Abstract
Background
The postoperative period is critical for a patient’s recovery, and postoperative hypotension, specifically, is associated with adverse clinical outcomes and significant harm to the patient. However, little is known about the association between postoperative hypotension in patients in the intensive care unit (ICU) after non-cardiac surgery, and morbidity and mortality, specifically among patients who did not experience intraoperative hypotension. The goal of this study was to assess the impact of postoperative hypotension at various absolute hemodynamic thresholds (≤ 75, ≤ 65 and ≤ 55 mmHg), in the absence of intraoperative hypotension (≤ 65 mmHg), on outcomes among patients in the ICU following non-cardiac surgery.
Methods
This multi-center retrospective cohort study included specific patient procedures from Optum® healthcare database for patients without intraoperative hypotension (MAP ≤ 65 mmHg) discharged to the ICU for ≥ 48 h after non-cardiac surgery with valid mean arterial pressure (MAP) readings. A total of 3185 procedures were included in the final cohort, and the association between postoperative hypotension and the primary outcome, 30-day major adverse cardiac or cerebrovascular events, was assessed. Secondary outcomes examined included all-cause 30- and 90-day mortality, 30-day acute myocardial infarction, 30-day acute ischemic stroke, 7-day acute kidney injury stage II/III and 7-day continuous renal replacement therapy/dialysis.
Results
Postoperative hypotension in the ICU was associated with an increased risk of 30-day major adverse cardiac or cerebrovascular events at MAP ≤ 65 mmHg (hazard ratio [HR] 1.52; 98.4% confidence interval [CI] 1.17–1.96) and ≤ 55 mmHg (HR 2.02, 98.4% CI 1.50–2.72). Mean arterial pressures of ≤ 65 mmHg and ≤ 55 mmHg were also associated with higher 30-day mortality (MAP ≤ 65 mmHg, [HR 1.56, 98.4% CI 1.22–2.00]; MAP ≤ 55 mmHg, [HR 1.97, 98.4% CI 1.48–2.60]) and 90-day mortality (MAP ≤ 65 mmHg, [HR 1.49, 98.4% CI 1.20–1.87]; MAP ≤ 55 mmHg, [HR 1.78, 98.4% CI 1.38–2.31]). Furthermore, we found an association between postoperative hypotension with MAP ≤ 55 mmHg and acute kidney injury stage II/III (HR 1.68, 98.4% CI 1.02–2.77). No associations were seen between postoperative hypotension and 30-day readmissions, 30-day acute myocardial infarction, 30-day acute ischemic stroke and 7-day continuous renal replacement therapy/dialysis for any MAP threshold.
Conclusions
Postoperative hypotension in critical care patients with MAP ≤ 65 mmHg is associated with adverse events even without experiencing intraoperative hypotension.
Similar content being viewed by others
Background
The postoperative period can be extremely dangerous for patients, and postoperative hypotension (POH), specifically, is associated with significant harm [1]. Mortality is 1000 times more common postoperatively than intraoperatively [2], and if mortality within 30 days of surgery was classified as a separate indication, it would be the third leading cause of death in the USA [3]. Myocardial infarction is the leading cause of attributable postoperative death [4], and other common pathologies include acute ischemic stroke (AIS) and acute kidney disease [3]. Furthermore, escalation of the complexity of postoperative critical care is not only costly, but is also associated with a decreased quality of life in the years following hospitalization [5].
Despite its association with significant patient harm and cost, only a limited number of studies have investigated POH, particularly none previously in cohorts without preceding intraoperative hypotension (IOH) [1, 6]. A mixed cohort of critically ill patients (including patients from the coronary care unit, general, medical, cardiac and surgical intensive care units (ICU)) revealed an increased risk of myocardial injury, mortality and kidney injury at MAP thresholds of 85 mmHg with a progressive increase at lower pressures [7]. A specific investigation in a postoperative population demonstrated that patients in the surgical ICU following non-cardiac surgery are especially sensitive to even mild amounts of hypotension [8]. However, taking a closer look at the data, post hoc analyses revealed that the relationship between ICU hypotension and the risk of adverse outcomes was dependent on the amount of IOH [8]. Moreover, there is a paucity of literature which examines various absolute blood pressure thresholds in postoperative ICU patients.
Understanding the impact of POH at different hemodynamic thresholds without the contribution from IOH on adverse clinical outcomes among post-surgical critical care patients can provide better insight into the importance of POH management and potentially inform strategies to assist in early intervention. For example, in a recent randomized clinical trial (HYPE), the use of a machine learning-derived early warning system resulted in less IOH and potentially fewer adverse outcomes (although not statistically significant) than the standard of care [9]. Furthermore, continuous hemodynamic monitoring and individualized blood pressure management might assist early intervention and thus result in overall better clinical outcomes, including risk reduction for postoperative organ dysfunction such as risk of AKI [10, 11].
This multi-center retrospective cohort study sought to evaluate the association of POH across multiple hemodynamic thresholds (≤ 75, ≤ 65 and ≤ 55 mmHg), in the absence of IOH (≤ 65 mmHg), among patients in the ICU after non-cardiac surgery. The primary outcome was major adverse cardiac or cerebrovascular events (MACCE) in the first 30 days. Additional clinical adverse outcomes investigated included: all-cause 30- and 90-day mortality, 30-day AMI, 30-day AIS, 7-day AKI stage II/III, 30-day readmissions and the need for continuous renal replacement therapy (CRRT)/dialysis in the first 7 days.
Materials and methods
Data source
The cohort for this study was obtained from the Optum® healthcare database (Optum®, Eden Prairie, MN), which integrates and standardizes de-identified electronic medical records from both ambulatory and inpatient settings of over 2000 hospitals and 7000 clinics. Optum® data cover diagnoses and procedure codes, clinical observations (i.e., vital signs), medications and laboratory tests [12]. In advance, a statistical analysis plan was submitted to the Western Institutional Review Board (Puyallup, WA), and the study was determined exempt from review as it does not meet the definition of human research as defined in 45 Code of Federal Regulations 46.102.
Cohort selection
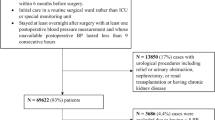
Patients were identified from an original cohort of 368,222 non-cardiac/non-obstetric surgical procedures (January 1, 2008, to December 31, 2017; based upon data availability) with valid intraoperative MAP readings (see Exposure section for calculations) and at least 1 year each of pre-surgical history and of follow-up (Additional file 1: Fig. S1, for initial attrition). No patient age restriction was applied.
The final cohort of 3185 non-cardiac/non-obstetric procedures for patients discharged to the ICU (with length of stay ≥ 48 h) with no evidence of IOH (MAP ≤ 65 mmHg) was selected by applying the following exclusion criteria: (a) patient died within 24 h of surgery; (b) procedures with greater than two 5-h gaps between MAP readings [13] within 48 h post-surgery; (c) missing or invalid MAP values within 48 h post-surgery; (d) discharge setting listed as the medical/surgical ward; (e) multiple conflicting discharge locations (“invalid”); (f) patients discharged to the ICU with greater than one 2-h interval between MAP readings over the 48 h post-surgery (this more stringent requirement for MAP readings [versus step (b)] was applied due to the ICU setting); and (g) IOH exposure (MAP ≤ 65 mmHg based on the literature [14, 15]) during qualifying surgery.
The surgeries were identified using procedures in the Center for Disease Control’s National Healthcare Safety Network Surgical Site Infection monitoring program, and the International Classification of Disease (ICD)-9 and 10, and Healthcare Common Procedure Coding System codes [16, 17]. For patients with multiple procedures within 30 days of each other, the last surgery was used as the index procedure. All procedures were included in cases where surgeries were > 30 days apart.
A machine learning approach was utilized to identify patients discharged to the ICU for patients with an undocumented post-surgery care location as previously described [18] (see Additional file 1: Method S1, for brief description of the machine learning approach). From the final cohort of 3185 patients, 46.7% of patients (n = 1486) had a formal documented discharge to the ICU, and 53.3% of patients (n = 1699) were identified as discharged to the ICU using the algorithm.
Determining MAP thresholds and exposures for postoperative hypotension
MAP was calculated by using the following formula: MAP = [(2 × diastolic blood pressure) + systolic blood pressure]/3. Invalid MAP data were identified using criteria described in previously published sources [14]. 0.7% (n = 844) of patient procedures were excluded due to missing or invalid MAP readings within the 48 h post-surgery (Fig. 1). Based on available literature, three a priori defined absolute MAP thresholds (≤ 75, ≤ 65 and ≤ 55 mmHg), were used [15]. POH was assessed as a binary variable (presence/absence; POH for the relevant threshold was defined as a single MAP reading below the threshold) with the defined absolute MAP thresholds over the first 48 h post-surgery (beginning from surgical stop time). Additionally, we accounted for the timing of the binary POH exposure to ensure that it occurred before any outcome of interest. We also report the incidence of the hypotension exposure by surgery type (Fig. 2).
Cumulative incidence of postoperative hypotension for overall surgeries and the top 10 surgeries, among patients discharged to the ICU for 48 h after non-cardiac/non-obstetric surgery. Patients included had no preceding IOH (MAP ≤ 65 mmHg). The incidence of lowest POH value recorded per patient by MAP thresholds of ≤ 55 mmHg, ≤ 65 mmHg, ≤ 75 mmHg and > 75 mmHg is shown for overall surgeries and the 10 most common surgery types and overall (craniotomy most common, knee prosthesis least common). Due to rounding, categories will not always add to 100%. surgeries in the top 10 cohort: AMP, limb amputation; CHOL, gallbladder surgery; COLO, colon surgery; CRAN, craniotomy; FUSN, spinal fusion; FUSN-LAM, spinal fusion laminectomy; FX, open reduction of fracture; HPRO, hip prosthesis; KPRO, knee prosthesis; THOR, thoracic surgery (non-cardiac, non-vascular); POH, postoperative hypotension
Potential confounding variables
Potential confounding variables were defined a priori based on previously described methods [13] and on clinical and operative factors that may affect the odds of experiencing MACCE (Tables 1 and 2; Additional file 1: Table S1). Briefly, patient demographics were determined, and comorbidities were identified in the year before surgery using ICD-9/10 codes except for valvular disease and severity, which were identified using physician notes (see Additional file 1: Table S2, for ICD codes). The Charlson comorbidity index (CCI) was utilized to determine baseline patient comorbidity severity [19]. Additional procedures in the year prior to surgery, besides the index procedure, were captured from relevant ICD-9/10 and Current Procedural Terminology (CPT) codes. The use of antihypertensive medication in the year before the procedure was captured from patient medication records (see Additional file 1: Table S3, for a list of antihypertensive drugs). Vasopressor agents were collected from patient records; evidence of major bleeding (ICD-9/10) and use of antihypertensives within 48 h after surgery were included as time-dependent confounding variables.
In order to determine new onset of outcomes and to adjust for patient status immediately prior to the index procedure, the following conditions were identified in the 7 days prior to index procedure using the appropriate ICD-9/10 codes: delirium, electrolyte disorders, AMI, AIS and new-onset AKI. Pre-existing dialysis or CRRT was identified for a patient over the year prior to the index procedure date.
Total surgery time was estimated using the high-frequency MAP datapoints defined as intervals of ≤ 5 min between readings. Time of surgery (day/night—defined as after 6PM/6AM local time) and date (weekday/weekend) were included in the association prediction models to adjust for the association between surgical/facility factors and the likelihood of an adverse event.
Primary, secondary and exploratory outcomes
The primary outcome was 30-day MACCE as defined by the composite measure of all-cause mortality, AMI or AIS [20,21,22]. Mortality was captured using the Social Security Index, and AMI and AIS were identified from ICD-9/10 codes. Additionally, AMI was captured using the Clinical Classifications Software diagnosis code 100.
Secondary outcomes investigated included: all-cause 30- and 90-day mortality, 30-day AMI, 30-day AIS, 7-day AKI stage II or III [23], 30-day readmissions and 7-day CRRT/dialysis. AKI stage II/III was chosen based on previous literature showing a significant increase in hospital mortality with stage II and III renal dysfunction [24] and defined as postoperative creatinine two times greater than the most recent preoperative value, an increase in serum creatinine ≥ 4 mg/dL, or initiation of dialysis therapy [25]. A new hospital admission within 30 days post-discharge from index hospitalization was counted within 30-day readmissions. The frequency of new-onset CRRT or intermittent hemodialysis, and delirium were assessed using ICD-9/10 codes or CPT codes.
Exploratory outcomes included readmission to ICU after 72 h post-ICU discharge, ICU-free days in the first 30 days (if a patient died in the hospital this variable counted as zero) and days alive and free (DAF) of vasopressors (calculated by subtracting days on vasopressors from the lesser of 28 or the number of days until death, as previously described) [26]. Readmission to ICU after 72 h was defined as ICU admission after 72 h after discharge from the ICU. Outcomes investigated in this study were not limited to events in the index visit.
Statistical analysis
The associations between POH thresholds and outcomes were evaluated using two-tailed hypothesis testing. The rate of adverse events for each POH threshold was evaluated, where the reference group was defined as all patients who did not experience POH at any MAP threshold (i.e., for ≤ 65 mmHg, this is > 65 mmHg). Additionally, the mean (SD) and median (25th and 75th percentile) of time below MAP < 55 mmHg were calculated. Patients with an outcome (within 7 days for AIS, AMI, AKI or delirium or within 1-year for dialysis or CRRT) in the 30 days before surgery were excluded from the corresponding outcome analyses.
The independent association of POH exposures on the primary and secondary outcomes was calculated using Cox proportional hazards models, where POH was modeled as a time-dependent covariate. For secondary outcomes that did not include mortality or a length-of-stay component, Fine–Gray regression models [27], which account for the competing risk of death, were performed, and sub-distribution hazards are reported. DAF of vasopressors was assessed via Poisson regression. ICU-free days in the first 30 days were assessed via Poisson regression as it broke the linearity assumption.
A sensitivity analysis for primary and secondary outcomes was performed to evaluate the magnitude of an unobserved or unaccounted confounding effect by calculating the E-values, defined as the effect required to reduce the observed odds ratio for an outcome to 1.0 [28].
Because outcomes were evaluated across three POH thresholds, a Bonferroni correction [29] with a p value of ≤ 0.05/3 or 0.016 was used. ICU-free days and DAF of vasopressors were reported as incidence rate ratios (IRRs) with 98.4% confidence intervals (CIs); all other outcomes were reported as hazard ratios (HRs) with 98.4% (CIs). All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC) and R 3.5.2.
Sample size considerations
A previous study in the USA (2004–2013) found that MACCE occurs in 3% of 10,581,621 hospitalizations for major non-cardiac surgery [20]. We would hypothesize that a 3% difference in the rate of MACCE would translate to an expected sample size of 999 with 95% confidence interval and 90% power; therefore, our study with 3185 procedures should be well above this threshold.
Results
Study cohort and patient characteristics
The final study cohort comprised 3185 procedures of patients (3169 unique patients) who were admitted to the ICU after non-cardiac/non-obstetric surgery with no evidence of IOH (MAP ≤ 65 mmHg) (Fig. 1). Among these patients, 47.9% were female (n = 1527), 77.3% were Caucasian (n = 2462), 36.0% (n = 1147) were classified with CCI category ≥ 4 and their mean (± SD) age was 63.5 (± 16.4) years (Tables 1 and 2). Patients ≤ 18 years comprised 1.2% (n = 37) of the population. Of the cohort, 2674 (84.0%) experienced a MAP ≤ 75 mmHg in the ICU; 1688 (53.0%) experienced hypotension with a MAP ≤ 65 mmHg and 654 (20.5%) a MAP ≤ 55 mmHg. Analysis of MAP readings suggested that ICU patients spent an average (SD) of 64.0 (123.6) minutes with MAP ≤ 55 mmHg [median (25th, 75th): 25.0 (7.9, 70.1) mins], an average of 250.6 (336.1) minutes with MAP ≤ 65 mmHg [median (25th, 75th): 108.9 (30.3, 332.2) and an average of 733.8 (716.8) minutes with MAP ≤ 75 mmHg [median (25th, 75th): 477.2 (135.3, 1161.8) mins]. Examination of the degree of hypotension experienced by patients who underwent the top 10 non-cardiac surgeries revealed that patients undergoing craniotomy procedures appear to experience less hypotension than other procedures (Fig. 2).
The overall incidence of 30-day MACCE was 17.1%. Stratified by MAP threshold, the incidence of MACCE among ICU patients without IOH was: 404 (17.7%) with MAP ≤ 75 mmHg and 293 (20.2%) and 142 (25.2%) with MAP ≤ 65 mmHg and ≤ 55 mmHg, respectively (Table 3). Descriptive statistics for patient characteristics and confounding variables stratified by POH MAP exposure thresholds, in nested groups, are shown in Tables 1 and 2 as well as Additional file 1: Table S1.
Association between POH and MACCE (with absence of IOH) in ICU setting
Adjusted models for the hazard ratios for ICU patients with POH without preceding IOH revealed the risk of 30-day MACCE progressively increased as the severity of hypotension increased. Both POH of MAP ≤ 65 mmHg (HR 1.52, 98.4% CI 1.17–1.96; p < 0.001) and MAP ≤ 55 mmHg (HR 2.02, 98.4% CI 1.50–2.72; p < 0.001) were associated with increased risk of MACCE. However, the association between MAP ≤ 75 mmHg (HR 1.19, 98.4% CI 0.84–1.68; p = 0.224) was not significant (Fig. 3 and Additional file 1: Table S4, for p values). Univariate (unadjusted) hazard ratios (HR) for MACCE were as follows for MAP thresholds: MAP ≤ 75 mmHg (HR 1.41, 98.4% CI 1.02–1.95; p < 0.011), MAP ≤ 65 mmHg (HR 1.64, 98.4% CI 1.29–2.08; p < 0.001), MAP ≤ 55 mmHg (HR 1.77, 98.4% CI 1.37–2.29; p < 0.001). Multivariate model results are included in Additional file 1: Table S4.
Adjusted hazard and sub-distribution hazard ratios for critical care patients with postoperative hypotension. Data shown for procedures (n = 3185) without preceding IOH (≤ 65 mmHg) at three absolute POH thresholds (≤ 55, ≤ 65 and ≤ 75 mmHg). *Significant after applying Bonferroni adjustment (p value of ≤ 0.05/3 or 0.016). POH, postoperative hypotension; MAP, mean arterial pressure; MACCE, major adverse cardiovascular or cerebrovascular events; AIS, acute ischemic stroke; AKI, acute kidney injury; AMI, acute myocardial infarction, Adj, adjusted; CRRT, continuous renal replacement therapy; HR, hazard ratio; SDHR, sub-distribution hazard ratio; CI, confidence interval
POH in the ICU and associations with secondary endpoints
POH with MAP thresholds ≤ 65 mmHg and ≤ 55 mmHg was associated with higher 30-day (MAP ≤ 65 mmHg, [HR 1.56, 98.4% CI 1.22–2.00; p < 0.001]; MAP ≤ 55 mmHg, [HR 1.97, 98.4% CI 1.48–2.60; p < 0.001]) and 90-day mortality (MAP ≤ 65 mmHg, [HR 1.49, 98.4% CI 1.20–1.87; p < 0.001]; MAP ≤ 55 mmHg, [HR 1.78, 98.4% CI 1.38–2.31; p < 0.001]). POH of MAP ≤ 55 mmHg was also associated with greater risk of 7-day AKI stage II/III (HR 1.68, 98.4% CI 1.02–2.77; p = 0.013). No associations were observed between POH and 30-day readmissions, 30-day AMI, 30-day AIS and 7-day CRRT/dialysis for any MAP threshold. Detailed results for primary and secondary outcomes for all thresholds are shown in Fig. 3 and Additional file 1: Table S5, for p values. Unmeasured confounders required to reduce the odds ratio to 1.0 were evaluated using E-values for primary and secondary endpoints (Additional file 1: Table S6). E-values for significant endpoints ranged from 3.46 to 2.34 (MACCE ≤ 55 mmHg threshold to 90-day mortality ≤ 65 mmHg threshold).
Association between POH and exploratory endpoints in the ICU
At any of the MAP thresholds investigated, no associations were found between POH and readmission to ICU after 72 h, ICU-free days in first 30 days and DAF of vasopressors (Additional file 1: Table S7 for exploratory outcomes).
Discussion
This is the first study to examine the association between multiple postoperative hemodynamic thresholds in the absence of prior IOH (≤ 65 mmHg) with adverse clinical outcomes in the ICU setting. We demonstrate that POH at various hemodynamic thresholds (≤ 75, ≤ 65 and ≤ 55 mmHg) in the absence of IOH is associated with a larger absolute number of adverse clinical events as compared to patients with no POH exposure. Our findings underscore the importance of the immediate postoperative period (48 h) on patient outcomes among patients without intraoperative hypotension. Overall, the risk of MACCE and 30- and 90-day mortality progressively increased with decreasing MAP thresholds. For example, we saw an 11.1% increase in MACCE and 13.6% and 15.1% increase in 30- and 90-day mortality, respectively, in patients who had exposure to POH in the ICU, despite being stable in the intraoperative period.
The risk of experiencing 30-day MACCE, 30-day or 90-day mortality was significantly increased at blood pressure thresholds of ≤ 65 mmHg and ≤ 55 mmHg. The high incidence of 30-day MACCE (17.1%) in the overall population is likely driven by 30-day mortality (16.3%), as 30-day AMI (1.2%) and AIS (2.6%) had a relatively low incidence in our population. This would also explain the lack of significant demonstrable associations of POH in the ICU with AMI or AIS. Our mortality rate in this critically ill population is supported by other recent studies in surgical and non-surgical ICU populations, which report rates of mortality between 16 and 20% [1, 30,31,32,33,34]. In particular, our mortality rate is consistent with a large cohort analysis of ~ 50,000 patients across 65 ICUs in Sweden which reported a 30-day mortality rate of 17% [32]. Indeed, our study population had 36% of overall patients (across all blood pressure thresholds) classified with CCI ≥ 4, suggesting that these patients were suffering from a number of comorbid conditions prior to their non-cardiac surgery, which could have contributed to adverse events with an associated increased risk of mortality.
Khanna and colleagues reported that increasing amounts of hypotension at pressures previously regarded as normal were associated with myocardial injury, mortality and renal injury among surgical ICU patients; however, there was an interaction between IOH and critical care outcomes related to POH, making it difficult to evaluate the selective contribution of POH among ICU patients in the absence of IOH [8]. Therefore, our study builds upon Khanna and colleagues as it effectively evaluated the association of POH with adverse clinical events in postoperative critical care patients that did not experience intraoperative hypotension. Our observations that POH in the ICU with MAP ≤ 65 mmHg is associated with increased risk of MACCE as well as 30- and 90-day mortality suggest that POH in the critically ill is hazardous and blood pressure should be carefully, and preferably, continuously monitored using invasive and non-invasive methods as clinically indicated. Furthermore, this study evaluated several hemodynamic thresholds and demonstrated that POH, even at a MAP of 75 mmHg, though not statistically significant, results in an increased rate of adverse clinical events (MACCE, mortality measures and AKI/CRRT) compared to maintaining MAP above 75 mmHg. This finding is novel and opens room to question conventional wisdom of MAP goal 65 mmHg in ICU patients, as illustrated by a recent editorial [35]. Other randomized trials of blood pressure in the ICU have not found a difference in outcomes comparing different blood pressure targets, though these were septic patients and different from our novel postoperative ICU population that had a stable intraoperative course prior to ICU admission [36, 37]. Overall, our data suggest the importance of aggressive monitoring and early intervention to correct blood pressure in the ICU which may decrease mortality and adverse clinical events.
Our study has several limitations which must be considered, the first being that as this study was observational in nature, the data were subject to reporting bias/data entry errors. While we calculated E-values to measure uncontrolled confounding, we cannot exclude that it may not exist. Overall, we controlled for numerous confounders available in our dataset, however, if hypotension was simply an indicator of the degree of severity of the underlying illness, our estimated risks could be overestimations [1]. Second, since this was a retrospective study, we had no control over the treatments chosen for the hypotension events and the specific protocols within and across various hospitals. Third, while we made every attempt to minimize gaps between blood pressure readings (limiting gaps to one 2-h gap in-between readings), this presents a limitation (therefore time below thresholds as time-weighted averages was not modeled). One reason for this is that only nurse or provider validated blood pressure readings were entered into electronic records; therefore, we sacrificed blood pressure data granularity at the cost of possibly greater accuracy. Additionally, since MAP recordings were intermittent in nature, there was the potential for unstable MAP readings especially at higher MAP values which might have resulted in higher average times below the MAP thresholds. Fourth, our study population was focused on ICU patients; therefore, our results cannot be generalized to surgical patients discharged to the ward after the operative procedure. Fifth, AKI stage II/III was chosen as an outcome for this study because at the time a large volume of literature suggested that hospital mortality was associated with stage II/III renal dysfunction [24]. Although, we realize now that AKI stage I has similar long-term renal dysfunction when compared with stage II/III [38]. Sixth, our study design allows for a patient to have multiple non-cardiac procedures in our cohort as procedures > 30 days prior to the index visit could also be included in this study. Therefore, any hypotension experienced during or after that procedure could have resulted in unknown long-term effects. Finally, because we utilized an algorithm with a positive predictive value of 0.97 to help assign patients without a known care location to the ICU, 3% of patients could be misidentified.
Conclusions
In conclusion, our analyses revealed a strong association between POH at several different hemodynamic thresholds in critical care patients with no hypotensive episodes during surgery (MAP > 65 mmHg), and adverse clinical outcomes in a multi-center retrospective cohort post-non-cardiac surgery. This study advances the field on POH among the critically ill as no study to date has evaluated the association among various POH thresholds in the absence of any prior hypotension with adverse clinical outcomes. The data here are hypothesis generating and should serve as a pilot for a larger interventional trial investigating the optimal blood pressure thresholds and need for improved hemodynamic monitoring for postoperative ICU patients, in order to improve short- and long-term outcomes of this vulnerable patient population.
Availability of data and materials
The data that support the findings of this study are available from Optum® (Eden Prairie, MN), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Optum®.
Abbreviations
- AIS:
-
Acute ischemic stroke
- AKI:
-
Acute kidney injury
- AMI:
-
Acute myocardial infarction
- BP:
-
Blood pressure
- CCI:
-
Charlson comorbidity index
- CRRT:
-
Continuous renal replacement therapy
- CI:
-
Confidence interval
- CPT:
-
Current procedural terminology
- DAF:
-
Days alive and free
- HR:
-
Hazard ratio
- ICD:
-
International Classification of Diseases
- ICU:
-
Intensive care unit
- IOH:
-
Intraoperative hypotension
- IRR:
-
Incidence rate ratio
- MACCE:
-
Major adverse cardiac or cerebrovascular events
- MAP:
-
Mean arterial pressure
- POH:
-
Postoperative hypotension
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SDHR:
-
Sub-distribution hazard ratio
References
Sessler DI, Meyhoff CS, Zimmerman NM, Mao G, Leslie K, Vasquez SM, et al. Period-dependent Associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128(2):317–27.
Sessler DI, Khanna AK. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018;44(6):811–22.
Bartels K, Karhausen J, Clambey ET, Grenz A, Eltzschig HK. Perioperative organ injury. Anesthesiology. 2013;119(6):1474–89.
Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642–51.
Timmers TK, Verhofstad MH, Moons KG, van Beeck EF, Leenen LP. Long-term quality of life after surgical intensive care admission. Arch Surg. 2011;146(4):412–8.
van Lier F, Wesdorp F, Liem VGB, Potters JW, Grune F, Boersma H, et al. Association between postoperative mean arterial blood pressure and myocardial injury after noncardiac surgery. Br J Anaesth. 2018;120(1):77–83.
Maheshwari K, Nathanson BH, Munson SH, Khangulov V, Stevens M, Badani H, et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 2018;44(6):857–67.
Khanna AK, Maheshwari K, Mao G, Liu L, Perez-Protto SE, Chodavarapu P, et al. Association between mean arterial pressure and acute kidney injury and a composite of myocardial injury and mortality in postoperative critically Ill patients: a retrospective cohort analysis. Crit Care Med. 2019;47(7):910–7.
Wijnberge M, Geerts BF, Hol L, Lemmers N, Mulder MP, Berge P, et al. Effect of a machine learning-derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: the HYPE randomized clinical trial. JAMA. 2020;323(11):1052–60.
Futier E, Lefrant JY, Guinot PG, Godet T, Lorne E, Cuvillon P, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–57.
Hori D, Hogue C, Adachi H, Max L, Price J, Sciortino C, et al. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interact Cardiovasc Thoracic Surg. 2016;22(4):445–51.
Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum labs: building a novel node in the learning health care system. Health Aff. 2014;33(7):1187–94.
Gregory A, Stapelfeldt WH, Khan A, Smischney NJ, Boero I, Chen Q, et al. Intraoperative hypotension is associated with adverse clinical outcomes after non-cardiac surgery (in Submission). 2019.
Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47–65.
Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706–21.
2019 NHSN Operative Procedure Code Mappings [Internet]. 2019. https://www.cdc.gov/nhsn/xls/icd10-pcs-pcm-nhsn-opc.xlsx.
ICD-10-PCS & CPT Codes - Guidance for HPRO & KPRO Procedure Details [Internet]. 2019. https://www.cdc.gov/nhsn/xls/guidance-for-hpro-kpro-procedure-details.xlsx.
Khanna AK, Shaw AD, Stapelfeldt WH, Boero IJ, Chen Q, Stevens M, et al. Postoperative hypotension is associated with adverse clinical outcomes in patients without intraoperative hypotension, after non-cardiac surgery (in submission). 2020.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol. 2017;2(2):181–7.
Xia J, Xu J, Li B, Liu Z, Hao H, Yin C, et al. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin Chim Acta. 2017;466:162–6.
Ham SY, Song SW, Nam SB, Park SJ, Kim S, Song Y. Effects of chronic statin use on 30-day major adverse cardiac and cerebrovascular events after thoracic endovascular aortic repair. J Cardiovasc Surg (Torino). 2018;59(6):836–43.
Koeze J, Keus F, Dieperink W, van der Horst ICC, Zijlstra JG, van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017;18(1):70.
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–23.
Ostermann M, Chang R. Correlation between the AKI classification and outcome. Crit Care (London, England). 2008;12(6):R144.
Russell JA, Lee T, Singer J, De Backer D, Annane D. Days alive and free as an alternative to a mortality outcome in pivotal vasopressor and septic shock trials. J Crit Care. 2018;47:333–7.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268.
Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502–8.
Lobo SM, Rezende E, Knibel MF, Silva NB, Páramo JA, Nácul FE, et al. Early determinants of death due to multiple organ failure after noncardiac surgery in high-risk patients. Anesth Analg. 2011;112(4):877–83.
Mukhopadhyay A, Tai BC, See KC, Ng WY, Lim TK, Onsiong S, et al. Risk factors for hospital and long-term mortality of critically ill elderly patients admitted to an intensive care unit. Biomed Res Int. 2014;2014:960575.
Rydenfelt K, Engerström L, Walther S, Sjöberg F, Strömberg U, Samuelsson C. In-hospital vs. 30-day mortality in the critically ill—a 2-year Swedish intensive care cohort analysis. Acta Anaesthesiol Scand. 2015;59(7):846–58.
Orban J-C, Walrave Y, Mongardon N, Allaouchiche B, Argaud L, Aubrun F, et al. Causes and characteristics of death in intensive care units: a prospective multicenter study. Anesthesiology. 2017;126(5):882–9.
Abelha FJ, Castro MA, Landeiro NM, Neves AM, Santos CC. Mortality and length of stay in a surgical intensive care unit. Rev Bras Anestesiol. 2006;56(1):34–45.
Asfar P, Radermacher P, Ostermann M. MAP of 65: target of the past? Intensive Care Med. 2018;44(9):1551–2.
Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, et al. Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA. 2020;323:938–49.
Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–93.
Turan A, Cohen B, Adegboye J, Makarova N, Liu L, Mascha EJ, et al. Mild acute kidney injury after noncardiac surgery is associated with long-term renal dysfunction: a retrospective cohort study. Anesthesiology. 2020;132(5):1053–61.
Acknowledgements
The authors thank Sibyl H. Munson, PhD, and Francie Moehring, PhD, of Boston Strategic Partners, Boston, MA, USA, for assistance with the manuscript.
Funding
Funding for this research was provided by Edwards Lifesciences.
Author information
Authors and Affiliations
Contributions
NJS, AKK and IJB helped design the study, supervise the data collection, analyze the data and prepare the manuscript. ADS and WHS helped supervise the data collection, analyze the data and prepare the manuscript. QC and MS helped analyze the data and prepare the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was determined exempt from review as it does not meet the definition of human research as defined in 45 Code of Federal Regulations 46.102.
Consent for publication
Not applicable.
Competing interests
NJS, AKK and WHS received consulting fees from Edwards Lifesciences. IJB and QC are employees of Boston Consulting Group, who received funds from Edwards Lifesciences to perform the research. MS is an employee of Edwards Lifesciences. AKK consults for Medtronic, Philips North America and Zoll Medical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Figure S1. Initial attrition diagram leading into cohort selection for current study. Method S1. Brief description of the machine learning approach to identify patients discharged to the ICU for patients with an undocumented post-surgery care location. Table S1. Comorbidities and additional cohort patient characteristics. Table S2. International Classification of Diseases (ICD) codes. Table S3. List of antihypertensive drug classes. Table S4. Multivariate model results. Table S5. P-values of hazard ratios for primary and secondary outcomes. Table S6. E-values to assess the magnitude of an unobserved or unaccounted confounding effect for postoperative hypotension in intensive care unit setting. Table S7. Hazard/Incidence rate ratios and p-values for length-of-stay and use of intensive care unit-specific endpoints for intensive care unit patients with postoperative hypotension (exploratory outcomes).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Smischney, N.J., Shaw, A.D., Stapelfeldt, W.H. et al. Postoperative hypotension in patients discharged to the intensive care unit after non-cardiac surgery is associated with adverse clinical outcomes. Crit Care 24, 682 (2020). https://doi.org/10.1186/s13054-020-03412-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-020-03412-5