple aspects of the pathway. Over the years, multiple case reports and case series [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] have produced encouraging results, demonstrating reduced short-term mortality and improved clinical outcomes in patients with sepsis-induced organ dysfunction receiving adjunct TPE. However, prospective, randomized data are scarce and inconclusive [35, 36]. Previous attempts to clarify have resulted in five meta-analyses [37,38,39,40,41] with the authors of these analyses concluding there is insufficient evidence to recommend TPE as routine therapy for patients with sepsis-induced organ dysfunction. Currently, the American Society for Apheresis (ASFA) 2023 guidelines [42] provide a category III, 2A recommendation for the use of TPE for patients with sepsis-induced organ dysfunction, allowing for individualized use on a case-by-case basis.
Our study aims to comprehensively review and analyze the currently available literature to re-evaluate the clinical impact of adjunct TPE on short-term mortality in critically ill adult septic patients with multiple organ dysfunction.
Materials and methods
Data source and search strategy
This systematic review and meta-analysis were performed by two investigators according to PRISMA (Preferred Reporting Items Systematic Reviews and Meta-Analysis) guidelines [43] and a pre-published protocol (PROSPERO database, CRD 42022377753). The National Library of Medicine’s Medline, Ovid (Embase), Cochrane library, and clinicaltrial.gov were searched for randomized, observational, and retrospective clinical studies of plasma exchange for treatment of septic patients with the following search terms: “plasma exchange, plasmapheresis, sepsis, and septic shock”. In addition, we hand searched references from retrieved articles to identify other eligible clinical studies. The search period took place from 01.01.1966 to 01.11.2022 and the language of the articles was limited to English. Initially, we planned to include only TPE by centrifuge technique. However, we deviated from the registered protocol (PROSPERO database, CRD 42022377753) due to the significant number of studies that performed TPE by filtration technique, which could have a significant impact on outcomes.
The assessed primary outcome was short-term mortality (14–35 days depending on individual study endpoints). Secondary outcomes included clinical (hemodynamics, noradrenaline dosing), laboratory, and severity of illness scores (SOFA, APACHE II, APACHE III). Post hoc subgroup analyses were performed based on TPE procedure type (membrane, centrifugal) and type of infection (non-COVID-19 versus COVID-19).
Selection of studies
PICO inclusion criteria (Patient/Population, Intervention, Comparison, Outcome) were used for inclusion in the meta-analysis: 1. Population: critically ill adult patients with sepsis-induced multi-organ dysfunction, 2. Intervention: therapeutic plasma exchange 3. Comparison intervention: standard therapy 4. Outcomes: clinical, laboratory markers, and short-term mortality 5. Study design: randomized, controlled trials, observational and retrospective studies.
All references were independently screened at the level of abstracts by two investigators (VK, MS) and then, if fulfilling inclusion criteria, the full-text articles were obtained and reviewed.
Data extraction and management
The first author extracted relevant information (authors, name of the article and journal, year of publication, patient demographics, illness severity scores, TPE treatment, short-term mortality, hemodynamic status/the dose of noradrenaline before and after TPE, laboratory values) from the selected articles. These data were checked independently by the second author. Discrepancies between the two investigators was resolved through consensus in discussion with the third author.
Quality assessment
The Cochrane Collaboration tool was used to assess the risk of bias (ROB2) in the RCTs [44], and the ROBINS-I tool was used to assess the risk of bias in MCSs [45].
The Cochrane tool assesses generation of the allocation sequence, concealment of the allocation sequence, blinding (participants/personnel and outcome assessors), incomplete outcome data, selective outcome reporting, and other biases [44]. All included RCTs were evaluated by two independent reviewers for the potential risk of bias by applying a rating of “Low”, “High” or “Unclear”.
The ROBINS-I tool assesses bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported result. The categories for risk of bias judgements by the ROBINS-I tool were assessed by two independent reviewers and rated a “Low risk,” or “Moderate risk,” or “Serious risk,” or “Critical risk,” or “No information.”
Two authors (VK, MS) independently reviewed the presence of authors’ possible conflict of interest and the funding source for each study, then rated each trial as of “Low,” “High,” or “Unclear” risk regarding those specific points.
Statistical analysis
The meta-analysis was performed using the R statistical program [46], using the “meta” [47] and “robvis” [48] packages. The meta-analysis only included MCSs that compared patients receiving adjunct TPE with controls. Additionally, multiple post hoc analyses were performed for subgroups of patients treated with centrifuge versus membrane filtration techniques of TPE, as well as subgroups of COVID-19 versus non-COVID-19 patients treated by TPE. Individual trials and summary results were reported as a relative risk (RR) with 95% confidential intervals (CI) of reported mortality in patients assigned to TPE versus controls. Random effects model were reported for all analyses. A RR of less than 1 suggests a lower rate of death among patients. Statistical heterogeneity among RCTs and MCSs included in the meta-analysis was assessed and quantified using the Cochran Q test and Higgins I2 metric correspondingly. P < 0.05 was considered statistically significant. A funnel plot and Peters’ linear regression test of asymmetry were used to evaluate the risk of publication bias.
Results
Study selection
We identified 1,305 publications from electronic and hand-searches (Fig. 1). After discarding duplicates and reviewing titles and abstracts, 1254 were excluded, leaving 51 records for analysis. Of these, 27 records were excluded for failing to meet the inclusion criteria. Of the remaining 24 records, three were excluded from analysis as they included pediatric patients, and one study was excluded because it was an abstract, leaving a total of 20 studies which were included in the analyses (5 RCTs and 15 MCSs) (Fig. 1).
The systematic review included all five randomized controlled trials (RCTs) [35, 36, 49,50,51] and 15 matched cohort studies (MCSs) [17, 32, 52,53,54,55,56,57,58,59,60,61,62,63,64], analyzing a total of 937 critically ill adult patients with sepsis and multiple organ dysfunction. Among these, 543 received adjunct therapeutic plasma exchange (TPE) in addition to standard sepsis management, while 394 patients received standard therapy alone.
The meta-analysis included only those trials comparing patients receiving adjunct TPE with controls, and included all five RCTs [35, 36, 49,50,51] and six MCSs [17, 55, 59, 61,62,63], reviewing a total of 627 critically ill adult patients with sepsis and multiple organ dysfunction. Among these, 300 received adjunct TPE in addition to standard sepsis management, while 327 patients received standard therapy alone.
Risk of publication bias assessment
Four of the five RCTs were rated as good quality with low risk of bias, while the fifth had a high risk of bias due to selection of reported results (Table 1, Additional file 1: Fig. S1A, B). Four of the six MCS studies were rated as good quality with low risk of bias. One MCS had serious risk of bias due to confounding and a moderate risk for bias due to selection of participants. The sixth MCS had moderate risk of bias due to confounding (Additional file 1: Fig. S2A, B). Due to the nature of a TPE procedure and the severity of the general condition of septic patients, blinding of these procedures/patients from medical personnel seemed difficult and illogical, therefore we did not judge it as a crucial factor for RCTs or MCSs.
We further evaluated the risk of publication bias with a funnel plot (Additional file 1: Fig. S3) and observed that the larger studies are evenly distributed around the random effects model estimate, while the smaller studies appear to be biased toward the larger effect sizes. The Peters’ regression test for funnel plot asymmetry [65] did not return a significant result (t = − 0.69, p = 0.507), indicating a low risk of publication bias (Table 2).
Characteristics and primary outcome of clinical studies
The median trial size was 43 patients, ranging from 7 to 106 participants. With the exception of one RCT, [36] (difference in mean age), there were no significant differences in baseline characteristics between controls and patients who underwent adjunct TPE in the RCTs. Inclusion criteria, TPE technique, choice and volume of replacement fluid, and number of TPE treatments varied among the trials (Table 3).
The five RCTs included 331 septic patients [35, 36, 49,50,51] with 166 receiving adjunct TPE (Table 3). Among these trials, patients treated with adjunct TPE had a lower mortality rate (RR: 0.62 [95% CI: 0.46, 0.83]) compared to those who received standard therapy alone (Fig. 2). In three RCTs [36, 49, 51], TPE was performed by centrifuge technique and the volume of plasma removed and replaced was between 30 and 45 ml/kg (Table 3). Membrane filtration was used in two RCTs [35, 50], and 30–150 ml/kg of plasma was removed (Table 3).
Risk ratios (RRs) of short-term mortality associated with therapeutic plasma exchange (TPE) treatment versus standard treatment of septic patients. Pooled risk ratios are from random effects model. Boxes and horizontal lines represent point estimates, varying in size according to the weight in the analysis, and 95% confidence intervals (CI). X2 = Chi-squared; df = degrees of freedom; I2 = I-squared; t2 = Tau-squared; Z = Z score; p = probability value
Five hundred and seventy patients were included in the 15 MCSs [17, 28, 52,53,54,55,56,57,58,59,60,61,62,63,64] with 377 of these patients receiving adjunct TPE (Table 3). Compared to those receiving standard therapy alone, patients receiving adjunct TPE had a non-significant decreased risk of short-term mortality in the subgroup of prospective studies (RR: 0.83 [95% CI: 0.44, 1.58]), while there was a significant reduction in the risk of short-term mortality in the subgroup of retrospective studies (RR: 0.33 [95% CI: 0.14, 0.76], Fig. 2). TPE by centrifugation was used in nine studies [52,53,54, 57, 59, 61,62,63,64] with removal and replacement of 30–45 ml/kg of plasma. The remaining six MCSs [17, 28, 55, 56, 58, 60] utilized the filtration technique, with an average of 30–45 ml/kg of plasma removed (Table 3).
Meta-analyses of pooled short-term mortality and subgroup analyses
We pooled data on short-term mortality from the 5 RCTs and 6 MCSs (n = 627) comparing those receiving standard therapy (n = 327) to those treated with adjunct TPE in addition to standard therapy (n = 300). The random effects model showed a significant reduction in the risk ratio of death (RR 0.59, [95% CI 0.47, 0.74]), with low overall heterogeneity (I2 = 3%, τ2 < 0.0001; Fig. 2).
We performed subgroup analyses by TPE method used. Filtration technique demonstrated a non-significant mortality reduction through random effects analysis (RR = 0.63, [95% CI 0.37, 1.07]) and very low heterogeneity (I2 = 0%, τ2 = 0.0527). In comparison, centrifugation technique demonstrated a decreased risk of short-term mortality with greater effect size and significance through random effects models (RR = 0.58, [95% CI 0.45, 0.75]) with quite low heterogeneity (I2 = 18%, τ2 < 0.0001), (Fig. 3).
Risk ratios (RRs) of short-term mortality associated with membrane filtration and centrifuge techniques of therapeutic plasma exchange (TPE) treatment in septic patients compared to standard treatment. Pooled risk ratios are from random effects model. Boxes and horizontal lines represent point estimates, varying in size according to the weight in the analysis, and 95% confidence intervals (CI). X2 = Chi-squared; df = degrees of freedom; I2 = I-squared; t2 = Tau-squared; Z = Z score; p = probability value
We also conducted a subgroup analysis comparing patients with sepsis caused by COVID-19 and those with sepsis from non-COVID-19 etiology (Fig. 4). Patients with sepsis caused by pathogens other than COVID-19 (referred to as non-COVID-19 sepsis) showed a reduced risk of short-term mortality in both randomized controlled trials (RCTs) (RR = 0.62, [95% CI 0.44, 0.86]) and observational studies (MCSs) (RR = 0.68, [95% CI 0.47, 0.97]). The random effects model indicated low heterogeneity (I2 = 0%, τ2 = 0) (Fig. 4). On the other hand, patients with sepsis due to COVID-19 exhibited a decreased risk of short-term mortality in MCSs (RR = 0.20, [95% CI 0.09, 0.47]) with low heterogeneity (I2 = 0%, τ2 = 0) (Fig. 4).
Risk ratios (RRs) of short-term mortality in septic patients with and without COVID-19 receiving therapeutic plasma exchange (TPE) or standard treatment. Pooled risk ratios are from random effects model. Boxes and horizontal lines represent point estimates, varying in size according to the weight in the analysis, and 95% confidence intervals (CI). X2 = Chi-squared; df = degrees of freedom; I2 = I-squared; t2 = Tau-squared; Z = Z score; p = probability value
Secondary clinical and laboratory outcomes
Multiple studies reported potentially relevant clinical and laboratory endpoints. Three RCTs [49,50,51] reported a significant reduction in noradrenaline doses required to maintain goal arterial blood pressure (ABP) following TPE. Two MCSs [58, 60] showed that TPE significantly improved the mean arterial pressure and stroke volume variance, enabling reduced noradrenaline doses. One MCS [56] found no change in hemodynamic status after TPE.
A significant reduction in Acute Physiology and Chronic Health Evaluation (APACHE) II [50], III [36] and Sequential Organ Failure Assessment (SOFA) [49, 50] scores following TPE were observed in four RCTs [35, 36, 49, 50], while one MCS [59] demonstrated a significant reduction in the SOFA score following TPE. One MCS [55] showed no change in the APACHE II score after TPE.
Three RCTs [49,50,51] demonstrated a significant decline in the plasma concentration of inflammatory cytokines, immune antibodies, serum lactate, lactate dehydrogenase (LDH), ferritin, D-dimer, and injurious mediators such as procalcitonin (PCT), von Willebrand factor antigen (vWF:Ag), angiopoietin-2 (Angpt-2) and a soluble receptor of tyrosine kinase with immunoglobulin-like and EGF-like domains (sTie-2) following TPE. In these trials, TPE also resulted in a significant increase in the number of lymphocytes, platelets, and repletion of protective factors such as antithrombin-III (AT III), protein C and a disintegrin and metalloprotease with thrombospondin type 1 motif 13 (ADAMTS-13) [49,50,51].
Two MCSs [56, 58] demonstrated a significant reduction in plasma concentration of inflammatory cytokines (interleukin (IL)-1 [58], IL-6 [58, 61], IL-8 [58], IL-10 [58]), C-reactive protein [61], procalcitonin [61], D-dimer [61], ferritin [61], and LDH [61] following TPE.
Discussion
Our meta-analysis demonstrates a significant reduction in short-term mortality when adjunct TPE is added to the standard therapy of critically ill patients with sepsis-induced organ dysfunction. These findings, combined with those of three recently published meta-analyses [66,67,68] add to the current body of evidence reflected in the 2023 ASFA guidelines which allow TPE to be considered on a case to case basis for sepsis with multiple organ dysfunction (category III, 2A recommendation) [41]. In addition, a large propensity-score matched analysis [69] demonstrating reduced 28-day and 1-year mortality associated with TPE in septic patients with MODS was published in November 2023, but was not included in the current analysis so as to avoid deviations from the predefined protocol and to avoid bias.
While these results are encouraging, it is important to acknowledge and address several limitations. As with any trial that includes retrospective observational reports, there is inherent bias that cannot be completely eliminated. It is again worth noting that in each trial, including the RCTs, a “sham” intervention was not performed because TPE requires an intervention and treatment that is difficult to “blind” from clinical providers. This lack of blinding could lead to bias among the treatment teams, which could affect management and would be difficult to eliminate due to the logistics of the intervention. Additionally, authors with negative and/or equivocal outcomes are less likely to publish their findings, so these outcomes may not be reported. In our efforts to identify published and unpublished studies, we conducted extensive searches of several databases, identifying those studies referenced in Table 3. In addition, we evaluated the risk of publication bias with a funnel plot (Additional file 1: Fig. S3), and the Peters’ regression test for funnel plot asymmetry [65] indicated a low risk of publication bias (t = − 0.69, p = 0.507). While these measures cannot guarantee that conflicting outcomes have not been observed clinically, our literature search and analysis were comprehensive.
By the nature of their design, meta-analyses and systematic reviews are limited by differing treatment protocols/algorithms and variable outcome measures among the included trials. While trials in critical care typically report 28–30 day mortality [2], the trials included in this analysis varied from 14 to 35 days (Table 3). Furthermore, the actual day of death is not reported in the individual trials, rather the data includes only short-term death or short-term survival as the outcome (Table 3). The authors of the current manuscript do not have access to outcomes beyond those reported in the original manuscripts, making it impossible to analyze whether the effect on mortality would differ if the same mortality endpoint were used in all trials.
Additionally, while long-term outcomes would be desirable, the design and endpoints of the included trials do not allow for the assessment of outcomes beyond 35 days.
A major limitation of our current review is the inability to assess the timing of TPE on mortality. The hallmark of sepsis management is early therapy, and it would seem intuitive that timely initiation of TPE would be paramount to response. Only one of the included trials included strict criteria in terms of timing, and none reported outcomes in relation to time. Similarly, a great deal of heterogeneity exists among the trials in terms of TPE eligibility criteria. Sepsis diagnostic criteria have evolved, and quantifying/analyzing the severity of illness among the trials is not possible with the available data. The absence of this information limits the generalizability of the results and should be a priority in future prospective trials.
The review is further limited by the variability in the number of TPE treatments performed (Table 3). Unfortunately, further analysis is not possible with the data available from each individual trial. Based on the authors’ clinical experience, we support that the number of procedures should be based on clinical response and not pre-determined. Although this cannot be confirmed or refuted by the current literature, this strategy is supported by the low incidence of severe adverse events attributed to the TPE treatment [70]. Future prospective trials should include this topic.
Guidelines and protocols also varied across the included trials in terms of technique. Both membrane and centrifuge modalities effectively remove pathological macromolecules, but some differences are worth noting. The membrane filtration technique has a lower plasma extraction ratio, requiring higher blood flow rates and a longer procedure. The centrifugation technique removes extracellular vesicles (EV) expelled as inflammatory mediators into plasma [71], while the membrane filtration technique results in partial deposition of the EV in the filter [72]. Additionally, the blood-membrane interaction itself activates cells and inflammation, which may require more procedures to achieve down-regulation of inflammation [73]. A subgroup analysis of TPE techniques suggests different efficacies of these techniques (Fig. 3). Future studies should include an emphasis on the efficacy of each technique.
The type and volume of replacement fluid also varied among the trials and may have impacted outcomes. Sepsis is characterized by decreased ADAMTS-13 activity, which results in increased thrombogenic ultra-large von Willebrand factor (ULvWF) multimers and potentially diffuse microcirculatory platelet thrombosis [2, 3]. Increased plasminogen activator inhibitor (PAI-1) activity leads to decreased fibrinolysis and disseminated fibrin-rich microcirculatory clotting [73,74,75]. The net result is a non-consumptive, platelet- and fibrin-rich microcirculatory thrombotic state with non-specific coagulation findings, often distinct from other thrombotic conditions.
Replacing plasma with that from healthy donors is crucial for replenishing essential protective anti-inflammatory mediators and coagulation factors, including ADAMTS-13, which may lead to improved tissue perfusion and recovery of organ dysfunction [51, 58]. Prior studies [21] have also identified circulating markers of endothelial injury that have been associated with electron microscopic changes to the endothelium [58]. Hypotension results not only from inflammatory vasodilation, but also from increased vascular permeability caused by endothelial injury [58]. Resuscitation with fresh frozen plasma (FFP) during the TPE procedure has shown restoration of endothelial integrity as assessed by improved levels of these circulating markers and an improved microscopic appearance of the endothelium [58]. Upon clinical response, retained fluid may redistribute into pulmonary edema. Fluid removal by dialysis now is necessary unless adequate diuresis exists.
Several studies [49,50,51, 58, 60] have demonstrated improved hemodynamics immediately following TPE, perhaps due to this effect on the endothelium, and as a result, may allow for management with a lower volume of intravenous fluids and lower doses of vasopressors—both of which are associated with improved survival and increased ventilator-free days [49]. Thus, while the current review is unable to address these differences, the authors strongly recommend using FFP as replacement fluid when performing TPE for sepsis (as reflected in the 2023 ASFA guidelines). Some data analyzed in our review is derived from studies in patients with COVID-19-induced sepsis, and Fig. 4 shows decreased short-term mortality in those receiving adjunct TPE. While effective vaccines and therapeutics have drastically reduced the number of patients developing critical illness from COVID-19, a small percentage will still develop sepsis. The focus of this review is on the treatment of sepsis with multiple organ dysfunction, not on specific inciting pathogens. Adjunct therapy, including TPE, should not be administered solely due to COVID-19 infection, but could be considered in cases of sepsis with MODS.
Despite limitations, our review found a decreased short-term mortality in critically ill patients with sepsis and MODS receiving adjunct TPE, regardless of inciting pathogen (Fig. 4). In the absence of well-designed, prospective, double-blinded RCTs, the clinical significance of these results should not be ignored. Rather, our findings, and the limitations observed, provide a solid foundation and an urgency for future studies.
Conclusions
Despite the small size of trials and heterogeneity of critically ill patients with sepsis and MODS, our meta-analysis demonstrates that adjunct therapeutic plasma exchange (TPE) using healthy donor plasma as replacement fluid is associated with a decreased risk of short-term mortality. While the results of this meta-analysis are encouraging, large, well-designed randomized trials are required to identify the optimal patient population and characteristics of TPE procedures prior to widespread adoption into practice.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10. https://doi.org/10.1001/jama.2016.0287.
Arina P, Singer M. Pathophysiology of sepsis. Curr Opin Anaesthesiol. 2021;34(2):77–84. https://doi.org/10.1097/ACO.0000000000000963.
Martí-Carvajal AJ, Solà I, Lathyris D, Cardona AF. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst Rev. 2011. https://doi.org/10.1002/14651858.CD004388.pub.
Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–44. https://doi.org/10.1056/NEJMoa1703058.
Scharfman WB, Tillotson JR, Taft EG, Wright E. Plasmapheresis for meningococcemia with disseminated intravascular coagulation. N Engl J Med. 1979;300(22):1277–8.
Vain NE, Mazlumian JR, Swarner OW, Cha CC. Role of exchange transfusion in the treatment of severe septicemia. Pediatrics. 1980;66(5):693–7.
Norska-Borowka I, Grzywna W, Monsiol A, Karczewska K, Godula-Stuglik U. Usefulness of exchange transfusions in the treatment of septicemia complicated by intravascular coagulation in newborn infants. Pediatr Pol. 1980;55:943–9.
Bjorvatn B, Bjertnaes L, Fadnes HO, Flaegstad T, Gutteberg TJ, Kristiansen BE, Pape J, Rekvig OP, Osterud B, Aanderud L. Meningococcal septicaemia treated with combined plasmapheresis and leucapheresis or with blood exchange. Br Med J. 1984;288(6415):439–41. https://doi.org/10.1136/bmj.288.6415.439.
Osterud B, Flaegstad T. Increased tissue thromboplastin activity in monocytes of patients with meningococcal infection: related to an unfavourable prognosis. Thromb Haemost. 1983;49(1):5–7.
Brandtzaeg P, Sirnes K, Folsland B, et al. Plasmapheresis in the treatment of severe meningococcal or pneumococcal septicaemia with DIC and fibrinolysis. Preliminary data on eight patients. Scand J Clin Lab Invest. 1985;178:53–5.
Ender LA, Lobakov AI, Lipats AA, Vatazin AV, Filizhanko VN. Plasmapheresis in the complex treatment of suppurative-septic complications of acute surgical diseases of the abdominal organs. Klin Med. 1985;63:98–101.
Drapkin MS, Wisch JS, Gelfand JA, Cannon JG, Dinarello CA. Plasmapheresis for fulminant meningococcemia. Pediatr Infect Dis J. 1989;8(6):399–400. https://doi.org/10.1097/00006454-198906000-00015.
McClelland P, Williams PS, Yaqoob M, et al. Multiple organ failure—a role for plasma exchange? Intensive Care Med. 1990;16:100–3. https://doi.org/10.1007/BF02575302.
Janbon B, Vuillez JP, Carpentier F, Barnoud D, André-Poyaud P, Barbe G, Guignier M. Removal of circulating tumor necrosis factor. Its role in septic shock treatment. Ann Med Interne. 1992;143(Suppl 1):13–6.
Westendorp RG, Brand A, Haanen J, van Hinsbergh VW, Thompson J, van Furth R, Meinders EA. Leukaplasmapheresis in meningococcal septic shock. Am J Med. 1992;92(5):577–8. https://doi.org/10.1016/0002-9343(92)90761-y.
van Deuren M, Santman FW, van Dalen R, Sauerwein RW, Span LF, van der Meer JW. Plasma and whole blood exchange in meningococcal sepsis. Clin Infect Dis. 1992;15(3):424–30. https://doi.org/10.1093/clind/15.3.424.
Gårdlund B, Sjölin J, Nilsson A, Roll M, Wickerts CJ, Wikström B, Wretlind B. Plasmapheresis in the treatment of primary septic shock in humans. Scand J Infect Dis. 1993;25(6):757–61. https://doi.org/10.3109/00365549309008575.
Churchwell KB, McManus ML, Kent P, Gorlin J, Galacki D, Humphreys D, Kevy SV. Intensive blood and plasma exchange for treatment of coagulopathy in meningococcemia. J Clin Apher. 1995;10(4):171–7. https://doi.org/10.1002/jca.2920100403.
Gårdlund B, Sjölin J, Nilsson A, Roll M, Wickerts CJ, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172(1):296–301. https://doi.org/10.1093/infdis/172.1.296.
Schött U, Björsell-Ostling E. Sonoclot coagulation analysis and plasma exchange in a case of meningococcal septicaemia. Can J Anaesth. 1995;42(1):64–8. https://doi.org/10.1007/BF03010573.
Haupt W, Fritzsche H, Hohenberger W, Zirngibl H. Selective cytokine release induced by serum and separated plasma from septic patients. Eur J Surg. 1996;162(10):769–76.
Mok Q, Butt W. The outcome of children admitted to intensive care with meningococcal septicaemia. Intensive Care Med. 1996;22(3):259–63. https://doi.org/10.1007/BF01712247.
Campbell WN, Joshi M, Sileo D. Osteonecrosis following meningococcemia and disseminated intravascular coagulation in an adult: case report and review. Clin Infect Dis. 1997;24(3):452–5. https://doi.org/10.1093/clinids/24.3.452.
Kumar A, Kanagasundaram NS, Collyns TA, Davison AM. Plasma exchange and haemodiafiltration in fulminant meningococcal sepsis. Nephrol Dial Transplant. 1998;13(2):484–7. https://doi.org/10.1093/oxfordjournals.ndt.a027853.
Darmon M, Azoulay E, Thiery G, Ciroldi M, Galicier L, Parquet N, Veyradier A, Le Gall JR, Oksenhendler E, Schlemmer B. Time course of organ dysfunction in thrombotic microangiopathy patients receiving either plasma perfusion or plasma exchange. Crit Care Med. 2006;34(8):2127–33.
Nguyen TC, Han YY, Kiss JE, Hall MW, Hassett AC, Jaffe R, Orr RA, Janosky J, Carcillo JA. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36(10):2878–87.
Sevketoglu E, Yildizdas D, Horoz OO, Kihtir HS, Kendirli T, Bayraktar S, Carcillo JA. Use of therapeutic plasma exchange in children with thrombocytopenia-associated multiple organ failure in the Turkish thrombocytopenia-associated multiple organ failure network. Pediatr Crit Care Med. 2014;15(8):e354-359.
Hadem J, Hafer C, Schneider AS, Wiesner O, Beutel G, Fuehner T, Welte T, Hoeper MM, Kielstein JT. Therapeutic plasma exchange as rescue therapy in severe sepsis and septic shock: retrospective observational single-centre study of 23 patients. BMC Anesthesiol. 2014;14:24. https://doi.org/10.1186/1471-2253-14-24.
De Simone N, Racsa L, Bevan S, Matevosyan K, Valley T, Girod C, Sarode R. Therapeutic plasma exchange in the management of sepsis and multiple organ dysfunction syndrome: a report of three cases. J Clin Apher. 2014;29(2):127–31. https://doi.org/10.1002/jca.21296.
Kawai Y, Cornell TT, Cooley EG, Beckman CN, Baldridge PK, Mottes TA, Luckritz KE, Plomaritas KS, Meade JM, Odetola FO, Han YY, Blatt NB, Annich GM. Therapeutic plasma exchange may improve hemodynamics and organ failure among children with sepsis-induced multiple organ dysfunction syndrome receiving extracorporeal life support. Pediatr Crit Care Med. 2015;16(4):366–74. https://doi.org/10.1097/PCC.0000000000000351.
Stegmayr BG. Plasmapheresis in severe sepsis or septic shock. Blood Purif. 1996;14(1):94–101. https://doi.org/10.1159/000170250.
Stegmayr BG. Apheresis as therapy for patients with severe sepsis and multiorgan dysfunction syndrome. Ther Apher. 2001;5(2):123–7. https://doi.org/10.1046/j.1526-0968.2001.005002123.x.
Stegmayr B. Apheresis in patients with severe sepsis and multi organ dysfunction syndrome. Transfus Apher Sci. 2008;38(3):203–8. https://doi.org/10.1016/j.transci.2008.03.009.
Garbero E, Livigni S, Ferrari F, Finazzi S, Langer M, Malacarne P, Meca MCC, Mosca S, Olivieri C, Pozzato M, Rossi C, Tavola M, Terzitta M, Viaggi B, Bertolini G. GiViTI High dose coupled plasma filtration and adsorption in septic shock patients. Results of the COMPACT-2: a multicentre, adaptive, randomised clinical trial. Intensive Care Med. 2021;47(11):1303–11. https://doi.org/10.1007/s00134-021-06501-3.
Reeves JH, Butt WW, Shann F, Layton JE, Stewart A, Waring PM, Presneill JJ. Continuous plasmafiltration in sepsis syndrome. Plasmafiltration in Sepsis Study Group. Crit Care Med. 1999;27(10):2096–104.
Busund R, Koukline V, Utrobin U, Nedashkovsky E. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med. 2002;28(10):1434–9. https://doi.org/10.1007/s00134-002-1410-7.
Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med. 2013;41(9):2209–20.
Rimmer E, Houston BL, Kumar A, Abou-Setta AM, Friesen C, Marshall JC, Rock G, Turgeon AF, Cook DJ, Houston DS, Zarychanski R. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014;18(6):699. https://doi.org/10.1186/s13054-014-0699-2.
Putzu A, Schorer R, Lopez-Delgado JC, Cassina T, Landoni G. Blood purification and mortality in sepsis and septic shock: a systematic review and meta-analysis of randomized trials. Anesthesiology. 2019;131(3):580–93. https://doi.org/10.1097/ALN.0000000000002820.
Snow TAC, Littlewood S, Corredor C, Singer M, Arulkumaran N. Effect of extracorporeal blood purification on mortality in sepsis: a meta-analysis and trial sequential analysis. Blood Purif. 2021;50(4–5):462–72. https://doi.org/10.1159/000510982.
Prakash S, Sahu A, Routray SS, Maiti R, Mitra JK, Mukherjee S. Efficacy of therapeutic plasma exchange in severe COVID-19 disease: a meta-analysis. Vox Sang. 2023;118(1):49–58. https://doi.org/10.1111/vox.13367.
Connelly-Smith L, Alquist CR, Aqui N, Hofmann J, Klingel R, Onwuemene O, Patriquin C, Pham H, Sanchez A, Schneiderman J, Witt V, Zantek N, Dunbar N. Guidelines on the use of therapeutic apheresis in clinical practice–evidence-based approach from the writing committee of the American society for apheresis: the ninth special issue. J Clin Apher. 2023;38(2):77–278. https://doi.org/10.1002/jca.22043.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. https://doi.org/10.1136/bmj.n71.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. https://doi.org/10.1136/bmj.d5928.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
R Core Team (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
McGuinness, LA robvis: An R package and web application for visualising risk-of-bias assessments. 2019, https://github.com/mcguinlu/robvis
Faqihi F, Alharthy A, Abdulaziz S, Balhamar A, Alomari A, AlAseri Z, Tamim H, Alqahtani SA, Kutsogiannis DJ, Brindley PG, Karakitsos D, Memish ZA. Therapeutic plasma exchange in patients with life-threatening COVID-19: a randomised controlled clinical trial. Int J Antimicrob Agents. 2021;57(5):106334. https://doi.org/10.1016/j.ijantimicag.2021.106334.
Weng J, Chen M, Fang D, Liu D, Guo R, Yang S. Therapeutic plasma exchange protects patients with sepsis-associated disseminated intravascular coagulation by improving endothelial function. Clin Appl Thromb Hemost. 2021. https://doi.org/10.1177/10760296211053313.
Stahl K, Wand P, Seeliger B, Wendel-Garcia PD, Schmidt JJ, Schmidt BMW, Sauer A, Lehmann F, Budde U, Busch M, Wiesner O, Welte T, Haller H, Wedemeyer H, Putensen C, Hoeper MM, Bode C, David S. Clinical and biochemical endpoints and predictors of response to plasma exchange in septic shock: results from a randomized controlled trial. Crit Care. 2022;26(1):134. https://doi.org/10.1186/s13054-022-04003-2.
Stegmayr BG, Jakobson S, Rydvall A, Björsell-Ostling E. Plasma exchange in patients with acute renal failure in the course of multiorgan failure. Int J Artif Organs. 1995;18(1):45–52.
Stegmayr BG. Plasma exchange in patients with septic shock including acute renal failure. Blood Purif. 1996;14(1):102–8. https://doi.org/10.1159/000170251.
Hjorth V, Stenlund G. Plasmapheresis as part of the treatment for septic shock. Scand J Infect Dis. 2000;32(5):511–4. https://doi.org/10.1080/003655400458794.
Schmidt J, Mann S, Mohr VD, Lampert R, Firla U, Zirngibl H. Plasmapheresis combined with continuous venovenous hemofiltration in surgical patients with sepsis. Intensive Care Med. 2000;26(5):532–7. https://doi.org/10.1007/s001340051200.
Ataman K, Jehmlich M, Kock S, Neumann S, Leischik M, Filipovic Z, Hopf HB. Short-term cardiovascular effects of plasmapheresis in norepinephrine-refractory septic shock. Intensive Care Med. 2002;28(8):1164–7. https://doi.org/10.1007/s00134-002-1375-6.
Stegmayr BG, Banga R, Berggren L, Norda R, Rydvall A, Vikerfors T. Plasma exchange as rescue therapy in multiple organ failure including acute renal failure. Crit Care Med. 2003;31(6):1730–6. https://doi.org/10.1097/01.CCM.0000064742.00981.14.
Knaup H, Stahl K, Schmidt BMW, Idowu TO, Busch M, Wiesner O, Welte T, Haller H, Kielstein JT, Hoeper MM, David S. Early therapeutic plasma exchange in septic shock: a prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care. 2018;22(1):285. https://doi.org/10.1186/s13054-018-2220-9.
Keith PD, Wells AH, Hodges J, Fast SH, Adams A, Scott LK. The therapeutic efficacy of adjunct therapeutic plasma exchange for septic shock with multiple organ failure: a single-center experience. Crit Care. 2020;24(1):518. https://doi.org/10.1186/s13054-020-03241-6.
Ahmed RM, Soliman AR, Yousry A, Marzouk K, Faris F. Efficacy of 4-hour rescue therapeutic plasma exchange in severe septic shock patients. Rom J Intern Med. 2020;58(2):75–80. https://doi.org/10.2478/rjim-2019-0026.
Gucyetmez B, Atalan HK, Sertdemir I, Cakir U, Telci L, COVID-19 Study Group. Therapeutic plasma exchange in patients with COVID-19 pneumonia in intensive care unit: a retrospective study. Crit Care. 2020;24(1):492. https://doi.org/10.1186/s13054-020-03215-8.
Khamis F, Al-Zakwani I, Al Hashmi S, Al Dowaiki S, Al Bahrani M, Pandak N, Al Khalili H, Memish Z. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–8. https://doi.org/10.1016/j.ijid.2020.06.064.
Kamran SM, Mirza ZE, Naseem A, Liaqat J, Fazal I, Alamgir W, Saeed F, Saleem S, Nisar S, Yousaf MA, Khan AZ, Hussain M, Azam R, Hussain M, Khan KA, Jamal Y, Iftikhar R. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS ONE. 2021;16(1): e0244853. https://doi.org/10.1371/journal.pone.0244853.
Jaiswal V, Nasa P, Raouf M, Gupta M, Dewedar H, Mohammad H, Al Rais Z, Ali Baqer M, Alsabbah A, Ibrahim Y, Salem M, Shammass D, Marashi M. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19-An exploratory study. Int J Infect Dis. 2021;102:332–4. https://doi.org/10.1016/j.ijid.2020.10.085.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–80. https://doi.org/10.1001/jama.295.6.676.
Lee OPE, Kanesan N, Leow EH, Sultana R, Chor YK, Gan CS, Lee JH. Survival benefits of therapeutic plasma exchange in severe sepsis and septic shock: a systematic review and meta-analysis. J Intensive Care Med. 2023;38(7):598–611. https://doi.org/10.1177/08850666231170775.
Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–9.
Chen JJ, Lai PC, Lee TH, Huang YT. Blood purification for adult patients with severe infection or sepsis/septic shock: a network meta-analysis of randomized controlled trials. Crit Care Med. 2023. https://doi.org/10.1097/CCM.0000000000005991.
Yan D, Yao R, Xie X, Fu X, Pei S, Wang Y, Xu D, Li N. The therapeutic efficacy of plasmapheresis for sepsis with multiple organ failure: a propensity score-matched analysis based on the MIMIC-IV database. Shock. 2023. https://doi.org/10.1097/SHK.0000000000002254.
Rock G, Weber V, Stegmayr B. Therapeutic plasma exchange (TPE) as a plausible rescue therapy in severe vaccine-induced immune thrombotic thrombocytopenia. Transfus Apher Sci. 2021;60(4):103174. https://doi.org/10.1016/j.transci.2021.103174.
Momen-Heravi F. Isolation of extracellular vesicles by ultracentrifugation. Methods Mol Biol. 2017;1660:25–32. https://doi.org/10.1007/978-1-4939-7253-1_3.
Stegmayr B, Abdel-Rahman EM, Balogun RA. Septic shock with multiorgan failure: from conventional apheresis to adsorption therapies. Semin Dial. 2012;25:171–5.
Fujimura Y, Holland LZ. COVID-19 microthrombosis: unusually large VWF multimers are a platform for activation of the alternative complement pathway under cytokine storm. Int J Hematol. 2022;115(4):457–69. https://doi.org/10.1007/s12185-022-03324-w.
Nedeva C, Menassa J, Puthalakath H. Sepsis: inflammation is a necessary evil. Front Cell Dev Biol. 2019;7:108.
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F, CRICS TRIGGERSEP Group. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–98.
Author information
Authors and Affiliations
Contributions
VK, MS, PK, and BS conceived and designed the study. VK and MS designed the search strategy. VK, JB, and MS collected the data. VK, MS, JB, and OMTT performed statistical analyses. VK, MS, PK and LKS drafted the manuscript. VK, MS, PK, and BS made substantial contributions to the design of the study. VK, MS, JB, PK, OMTT, LKS, WS, GR, and BS revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental figures and figure legends.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kuklin, V., Sovershaev, M., Bjerner, J. et al. Influence of therapeutic plasma exchange treatment on short-term mortality of critically ill adult patients with sepsis-induced organ dysfunction: a systematic review and meta-analysis. Crit Care 28, 12 (2024). https://doi.org/10.1186/s13054-023-04795-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04795-x