Abstract
Organ dysfunction or overt failure is a commonplace event in the critically ill affecting up to 70% of patients during their stay in the ICU. The outcome depends on the resolution of impaired organ function, while a domino-like deterioration of organs other than the primarily affected ones paves the way for increased mortality. “Acute Liver Failure” was defined in the 1970s as a rare and potentially reversible severe liver injury in the absence of prior liver disease with hepatic encephalopathy occurring within 8 weeks. Dysfunction of the liver in general reflects a critical event in “Multiple Organ Dysfunction Syndrome” due to immunologic, regulatory and metabolic functions of liver parenchymal and non-parenchymal cells. Dysregulation of the inflammatory response, persistent microcirculatory (hypoxic) impairment or drug-induced liver injury are leading problems that result in “secondary liver failure,” i.e., acquired liver injury without underlying liver disease or deterioration of preexisting (chronic) liver disease (“Acute-on-Chronic Liver Failure”). Conventional laboratory markers, such as transaminases or bilirubin, are limited to provide insight into the complex facets of metabolic and immunologic liver dysfunction. Furthermore, inhomogeneous definitions of these entities lead to widely ranging estimates of incidence. In the present work, we review the different definitions to improve the understanding of liver dysfunction as a perpetrator (and therapeutic target) of multiple organ dysfunction syndrome in critical care.
Graphic Abstract
Similar content being viewed by others
Introduction
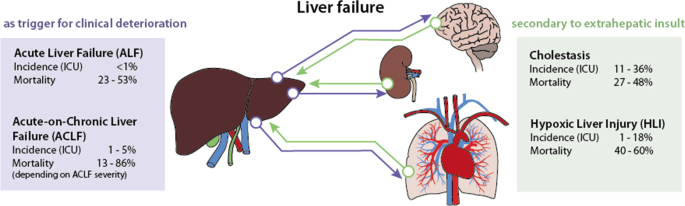
The definition of liver dysfunction is far from a global consensus. Typically, liver failure is divided into two major entities depending on the presence or absence of preexisting liver disease. Acute liver failure (ALF) is rare, occurs in the absence of previous liver damage, has a clear etiology and is classified according to the interval between the appearance of jaundice and the onset of hepatic encephalopathy into acute, subacute and hyperacute processes [1,2,3]. ALF is managed according to guidelines, which account for transplant needs [1, 4]. In contrast, acute-on-chronic liver failure (ACLF) is triggered by acute hepatic decompensation in patients with preexisting chronic disease [5]. ACLF definitions vary depending on the issuing consortium [6,7,8]. Apart from ALF and ACLF, secondary liver injury without underlying liver disease in response to hypoxic, toxic or inflammatory insults represents the most common form of hepatic dysfunction in the intensive care unit (ICU), and it commonly manifests as cholestasis and hypoxic liver injury [9, 10].
In ALF, the liver is triggering clinical deterioration, i.e., extrahepatic organ dysfunction develops due to impaired liver function. The situation in ACLF is more complex: A previous chronic liver disease worsens to liver failure by primary hepatic deterioration [alcoholic hepatitis, viral hepatitis, drug-induced liver injury (DILI)] or by secondary hepatic deterioration due to extrahepatic events (for example, sepsis). Whether alterations are classified as “dysfunction,” “injury” or “failure” depends on the surrogate marker and the score used. For instance, when applying bilirubin as a common marker, the definition of acute liver injury (ALI) by Koch et al. uses a value higher than 3 mg/dl (51.3 µmol/l) to define injury [11]. The Sequential Organ Failure Assessment (SOFA) Score divides dysfunction and failure using thresholds of 1.2 mg/dl (20.5 µmol/l) and 6.0 mg/dl (102.6 µmol/l) [12], respectively. Levels above 2 mg/dl are otherwise frequently used as pragmatic cutoffs to assess jaundice and cholestasis [6, 9].
Experimental data on immunologic, regulatory and metabolic functions of the liver suggest a role of the liver as a “perpetrator” rather than a “victim” of “host response failure” and multiple organ failure [13,14,15]. In any case, liver dysfunction and failure are clearly of utmost importance in the ICU as they affect at least 20% of patients and significantly increase the risk of death [16, 17].
The following overview will discuss ALF, ACLF and secondary liver failure. A thorough review and description of the pathophysiology and management can be found elsewhere [3, 9, 18,19,20].
Acute liver failure (ALF)
Definition
Acute liver failure was first defined in 1970 to describe a rare and potentially reversible critical illness caused by “severe liver injury in the absence of prior liver disease with hepatic encephalopathy occurring within 8 weeks from the appearance of first symptoms” [21]. In general, the duration of the symptoms should not be longer than 26 weeks to be considered ALF [22].
Since the first definition of ALF, several authors have classified ALF according to the interval between the appearance of jaundice and the onset of hepatic encephalopathy (HE) (Table 1), e.g., defining “fulminant” for appearance of hepatic encephalopathy in the first 2 weeks and “subfulminant” when occurring until week 12 [23]. O´Grady employed the terms “hyperacute,” “acute” and “subacute” for an onset of hepatic encephalopathy within 1, 4 or between 5 and 12 weeks, respectively [2]. The Japanese consensus classifies ALF into “acute liver failure without hepatic coma” (HE < Grade II) and “acute liver failure with hepatic coma” (HE ≥ Grade II). The latter distinguishes between the “acute type” and “subacute type,” with hepatic encephalopathy developing within 10 days or between 11 and 56 days, respectively [24]. If hepatic encephalopathy occurs later, the disease is known as late-onset hepatic failure (LOHF) [4]. All these classifications can even be further complicated if the etiology of ALF [22, 25] is taken into consideration, which can lead to lack of comparability of clinical trials [26].
Incidence and mortality
Estimates based on data from transplant units from the European Union suggest that 8% of liver transplants (LTx) are due to ALF, either caused by viral infection (19%), DILI (18%), toxic insults (4%), traumatic events (3%) or unknown causes (56%) [27]. However, a trend toward an increase in DILI and a decrease in viral etiologies has been documented [1, 28, 29]. Overall, ALF occurs in less than 10 cases per million inhabitants per year [3]. In-hospital mortality remains high with rates between 23 and 53% [9].
Prognostic clinical features
Wlodzimirow et al. identified and characterized different prognostic models for mortality in ALF patients [30]. The variables more commonly included in the final model of the studies investigated were hepatic encephalopathy (45%), prothrombin time (45%), bilirubin (40%), age (40%) and creatinine (25%) [30]. A recent study revealed that M30, a cleavage product of cytokeratin-18 caspase, most accurately identified patients who would require LTx or die [31].
-
Hepatic encephalopathy HE remains the essential clinical hallmark, and its presence, even at low grade, is indicative of poor prognosis [1]. HE is primarily a clinical diagnosis. The joint EASL/AASLD guidelines suggest that if ammonia levels are normal, the diagnosis of HE is in question [32]. EEG provides information on the severity of HE in both cooperative and especially in uncooperative patients but is nonspecific [33]. Arterial ammonia concentration in whole blood on admission to the ICU is an independent risk factor for both encephalopathy and intracranial hypertension [34].
-
Creatinine The presence of extrahepatic organ failure, especially acute kidney injury, has been shown to increase mortality rates [3, 35, 36]. The occurrence of concomitant kidney failure (i.e., multiple organ failure) urges admission to the ICU [1].
-
Severity of liver injury (reflected in prothrombin time or bilirubin) Changes in coagulation factors, such as INR, reflecting injury to the hepatocellular synthesis machinery, are of prognostic value. Similarly, serum bilirubin, reflecting injury to the excretory machinery of the hepatocyte, serves as a prognostic marker in ALF of non-paracetamol etiology, but has no value in paracetamol-induced ALF or even other causes of hyperacute liver failure due to the time required for bilirubin levels to build up [37].
-
Other factors These may include age (e.g., < 10 or > 40 years) and lactate levels (e.g., > 4 mmol/l), as indicated in the King’s College Criteria or the recent guidelines in the UK for the assessment of the need for LTx [2, 38]. Lactate, together with bilirubin and etiology, is part of the BiLE score, which has recently shown a good predictive value in a cohort of 102 ALF patients [37]. Other scoring systems are available for rare diseases, for example, the TIPS-BSC prognostic index [39].
Acute-on-chronic liver failure (ACLF)
Definition
ACLF is a syndrome affecting multiple organs, including new worsening of liver function, defined by an acute hepatic decompensation in patients with preexisting chronic liver disease [5]. Even if the definition provided by the European Association for the Study of the Liver (EASL) and the International Chronic Liver Failure (CLIF) Consortium is the most employed [6], there is no single agreed definition for ACLF. Since the first report in 1995 [40], different attempts have been made in Europe [6, 41], Asia [7, 42, 43] and North America [8]. A consensus definition has been proposed, but has not yet been clinically validated [5]. Table 1 summarizes the current definitions of ACLF. A recent paper even highlighted three different stages in acutely decompensated cirrhosis, i.e., stable decompensated cirrhosis, unstable decompensated cirrhosis and ACLF [44]. In this study, ACLF was differentiated from decompensated cirrhosis by the presence of organ failure and systemic inflammation.
According to an investigation of 1343 patients with decompensated cirrhosis (CANONIC study), the EASL included the presence of at least renal dysfunction (creatinine > 1.5 mg/dl) or hepatic encephalopathy (grade I or II), together with a high risk of death due to multiple organ failure, as criteria to define each of the subgroups of ACLF [6]. These organ failures were first defined according to the SOFA score (Table 2). Depending on the number of failing organs, different subgroups were established (Table 1).
Similar to the EASL description, the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) includes extrahepatic organ failure in its definition [8] (summarized in Table 2). This stratification was validated in a study with more than 500 cirrhotic patients [45].
In contrast, the Asian Pacific Association for the Study of the Liver (APASL) does not include extrahepatic organ failure in its definition, arguing with potential delay in identification of the potential therapeutic window for reversal [7]. In any case, there is no consistent definition of organ failure, as illustrated in Table 2. Moreover, neither patients with an extrahepatic insult as a precipitating factor for ACLF nor patients with prior decompensation are considered in the definition [46].
Incidence and mortality
Data reported from hospital registries indicate that 24–40% of patients with cirrhosis admitted to the hospital develop ACLF [19]. This accounts for approximately 1–5% of patients admitted to the ICU [9]. Up to 44% and 53% of patients with ACLF died by day 28 and day 90, respectively. In the first 4 weeks, mild ACLF leads to death in 13–22% of patients, whereas moderate and severe failure results in mortality rates of 32–44% and 77–86%, respectively [6, 7, 43, 47]. A recent meta-analysis using the EASL definition to assess the global burden of ACLF demonstrated that 35% of patients with decompensated cirrhosis already presented ACLF at hospital admission, showing a 60% mortality at 90 days [48, 49]. Proven bacterial infection and severe alcoholic hepatitis accounted for almost all (approximately 97%) of acute decompensation and ACLF [50].
Prognostic clinical features
EASL and APASL use different criteria to predict higher mortality rates. The European Society first used the CLIF-SOFA score, a modified SOFA score for patients with cirrhosis, to define organ “failure” (Table 2). Parameters such as bilirubin, creatinine, HE, INR, PaO2/FiO2 or SPO2/FiO2 ratios, mean arterial pressure (MAP) and the need for vasopressors were employed to assess damage to liver and extrahepatic organ systems [6]. In the CANONIC study, four groups of patients were defined: the occurrence of ≥ 2 organ failures, the presence of kidney failure alone and the combination of a single non-renal organ failure with kidney dysfunction and/or mild-to-moderate HE [6]. In addition, the impact of the inflammatory response (as reflected in an increased white blood cell (WBC) count) and its early recognition and aggressive management were also recognized to influence the outcome [18]. Afterward, using the same parameters, the score was adapted into the more specific CLIF-C OF score, which was then combined with age and WBC count into the CLIF-C ACLF score, which improved prediction of mortality [51].
The Asian Pacific societies developed their own score based on bilirubin, HE grade, INR, lactate and creatinine (Table 2). This APASL-ACLF Research Consortium (AARC) score was only thought to stratify the degree of liver failure into mild, severe and very severe [47]. The definition of kidney failure follows the acute kidney injury network (AKIN) criteria [52], while no specific reference exists for circulatory or respiratory failures [7]. Prognostic factors such as bilirubin, HE or creatinine are considered, even if different cutoff values are used (Table 2). In the last update, the APASL also included obesity as a risk factor [7].
The approach of NACSELD is even simpler; only the number of organ failures being ≥ 2 is considered the main prognostic factor [8]. In fact, this seems to predict increased mortality better than age, WBC count, serum albumin, MELD score and/or presence of infection [45, 53]. The definition of “failure” does not follow any specific score and is based on the presence of severe HE, low MAP and the need for hemodialysis or mechanical ventilation (Table 2). Hepatic encephalopathy grade and the presence of infection have also been shown to be associated with higher mortality rates [54].
Secondary acquired liver injury
Definition
Acquired liver injury without underlying liver disease represents the most common form of hepatic dysfunction in the ICU [9, 10]. It can occur after a hypoxic (e.g., shock), toxic (i.e., hepatotoxic drugs) or inflammatory insult (e.g., sepsis) [55]. There is no common definition for this syndrome, and its diagnosis is mainly based on the elevation of liver parameters, such as transaminases or bilirubin [56].
Cholestasis is characterized by altered bile excretion (i.e., extrahepatic cholestasis due to mechanical bile duct obstruction) or impaired conjugation and/or secretion (i.e., intrahepatic cholestasis owing to altered hepatocellular signaling and transport) [9, 55, 57]. This results in an accumulation of bile acids and conjugated bilirubin, together with increased enzymes that indicate cholestasis, such as alkaline phosphatase (AP) or γ-glutamyl transpeptidase (GGT). A consensus on how to define cholestasis in critically ill patients using these routine laboratory parameters does not exist. As defined by the SOFA score [12], a bilirubin threshold > 2 mg/dl is included in the guidelines of the Surviving Sepsis Campaign [58] and has been adopted by many authors [9, 14, 15, 56, 59, 60]. However, elevated bilirubin levels are not specific to cholestasis and might also reflect hemolysis. Lyu et al. [61] showed that 73% of adult cardiac patients supported by veno-arterial ECMO had hyperbilirubinemia (> 3 mg/dl), which may be due to hemolysis in up to 42% of the cases. The combination of AP and GGT with or without bilirubin has been suggested to diagnose cholestasis more specifically [62, 63].
Hypoxic liver injury is generally defined based on a clinical setting of circulatory, cardiac or respiratory failure, a substantial increase in transaminases, ranging from > 5 to > 20 times the upper limit of normal (ULN), and the absence of other causes of liver damage [9, 64,65,66,67,68,69,70]. It was first reported in 1901 as “central necrosis” [71] and is known as “ischemic hepatitis,” “shock liver” or “hypoxic hepatitis” [67]. The cause is an imbalance of oxygen supply and demand in the liver that results in cell death [64]. More specifically, insufficient hepatic perfusion, including Budd–Chiari perfusion, hypoxemia, poor global oxygen delivery, inadequate oxygen extraction by hepatocytes or an increased metabolic demand, can cause hypoxic liver injury [72,73,74,75,76]. Three subgroups of causes may be distinguished: respiratory failure, cardiac failure and shock/hypotension [1, 67].
Incidence and mortality
Due to the absence of a consensus definition, heterogeneous epidemiological data for secondary liver injury exist. Cholestasis is present in 11–36% of ICU patients [9], while hypoxic liver injury occurs in 1–18% of cases [69, 70, 77]. For the latter, the incidence increases over 20% in patients with shock [75]. The overall mortality for secondary acquired liver injury is high, ranging between 27 and 48% for ICU patients with cholestasis [56, 76] and between 40 and 60% for hypoxic liver injury [9, 69, 70, 72, 74, 75]. Moreover, in a single-center cohort with 1116 critically ill patients, mortality rates were significantly correlated with the magnitude of transaminases (33.2, 44.4 and 55.4% for peak AST 5–10 × ULN, 10–20 × ULN and > 20 × ULN, respectively) [70].
Prognostic clinical features
Serum bilirubin is a stable and prevailing marker of liver impairment in the ICU. Its concentration is influenced by bilirubin synthesis, transport, uptake, conjugation and excretion. Ischemic and sepsis-associated cholestasis, drug-induced liver injury and parenteral nutrition are predominant causes of hyperbilirubinemia in the ICU [55]. Bilirubin is a marker of liver dysfunction and a powerful prognostic factor. It has been shown to be linked to infections in surgical patients [78, 79], to an increased mortality among trauma patients [80, 81], sepsis [82] or hematological malignancies [83], to a poor prognosis among ARDS patients [84], or simply to a worse outcome in the ICU [56, 85] (summarized in Table 3).
In the case of cholestasis, total bile acids are also an independent prognostic factor of disease severity [57, 86]. An elevation of 5.2 µmol/l of total bile acids from baseline data demonstrated discriminating value, while mortality was specifically augmented with increases > 10 µmol/l [86]. Horvatits et al. suggested that bile acids could be a better prognostic factor than bilirubin in the ICU. Nevertheless, it remains unclear whether the elevation of bile acids in critically ill patients is a distinct pathophysiological entity or a compensatory mechanism [86].
Regarding hypoxic liver injury, the indocyanine green plasma disappearance rate is an effective tool for assessing liver function. In an observational study with 97 patients, a cutoff value of 9% 48 h after ICU admission demonstrated a significant prognostic accuracy for 28-day mortality [87]. In contrast, bilirubin, due to the later peak time, might not be a reliable marker and is elevated in only one-third of patients with hypoxic liver injury [76]. The indocyanine green plasma disappearance rate outperforms bilirubin as a more sensitive combined indicator of perfusion and excretory liver function in the ICU [88].
Finally, underlying and concomitant syndromes will impact the outcome, such as sepsis or need for organ support [89]. Moreover, the presence of coagulopathy or hepatic encephalopathy has been shown to predict mortality in critically ill patients with hypoxic liver injury [74, 90]. As expected, the severity of organ failure results in higher mortality rates [9, 74].
Liver injury as a core component of multiple organ failure
The role of the liver in multiple organ failure has been widely studied, especially in sepsis [15]. Due to the extensive number of immunologic, regulatory and metabolic functions, the liver can take the role of the “perpetrator,” triggering an inflammatory response, or that of the “victim” of the host response [13,14,15].
ALF is rare but frequently results in damage to other organ systems. These include the cardiovascular and respiratory systems, the central nervous system, the kidney, coagulation and the immune system [20]. This also applies to secondary acquired liver injury, since a hypoxic, toxic (e.g., due to commonly used drugs in the ICU [91]) or inflammatory extrahepatic insult triggers hepatic dysfunction.
Each of the organs affected may act either as a precipitating factor or as a target, both paving the way to the worsening of multiple organ failure. This is especially true for the inflammatory response accompanying infection as a prototypical trigger [13, 15, 92].
During ACLF, the release of both damage-associated molecular patterns (DAMPs) linked to inflammation and pathogen-associated molecular patterns (PAMPs) is common [46]. The intensity of inflammation directly correlates with the number of failing organs and outcome [93].
The progressive impairment of circulation is considered as a risk factor for the development of multiple organ failure [94, 95]. The “peripheral arterial vasodilation hypothesis” suggests that during liver failure, portal hypertension and splanchnic vasodilatation emerge [96]. As a result, a reduction in systemic vascular resistance and central hypovolemia arises. Moreover, activation of hormone systems, such as the renin–angiotensin–aldosterone system (RAAS), sympathetic nervous system (SNS) and antidiuretic hormone (ADH), occurs.
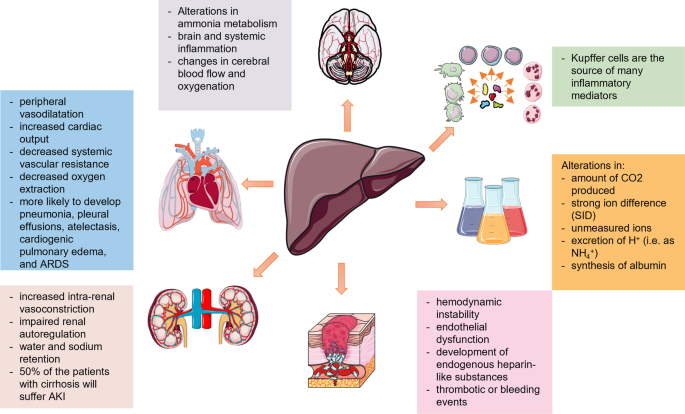
Critically ill patients with liver disease are also more likely to develop pulmonary complications, e.g., acute respiratory distress syndrome (ARDS) [97]. The main mechanisms initiating dysfunction or failure of different organs are further described in [15, 96, 98]. A consensus paper from a North American and European expert panel gives a thorough description of possible organ failures and recommendations for their management [99] (Fig. 1).
The silent period of compensated cirrhosis may turn into a decompensated period (with, e.g., ascites, bleeding from esophageal varices, HE) and is further complicated by cardiopulmonary and renal failure [96]. As described above, the main mechanism triggering renal failure is an alteration in the arterial circulation and volume, which is combined with increased intrarenal vasoconstriction and impaired renal autoregulation. Factors affecting the circulatory status, such as bacterial infections and gastrointestinal bleeding, can reduce renal perfusion and precipitate HRS-AKI [100]. In the case of cardiorespiratory failure, the occurring circulatory changes (increased cardiac output, peripheral vasodilatation, decreased systemic vascular resistance and decreased oxygen extraction) are associated with hypovolemia and impaired tissue perfusion, together with water and sodium retention [100].
The majority of the macrophage population of the body is represented by Kupffer cells in the liver [14]. They act as the first defensive barrier against gut-derived bacteria [101]. The activation of Kupffer cells triggers the release of proinflammatory mediators [102], including TNF-α, IL-1, IL-6 and IL-12. TNF-α and IL-1 can act synergistically to activate the cytokine network, the coagulation cascade, fibrinolysis and neutrophils [103]. IL-6 triggers the synthesis of acute-phase proteins, the production of immunoglobulins, the proliferation and differentiation of T cells, enhanced activity of natural killer cells and the maturation of megakaryocytes [103]. Finally, IL-12 induces the production of interferon-γ in T cells and natural killer cells [14]. These processes can trigger cholestasis [14]. The maximal reduction in bile flow has been reported to occur within the first 24 h after cytokine challenge and can be accompanied by a posttranscriptional mechanism affecting the expression of the hepatobiliary transporters BSEP and MRP2 [104, 105]. In this regard, the development of secondary liver injury, i.e., cholestasis or hypoxic liver injury, is common during sepsis, microcirculatory impairment or drug exposure [106].
Other systems, such as the hemostatic balance in patients with liver injury, might be altered by hemodynamic instability, endothelial dysfunction, the development of endogenous heparin-like substances, leading to either thrombotic or bleeding events [99, 107, 108].
Neurological dysfunction is mainly caused by alterations in ammonia metabolism, brain and systemic inflammation and changes in cerebral blood flow and oxygenation, due to hepatic encephalopathy and/or concomitant infections and electrolyte abnormalities [90, 109]. This might include cerebral edema and intracranial hypertension [97].
Finally, the liver is also responsible for substrate oxidation, metabolism of organic acids (e.g., lactate, ketones, amino acids), metabolism of ammonium and production of plasma proteins [110]. Any alteration of these functions may change the acid–base balance by modifying the amount of CO2 produced, the strong ion difference (SID), the concentration of unmeasured ions, the excretion of H+ (i.e., as NH4+) or the synthesis of albumin [111,112,113,114,115]. A retrospective study of 23,795 patients showed that liver “dysfunction” (i.e., SOFA score for liver 1 or 2) was already present on admission in at least 20% of the cases. In the same study, 80% of the non-survivors had an increase in at least one individual organ SOFA score in the four days prior to death [17]. Among patients with organ failure, the highest risk was associated with liver failure (OR 2.587; CI 2.098–3.189).
The association of liver dysfunction and mortality has been suggested by many authors [16, 56, 76, 79,80,81,82,83,84,85, 116,117,118,119]. Regarding patterns of organ involvement in sepsis, Seymour et al. defined four phenotypes, among which a “hepatic” phenotype with a 28-day mortality rate of 40% among adult patients with sepsis was prognostically the worst [120].
Conclusions
The definition of liver injury, dysfunction and/or failure is far from a global consensus. Similarly, the cutoff values of prognostic parameters vary. We provided current definitions, epidemiological data and prognostic factors for acute, acute-on-chronic liver failure and secondary liver injury with a focus on the intensive care unit. The reviewed data show an association of liver impairment with extrahepatic organ failure and present liver dysfunction as an underappreciated component for the development of multiple organ failure.
Availability of data and materials
Not applicable.
References
Wendon J, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–81. https://doi.org/10.1016/j.jhep.2016.12.003.
O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–5. https://doi.org/10.1016/0140-6736(93)91818-7.
Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–34. https://doi.org/10.1056/NEJMra1208937.
Mochida S, Nakayama N, Matsui A, Nagoshi S, Fujiwara K. Re-evaluation of the Guideline published by the Acute Liver Failure Study Group of Japan in 1996 to determine the indications of liver transplantation in patients with fulminant hepatitis. Hepatol Res. 2008;38:970–9. https://doi.org/10.1111/j.1872-034X.2008.00368.x.
Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin H-C, et al. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4–10. https://doi.org/10.1053/j.gastro.2014.05.005.
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(1426–37):1437.e1-9. https://doi.org/10.1053/j.gastro.2013.02.042.
Leao GS, Lunardi FL, Picon RV, Tovo CV, de Mattos AA, de Mattos AZ. Acute-on-chronic liver failure: a comparison of three different diagnostic criteria. Ann Hepatol. 2019;18:373–8. https://doi.org/10.1016/j.aohep.2019.01.001.
Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–6. https://doi.org/10.1002/hep.27077.
Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70:2204–15. https://doi.org/10.1002/hep.30824.
Jenniskens M, Langouche L, Vanwijngaerden Y-M, Mesotten D, van den Berghe G. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 2016;42:16–27. https://doi.org/10.1007/s00134-015-4054-0.
Koch DG, Speiser JL, Durkalski V, Fontana RJ, Davern T, McGuire B, et al. The natural history of severe acute liver injury. Am J Gastroenterol. 2017;112:1389–96. https://doi.org/10.1038/ajg.2017.98.
Vincent JL, Moreno R, Takala J, Willatts S, de Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Caraballo C, Jaimes F. Organ dysfunction in sepsis: an ominous trajectory from infection to death. Yale J Biol Med. 2019;92:629–40.
Fuchs M, Sanyal AJ. Sepsis and cholestasis. Clin Liver Dis. 2008;12(151–72):ix. https://doi.org/10.1016/j.cld.2007.11.002.
Strnad P, Tacke F, Koch A, Trautwein C. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. https://doi.org/10.1038/nrgastro.2016.168.
Sakr Y, Lobo SM, Moreno RP, Gerlach H, Ranieri VM, Michalopoulos A, Vincent J-L. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care. 2012;16:R222. https://doi.org/10.1186/cc11868.
Bingold TM, Lefering R, Zacharowski K, Meybohm P, Waydhas C, Rosenberger P, Scheller B. Individual organ failure and concomitant risk of mortality differs according to the type of admission to ICU—a retrospective study of SOFA score of 23,795 patients. PLoS ONE. 2015;10:e0134329. https://doi.org/10.1371/journal.pone.0134329.
Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. 2015;386:1576–87. https://doi.org/10.1016/S0140-6736(15)00309-8.
Hernaez R, Sola E, Moreau R, Gines P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–53. https://doi.org/10.1136/gutjnl-2016-312670.
Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869–81. https://doi.org/10.1016/S0140-6736(19)31894-X.
Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–98.
Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965–7. https://doi.org/10.1002/hep.25551.
Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97–106. https://doi.org/10.1055/s-2008-1040593.
Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol. 2012;47:849–61. https://doi.org/10.1007/s00535-012-0624-x.
Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SHB, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. https://doi.org/10.7326/0003-4819-137-12-200212170-00007.
Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RAFM. Systematic review: acute liver failure—one disease, more than 40 definitions. England; 2012.
Germani G, Theocharidou E, Adam R, Karam V, Wendon J, O’Grady J, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012;57:288–96. https://doi.org/10.1016/j.jhep.2012.03.017.
Hey P, Hanrahan TP, Sinclair M, Testro AG, Angus PW, Peterson A, et al. Epidemiology and outcomes of acute liver failure in Australia. World J Hepatol. 2019;11:586–95. https://doi.org/10.4254/wjh.v11.i7.586.
Rajaram P, Subramanian R. Acute liver failure. Semin Respir Crit Care Med. 2018;39:513–22. https://doi.org/10.1055/s-0038-1673372.
Wlodzimirow KA, Eslami S, Chamuleau RAFM, Nieuwoudt M, Abu-Hanna A. Prediction of poor outcome in patients with acute liver failure-systematic review of prediction models. United States; 2012.
Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W, Lee WM, Chung RT. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology. 2012;143:1237–43. https://doi.org/10.1053/j.gastro.2012.07.113.
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35. https://doi.org/10.1002/hep.27210.
Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–47. https://doi.org/10.1016/j.jhep.2014.09.005.
Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–52. https://doi.org/10.1002/hep.21838.
Bagshaw SM, Uchino S, Kellum JA, Morimatsu H, Morgera S, Schetz M, et al. Association between renal replacement therapy in critically ill patients with severe acute kidney injury and mortality. J Crit Care. 2013;28:1011–8. https://doi.org/10.1016/j.jcrc.2013.08.002.
Hadem J, Kielstein JT, Manns MP, Kumpers P, Lukasz A. Outcomes of renal dysfunction in patients with acute liver failure. United Eur Gastroenterol J. 2019;7:388–96. https://doi.org/10.1177/2050640618817061.
Hadem J, Stiefel P, Bahr MJ, Tillmann HL, Rifai K, Klempnauer J, et al. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin Gastroenterol Hepatol. 2008;6:339–45. https://doi.org/10.1016/j.cgh.2007.12.039.
Tavabie OD, Bernal W. How to manage: acute liver failure. Frontline Gastroenterol. 2020;11:70–4. https://doi.org/10.1136/flgastro-2018-101105.
Hernández-Gea V, de Gottardi A, Leebeek FWG, Rautou P-E, Salem R, Garcia-Pagan JC. Current knowledge in pathophysiology and management of Budd-Chiari syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J Hepatol. 2019;71:175–99. https://doi.org/10.1016/j.jhep.2019.02.015.
Ohnishi H, Sugihara J, Moriwaki H, Muto Y. Acute-on-chronic liver failure. Ryoikibetsu Shokogun Shirizu. 1995:217–9.
Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–61. https://doi.org/10.1159/000047017.
Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269–82. https://doi.org/10.1007/s12072-008-9106-x.
Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–71. https://doi.org/10.1007/s12072-014-9580-2.
Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–54. https://doi.org/10.1016/j.jhep.2020.06.013.
O’Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 2018;67:2367–74. https://doi.org/10.1002/hep.29773.
Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–45. https://doi.org/10.1056/NEJMra1914900.
Choudhury A, Jindal A, Maiwall R, Sharma MK, Sharma BC, Pamecha V, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int. 2017;11:461–71. https://doi.org/10.1007/s12072-017-9816-z.
Schulz M, Trebicka J. Acute-on-chronic liver failure: a global disease. Gut. 2021. https://doi.org/10.1136/gutjnl-2020-323973.
Mezzano G, Juanola A, Cardenas A, Mezey E, Hamilton JP, Pose E, et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut. 2021. https://doi.org/10.1136/gutjnl-2020-322161.
Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097–108. https://doi.org/10.1016/j.jhep.2020.11.019.
Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Gines P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–47. https://doi.org/10.1016/j.jhep.2014.06.012.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. https://doi.org/10.1186/cc5713.
Rosenblatt R, Shen N, Tafesh Z, Cohen-Mekelburg S, Crawford CV, Kumar S, et al. The North American consortium for the study of end-stage liver disease-acute-on-chronic liver failure score accurately predicts survival: an external validation using a national cohort. Liver Transpl. 2020;26:187–95. https://doi.org/10.1002/lt.25696.
Bajaj JS, O’Leary JG, Tandon P, Wong F, Garcia-Tsao G, Kamath PS, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol. 2017;15:565-574.e4. https://doi.org/10.1016/j.cgh.2016.09.157.
Lescot T, Karvellas C, Beaussier M, Magder S. Acquired liver injury in the intensive care unit. Anesthesiology. 2012;117:898–904. https://doi.org/10.1097/ALN.0b013e318266c6df.
Kramer L, Jordan B, Druml W, Bauer P, Metnitz PGH. Incidence and prognosis of early hepatic dysfunction in critically ill patients—a prospective multicenter study. Crit Care Med. 2007;35:1099–104. https://doi.org/10.1097/01.CCM.0000259462.97164.A0.
Recknagel P, Gonnert FA, Westermann M, Lambeck S, Lupp A, Rudiger A, et al. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: experimental studies in rodent models of peritonitis. PLoS Med. 2012;9:e1001338. https://doi.org/10.1371/journal.pmed.1001338.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. https://doi.org/10.1001/jama.2016.0287.
Chen H-L, Wu S-H, Hsu S-H, Liou B-Y, Chen H-L, Chang M-H. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018;25:75. https://doi.org/10.1186/s12929-018-0475-8.
Woznica EA, Inglot M, Woznica RK, Lysenko L. Liver dysfunction in sepsis. Adv Clin Exp Med. 2018;27:547–51. https://doi.org/10.17219/acem/68363.
Lyu L, Yao J, Gao G, Long C, Hei F, Ji B, et al. Incidence, risk factors, and outcomes of hyperbilirubinemia in adult cardiac patients supported by veno-arterial ECMO. Artif Organs. 2018;42:148–54. https://doi.org/10.1111/aor.12979.
de Tymowski C, Dépret F, Soussi S, Nabila M, Vauchel T, Chaussard M, et al. Contributing factors and outcomes of burn-associated cholestasis. J Hepatol. 2019;71:563–72. https://doi.org/10.1016/j.jhep.2019.05.009.
EASL Clinical Practice Guidelines. Management of cholestatic liver diseases. J Hepatol. 2009;51:237–67. https://doi.org/10.1016/j.jhep.2009.04.009.
Ebert EC. Hypoxic liver injury. Mayo Clin Proc. 2006;81:1232–6. https://doi.org/10.4065/81.9.1232.
Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg. 2000;63:336–47.
Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care. 2013;19:128–32. https://doi.org/10.1097/MCC.0b013e32835ec9e6.
Henrion J. Hypoxic hepatitis. Liver Int. 2012;32:1039–52. https://doi.org/10.1111/j.1478-3231.2011.02655.x.
Waseem N, Chen P-H. Hypoxic hepatitis: a review and clinical update. J Clin Transl Hepatol. 2016;4:263–8. https://doi.org/10.14218/JCTH.2016.00022.
Jonsdottir S, Arnardottir MB, Andresson JA, Bjornsson HK, Lund SH, Bjornsson ES. Prevalence, clinical characteristics and outcomes of hypoxic hepatitis in critically ill patients. Scand J Gastroenterol. 2022;57:311–8. https://doi.org/10.1080/00365521.2021.2005136.
van den Broecke A, van Coile L, Decruyenaere A, Colpaert K, Benoit D, van Vlierberghe H, Decruyenaere J. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann Intensive Care. 2018;8:15. https://doi.org/10.1186/s13613-018-0356-z.
Mallory FB. Necroses of the liver. J Med Res. 1901;6:264–80.
Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–70.
Drolz A, Horvatits T, Roedl K, Fuhrmann V. Shock liver and cholestatic liver in critically ill patients. [Schockleber und Cholestase beim kritisch Kranken]. Med Klin Intensivmed Notfmed. 2014;109:228–34. https://doi.org/10.1007/s00063-013-0320-5.
Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, et al. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009;35:1397–405. https://doi.org/10.1007/s00134-009-1508-2.
Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore). 2003;82:392–406. https://doi.org/10.1097/01.md.0000101573.54295.bd.
Jager B, Drolz A, Michl B, Schellongowski P, Bojic A, Nikfardjam M, et al. Jaundice increases the rate of complications and one-year mortality in patients with hypoxic hepatitis. Hepatology. 2012;56:2297–304. https://doi.org/10.1002/hep.25896.
Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011;37:1302–10. https://doi.org/10.1007/s00134-011-2248-7.
Field E, Horst HM, Rubinfeld IS, Copeland CF, Waheed U, Jordan J, et al. Hyperbilirubinemia: a risk factor for infection in the surgical intensive care unit. Am J Surg. 2008;195:304–6. https://doi.org/10.1016/j.amjsurg.2007.12.010 (discussion 306–7).
Diab M, Sponholz C, von Loeffelholz C, Scheffel P, Bauer M, Kortgen A, et al. Impact of perioperative liver dysfunction on in-hospital mortality and long-term survival in infective endocarditis patients. Infection. 2017;45:857–66. https://doi.org/10.1007/s15010-017-1064-6.
Harbrecht BG, Zenati MS, Doyle HR, McMichael J, Townsend RN, Clancy KD, Peitzman AB. Hepatic dysfunction increases length of stay and risk of death after injury. J Trauma. 2002;53:517–23. https://doi.org/10.1097/00005373-200209000-00020.
Saloojee A, Skinner DL, Loots E, Hardcastle TC, Muckart DJJ. Hepatic dysfunction: a common occurrence in severely injured patients. Injury. 2017;48:127–32. https://doi.org/10.1016/j.injury.2016.08.017.
Juschten J, Bos LDJ, de Grooth H-J, Beuers U, Girbes ARJ, Juffermans NP, et al. Incidence, clinical characteristics and outcomes of early hyperbilirubinemia in critically ill patients: insights from the MARS study. Shock. 2022;57:161–7. https://doi.org/10.1097/SHK.0000000000001836.
Bisbal M, Darmon M, Saillard C, Mallet V, Mouliade C, Lemiale V, et al. Hepatic dysfunction impairs prognosis in critically ill patients with hematological malignancies: A post-hoc analysis of a prospective multicenter multinational dataset. J Crit Care. 2021;62:88–93. https://doi.org/10.1016/j.jcrc.2020.11.023.
Dizier S, Forel J-M, Ayzac L, Richard J-C, Hraiech S, Lehingue S, et al. Early hepatic dysfunction is associated with a worse outcome in patients presenting with acute respiratory distress syndrome: a post-hoc analysis of the ACURASYS and PROSEVA studies. PLoS ONE. 2015;10:e0144278. https://doi.org/10.1371/journal.pone.0144278.
Brienza N, Dalfino L, Cinnella G, Diele C, Bruno F, Fiore T. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med. 2006;32:267–74. https://doi.org/10.1007/s00134-005-0023-3.
Horvatits T, Drolz A, Rutter K, Roedl K, Langouche L, van den Berghe G, et al. Circulating bile acids predict outcome in critically ill patients. Ann Intensive Care. 2017;7:48. https://doi.org/10.1186/s13613-017-0272-7.
Horvatits T, Kneidinger N, Drolz A, Roedl K, Rutter K, Kluge S, et al. Prognostic impact of ICG-PDR in patients with hypoxic hepatitis. Ann Intensive Care. 2015;5:47. https://doi.org/10.1186/s13613-015-0092-6.
Kortgen A, Paxian M, Werth M, Recknagel P, Rauchfuss F, Lupp A, et al. Prospective assessment of hepatic function and mechanisms of dysfunction in the critically ill. Shock. 2009;32:358–65. https://doi.org/10.1097/SHK.0b013e31819d8204.
Drolz A, Horvatits T, Roedl K, Rutter K, Staufer K, Haider DG, et al. Outcome and features of acute kidney injury complicating hypoxic hepatitis at the medical intensive care unit. Ann Intensive Care. 2016;6:61. https://doi.org/10.1186/s13613-016-0162-4.
Drolz A, Jager B, Wewalka M, Saxa R, Horvatits T, Roedl K, et al. Clinical impact of arterial ammonia levels in ICU patients with different liver diseases. Intensive Care Med. 2013;39:1227–37. https://doi.org/10.1007/s00134-013-2926-8.
Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5:58. https://doi.org/10.1038/s41572-019-0105-0.
Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33:498–510. https://doi.org/10.3109/08830185.2014.889129.
Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–64. https://doi.org/10.1002/hep.28740.
Gines P, Fernandez J, Durand F, Saliba F. Management of critically-ill cirrhotic patients. J Hepatol. 2012;56(Suppl 1):S13-24. https://doi.org/10.1016/S0168-8278(12)60003-8.
Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. https://doi.org/10.1055/s-2008-1040319.
Moller S, Bendtsen F. Cirrhotic multiorgan syndrome. Dig Dis Sci. 2015;60:3209–25. https://doi.org/10.1007/s10620-015-3752-3.
Damm TW, Kramer DJ. The liver in critical illness. Crit Care Clin. 2016;32:425–38. https://doi.org/10.1016/j.ccc.2016.02.002.
Olson JC, Karvellas CJ. Critical care management of the patient with cirrhosis awaiting liver transplant in the intensive care unit. Liver Transpl. 2017;23:1465–76. https://doi.org/10.1002/lt.24815.
Nadim MK, Durand F, Kellum JA, Levitsky J, O’Leary JG, Karvellas CJ, et al. Management of the critically ill patient with cirrhosis: a multidisciplinary perspective. J Hepatol. 2016;64:717–35. https://doi.org/10.1016/j.jhep.2015.10.019.
Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–90. https://doi.org/10.1056/NEJMra0809139.
Katz S, Jimenez MA, Lehmkuhler WE, Grosfeld JL. Liver bacterial clearance following hepatic artery ligation and portacaval shunt. J Surg Res. 1991;51:267–70. https://doi.org/10.1016/0022-4804(91)90105-u.
O’Reilly M, Newcomb DE, Remick D. Endotoxin, sepsis, and the primrose path. Shock. 1999;12:411–20. https://doi.org/10.1097/00024382-199912000-00001.
van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin N Am. 1999;13(413–26):ix. https://doi.org/10.1016/s0891-5520(05)70083-0.
Elferink MGL, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJH, et al. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1008–16. https://doi.org/10.1152/ajpgi.00071.2004.
Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163–72. https://doi.org/10.1016/s0016-5085(00)70425-2.
Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med. 2001;29:S42–7. https://doi.org/10.1097/00003246-200107001-00016.
Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–46. https://doi.org/10.1002/hep.21303.
Tripodi A, Primignani M, Chantarangkul V, Dell’Era A, Clerici M, de Franchis R, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137:2105–11. https://doi.org/10.1053/j.gastro.2009.08.045.
Cordoba J, Ventura-Cots M, Simon-Talero M, Amoros A, Pavesi M, Vilstrup H, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol. 2014;60:275–81. https://doi.org/10.1016/j.jhep.2013.10.004.
Hasan A. Buffer Systems. In: Hasan A, editor. Handbook of blood gas/acid–base interpretation. London: Springer; 2009. p. 143–64. https://doi.org/10.1007/978-1-84800-334-7_5.
Adeva MM, Souto G, Blanco N, Donapetry C. Ammonium metabolism in humans. Metabolism. 2012;61:1495–511. https://doi.org/10.1016/j.metabol.2012.07.007.
Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014;37:541–51. https://doi.org/10.1007/s10545-014-9704-9.
Guder WG, Häussinger D, Gerok W. Renal and hepatic nitrogen metabolism in systemic acid base regulation. J Clin Chem Clin Biochem. 1987;25:457–66. https://doi.org/10.1515/cclm.1987.25.8.457.
Häussinger D, Steeb R, Gerok W. Ammonium and bicarbonate homeostasis in chronic liver disease. Klin Wochenschr. 1990;68:175–82. https://doi.org/10.1007/bf01649081.
Scheiner B, Lindner G, Reiberger T, Schneeweiss B, Trauner M, Zauner C, Funk G-C. Acid-base disorders in liver disease. J Hepatol. 2017;67:1062–73. https://doi.org/10.1016/j.jhep.2017.06.023.
Han HS, Park C-M, Lee D-S, Sinn DH, Gil E. Evaluating mortality and recovery of extreme hyperbilirubinemia in critically ill patients by phasing the peak bilirubin level: a retrospective cohort study. PLoS ONE. 2021;16:e0255230. https://doi.org/10.1371/journal.pone.0255230.
Pierrakos C, Velissaris D, Felleiter P, Antonelli M, Vanhems P, Sakr Y, Vincent J-L. Increased mortality in critically ill patients with mild or moderate hyperbilirubinemia. J Crit Care. 2017;40:31–5. https://doi.org/10.1016/j.jcrc.2017.01.017.
Gupta T, Puskarich MA, Devos E, Javed A, Smotherman C, Sterling SA, et al. Sequential organ failure assessment component score prediction of in-hospital mortality from sepsis. J Intensive Care Med. 2018. https://doi.org/10.1177/0885066618795400.
Umegaki T, Ikai H, Imanaka Y. The impact of acute organ dysfunction on patients’ mortality with severe sepsis. J Anaesthesiol Clin Pharmacol. 2011;27:180–4. https://doi.org/10.4103/0970-9185.81816.
Seymour CW, Kennedy JN, Wang S, Chang C-CH, Elliott CF, Xu Z, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321:2003–17. https://doi.org/10.1001/jama.2019.5791.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. https://doi.org/10.1053/jhep.2001.22172.
Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26. https://doi.org/10.1056/NEJMoa0801209.
Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. https://doi.org/10.1002/bjs.1800600817.
Guo K, Ren J, Wang G, Gu G, Li G, Wu X, et al. Early liver dysfunction in patients with intra-abdominal infections. Medicine (Baltimore). 2015;94:e1782–e1782. https://doi.org/10.1097/MD.0000000000001782.
Champigneulle B, Geri G, Bougouin W, Dumas F, Arnaout M, Zafrani L, et al. Hypoxic hepatitis after out-of-hospital cardiac arrest: incidence, determinants and prognosis. Resuscitation. 2016;103:60–5. https://doi.org/10.1016/j.resuscitation.2016.03.021.
Jung C, Fuernau G, Eitel I, Desch S, Schuler G, Kelm M, et al. Incidence, laboratory detection and prognostic relevance of hypoxic hepatitis in cardiogenic shock. Clin Res Cardiol. 2017;106:341–9. https://doi.org/10.1007/s00392-016-1060-3.
Iesu E, Franchi F, Zama Cavicchi F, Pozzebon S, Fontana V, Mendoza M, et al. Acute liver dysfunction after cardiac arrest. PLoS ONE. 2018;13:e0206655–e0206655. https://doi.org/10.1371/journal.pone.0206655.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MB developed the initial idea for the review. All authors were responsible for the literature search, manuscript writing and editing. All authors critically reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Aritz Perez Ruiz de Garibay is affiliated with ADVITOS, GmbH, a company that offers extracorporeal support devices including liver support. No statements regarding the use of devices for liver support are made in this review. None of the other authors reports a link with ADVITOS or any other link to commercial entities that would be perceived as conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Perez Ruiz de Garibay, A., Kortgen, A., Leonhardt, J. et al. Critical care hepatology: definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit Care 26, 289 (2022). https://doi.org/10.1186/s13054-022-04163-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04163-1