Abstract
Monitoring with electrical impedance tomography (EIT) during a decremental PEEP trial has been used to identify the PEEP that yields the optimal balance of pulmonary overdistension and collapse. This method is based on pixel-level changes in respiratory system compliance and depends on fixed or measured airway driving pressure. We developed a novel approach to quantify overdistension and collapse during pressure support ventilation (PSV) by integrating transpulmonary pressure and EIT monitoring and performed pilot tests in three hypoxemic patients. We report that our experimental approach is feasible and capable of identifying a PEEP that balances overdistension and collapse in intubated hypoxemic patients undergoing PSV.
Similar content being viewed by others
Introduction
Selection of physiology-based personalized positive end-expiratory pressure (PEEP) is key to the management of intubated patients with acute hypoxemic respiratory failure. PEEP should balance alveolar recruitment (which decreases volu- and atelectrauma) with the risk of overdistension (which increases barotrauma) [1].
Conventional pulmonary monitoring with electrical impedance tomography (EIT) during a decremental PEEP trial has been used to quantify regional overdistension and collapse at each level. The PEEP that yields the lowest difference (crossover PEEP) between the two phenomena could be considered as an optimal balance. This method is based on pixel-level changes in respiratory system compliance and depends on fixed or measured driving pressure during the decremental trial [2, 3]. Considering only the airway pressure (Paw) during assisted ventilation can be flawed because the driving pressure is composed of both ventilator support and the patient’s inspiratory effort.
We developed a modified approach to quantify lung overdistension and collapse by integrating esophageal pressure (Pes) and EIT monitoring during a decremental PEEP trial. We assessed pixel-level changes in lung compliance by using the dynamic transpulmonary driving pressure (∆PLdyn) instead of airway driving pressure. Here, we describe the novel methodology and report the feasibility of the modified approach based on pilot tests in three intubated patients with acute hypoxemic respiratory failure of different etiologies undergoing pressure support ventilation (PSV).
Methods
Novel experimental approach to identify crossover PEEP during PSV
Paw, measured at the endotracheal tube, and Pes were recorded simultaneously with the EIT signal. A transpulmonary pressure (PL) waveform was generated offline as the difference between Paw and Pes. Automated breath detection was performed on PL tracings by adapting a previously published algorithm [4]. Within each breath, ∆PLdyn was calculated as the difference between the maximal PL (peak inspiration) and PL at end-expiration.
The EIT signal was expressed as a relative impedance change from end-expiration (∆Z). Linear regression between impedance within each pixel and global impedance in the whole image was estimated. Pixels with a regression coefficient of at least 20% of the maximum at any step were classified as ventilated [5]. Within each ventilated pixel, the tidal variation in impedance (∆Ztidal,px) was calculated as the difference between the maximum and minimum value of the impedance signal for each breath.
Dynamic lung compliance for each breath and for each pixel (CL,px) was calculated as:
To ensure that the inadvertent inclusion of brief artifacts (e.g., patient–ventilator dyssynchronies, air leaks, coughing) leading to incorrect breath detection or compliance estimations would not affect our findings, breaths yielding a global dynamic lung compliance value above or below 3 scaled mean absolute deviations from the median were rejected as outliers. Then, for each PEEP step, segments comprising ~ 10 breaths were manually selected toward the end of the step and compliance for each pixel was averaged over breaths to obtain a compliance map. As previously described [2], relative compliance for each pixel and step was calculated as the percentage departure from the highest compliance obtained at any step within that pixel. Each relative compliance change was categorized as lung collapse or overdistension according to the decreasing PEEP trend [2, 3]. The crossover PEEP was defined as the tested PEEP step that yielded the smallest difference between the percentages of overdistension and collapse in the whole image. MATLAB R2021a was used to implement the algorithm.
Patients test
Data were recorded from three intubated hypoxemic patients already monitored by EIT and esophageal pressure and undergoing a decremental PEEP trial during PSV for clinical purposes. The trial was performed by setting PEEP to 12 cm H2O and decreasing it to 6 cm H2O, in 2 cm H2O steps, maintained for 2 min each. The PEEP range was selected considering the severity of the respiratory failure and the assisted ventilation mode. EIT data were continuously recorded at a 50 Hz sampling frequency via a 16-electrode belt placed around the chest in the axial plane at the 5th intercostal space (Dräger, Lübeck, Germany). At the end of the trial, data were downloaded and analyzed offline using the Dräger EIT Data Analysis Tool version 6.3 (Dräger, Lübeck, Germany).
Results
Patient 1 was a 43-year-old woman with primary graft dysfunction following a bilateral lung transplant; her PaO2/FiO2 was 240 mmHg on clinical PSV 8 cm H2O and PEEP 8 cm H2O. Patient 2 was a 46-year-old man with extensive left lung pneumonia and empyema; his PaO2/FiO2 was 188 mmHg with PSV 6 cm H2O and PEEP 10 cm H2O. Patient 3 was a 72-year-old woman with ARDS due to pneumonia; her PaO2/FiO2 was 204 mmHg with PSV 2 cm H2O and PEEP 12 cm H2O.
Respiratory parameters and the results from the experimental combined EIT-∆PLdyn analyses during the decremental trial are displayed in Table 1.
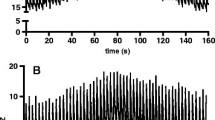
Inspiratory effort (and thus ∆PLdyn) varied across the PEEP trial, confirming the physiological rationale behind our novel method. Identification of the PEEP level associated with the lowest difference between overdistension and collapse was feasible in all the three patients (Fig. 1). Explorative data also showed that, in all patients, the PEEP that balanced overdistension and collapse based on the experimental approach was lower than the PEEP identified using the conventional approach (Fig. 1).
Decremental PEEP trial performed with the conventional and experimental approach. Results from a decremental PEEP trial performed during pressure support ventilation in patients 1, 2, and 3 using the conventional approach that assumes a fixed airway driving pressure (Panels A, B, and C) and the experimental approach that uses the measured dynamic transpulmonary driving pressure (Panels D, E, and F). Regional compliance maps and the percentage of overdistension and collapse are plotted against PEEP steps for both approaches. PEEP Positive end-expiratory pressure
Discussion
We developed a novel experimental approach to select personalized PEEP by integrating EIT and Pes monitoring in patients undergoing PSV. Our approach could be different from the conventional approach because it uses measured ∆PLdyn instead of airway driving pressure to calculate the extent of overdistension and collapse at each PEEP. Pilot testing in three patients with different severities and etiologies of hypoxemic respiratory failure demonstrates that the novel method is physiologically rational and clinically feasible.
Our experimental approach may represent a more accurate assessment of regional lung mechanics in the setting of assisted ventilation as it accounts for variations in the patient effort that can occur due to changes in PEEP [6] and uses ∆PLdyn instead of the airway driving pressure. Indeed, the experimental crossover PEEP tended to be associated with the lowest inspiratory effort and, thus, ∆PLdyn. This suggests that the PEEP which results in the lowest difference between lung collapse and overdistension may minimize respiratory drive and effort for a given inspiratory support level [7].
Our method can be applied at the bedside in patients in whom Pes is monitored. However, it also has some limitations: It uses a dynamic measurement of ∆PL, and should be used with caution when airway resistance might vary significantly between PEEP steps (i.e., very high PEEP or obstructive lung disease); peristalsis waves should be carefully monitored during the decremental PEEP trial and, theoretically, each step might need to be maintained longer than during passive conditions; it is not feasible in patients with contraindication to Pes monitoring (e.g., esophageal surgery or bleeding). Finally, our pilot tests studied a relatively narrow range of PEEP steps and we acknowledge the possibility that higher lung compliance may have existed at a PEEP > 12 cm H2O.
To conclude, in patients undergoing PSV, a simultaneous EIT- and Pes-based decremental PEEP titration method that accounts for variations in the inspiratory effort is feasible.
Availability of data and materials
The datasets used and analyzed in the current report are available from the corresponding author on reasonable request.
Abbreviations
- PEEP:
-
Positive end-expiratory pressure
- EIT:
-
Electrical impedance tomography
- Paw:
-
Airway pressure
- Pes:
-
Esophageal pressure
- ∆PLdyn :
-
Dynamic transpulmonary driving pressure
- PSV:
-
Pressure support ventilation
- PL :
-
Transpulmonary pressure
- ∆Z:
-
Impedance change
- ∆Ztidal,px:
-
Tidal impedance change per pixel
- CL,px:
-
Dynamic lung compliance per pixel
References
Scaramuzzo G, Spadaro S, Dalla Corte F, Waldmann AD, Bohm SH, Ragazzi R, et al. Personalized positive end-expiratory pressure in acute respiratory distress syndrome: comparison between optimal distribution of regional ventilation and positive transpulmonary pressure. Crit Care Med. 2020;48(8):1148–56.
Costa EL, Borges JB, Melo A, Suarez-Sipmann F, Toufen C Jr, Bohm SH, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35(6):1132–7.
Frerichs I, Amato MB, van Kaam AH, Tingay DG, Zhao Z, Grychtol B, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax. 2017;72(1):83–93.
Nguyen CD, Amatoury J, Carberry JC, Eckert DJ. An automated and reliable method for breath detection during variable mask pressures in awake and sleeping humans. PLoS One. 2017;12(6): e0179030.
Pulletz S, van Genderingen HR, Schmitz G, Zick G, Schadler D, Scholz J, et al. Comparison of different methods to define regions of interest for evaluation of regional lung ventilation by EIT. Physiol Meas. 2006;27(5):S115–27.
Perez J, Dorado JH, Navarro E, Morais CCA, Accoce M. Mechanisms of lung and diaphragmatic protection by high PEEP in obese COVID-19 ARDS: role of the body mass index. Crit Care. 2022;26(1):182.
Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46(4):606–18.
Acknowledgements
Not applicable.
Funding
This study was (partially) funded by Italian Ministry of Health—Current research IRCCS to Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy.
Author information
Authors and Affiliations
Contributions
TM conceived of the work. DS, TM, and ES collected and interpreted patient data. ML created the experimental software. DS, TM, and ML wrote the manuscript. ES, DLG, and SS revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Data were collected as part of routine clinical practice. Consent for the publication of data was obtained from all patients.
Competing interests
DS, ML, ES, and SS report no conflicts of interest. DLG reports a research grant from General Electric Healthcare, personal speaking fees from Intersurgical, General Electric, Fisher and Paykel, and Gilead, and personal travel expenses from Getinge and Air Liquide. TM reports receiving personal fees from Fisher and Paykel, Dräger, Mindray, and B. Braun.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Slobod, D., Leali, M., Spinelli, E. et al. Integrating electrical impedance tomography and transpulmonary pressure monitoring to personalize PEEP in hypoxemic patients undergoing pressure support ventilation. Crit Care 26, 314 (2022). https://doi.org/10.1186/s13054-022-04198-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04198-4