Abstract
Introduction
Direct comparison of the relative efficacy of different recruitment maneuvers (RMs) for patients with acute respiratory distress syndrome (ARDS) via clinical trials is difficult, due to the heterogeneity of patient populations and disease states, as well as a variety of practical issues. There is also significant uncertainty regarding the minimum values of positive end-expiratory pressure (PEEP) required to ensure maintenance of effective lung recruitment using RMs. We used patient-specific computational simulation to analyze how three different RMs act to improve physiological responses, and investigate how different levels of PEEP contribute to maintaining effective lung recruitment.
Methods
We conducted experiments on five ‘virtual’ ARDS patients using a computational simulator that reproduces static and dynamic features of a multivariable clinical dataset on the responses of individual ARDS patients to a range of ventilator inputs. Three recruitment maneuvers (sustained inflation (SI), maximal recruitment strategy (MRS) followed by a titrated PEEP, and prolonged recruitment maneuver (PRM)) were implemented and evaluated for a range of different pressure settings.
Results
All maneuvers demonstrated improvements in gas exchange, but the extent and duration of improvement varied significantly, as did the observed mechanism of operation. Maintaining adequate post-RM levels of PEEP was seen to be crucial in avoiding cliff-edge type re-collapse of alveolar units for all maneuvers. For all five patients, the MRS exhibited the most prolonged improvement in oxygenation, and we found that a PEEP setting of 35 cm H2O with a fixed driving pressure of 15 cm H2O (above PEEP) was sufficient to achieve 95% recruitment. Subsequently, we found that PEEP titrated to a value of 16 cm H2O was able to maintain 95% recruitment in all five patients.
Conclusions
There appears to be significant scope for reducing the peak levels of PEEP originally specified in the MRS and hence to avoid exposing the lung to unnecessarily high pressures. More generally, our study highlights the huge potential of computer simulation to assist in evaluating the efficacy of different recruitment maneuvers, in understanding their modes of operation, in optimizing RMs for individual patients, and in supporting clinicians in the rational design of improved treatment strategies.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is a severe condition that affects around 1 in 10,000 people every year with life-threatening consequences [1]. The pathophysiology of ARDS is characterized by bronchoalveolar injury and alveolar collapse (atelectasis) [2-5]. The use of recruitment maneuvers (RMs) in ARDS to open up unstable, collapsed alveoli using a brief increase in transpulmonary pressure has become common practice in intensive care units [3], and a large variety of RMs has been proposed in the literature [3,6-12]. However, there remains a great deal of confusion regarding the optimal way to achieve and maintain alveolar recruitment in ARDS and, in many cases, the precise mode of action of particular RMs is not well understood [7,8,13,14].
The most frequently used recruitment maneuver in ARDS treatment is sustained inflation (SI) [8]. Studies have shown varying degrees of success, with several reporting post RM improvement in oxygenation [15,16] and reduction in lung atelectasis [17]. However, SI has also been shown to result in increased risk of hypotension [14,16] and barotrauma [18], decline in oxygenation [19] and has even been reported to be ineffective [20].
An alternative recruitment strategy that has been recently proposed is the prolonged recruitment maneuver (PRM) [21], in which positive end-expiratory pressure (PEEP) is fixed to a higher than baseline level and the positive inspiratory pressure is progressively increased. When PRM was compared with SI in an experimental model of mild acute lung injury (ALI) induced in a rat lung, it showed improved alveolar recruitment, gas exchange and a reduced level of lung damage. To date, however, no further evidence is available to support the use of PRM in ARDS patients.
The third RM considered in this paper is the maximal recruitment strategy (MRS), which was evaluated via patient trials in [6,22]. When this strategy was followed by ventilation with low tidal volumes and titrated PEEP, a median of 45% relative lung tissue recruitment was observed in quantitative computed tomography (CT) scan analysis. However, as noted in [13], the final PEEP levels applied at the end of the titration phase in the MRS resulted in inspiratory plateau pressures in the patient population of approximately 40 cm H2O, on average. This far exceeds the 28 cm H2O safety limit, which had been associated with increased inflammatory response in a previous study [23], and even the 30 cm H2O cutoff proposed by the ARDS Network. As noted in [6], it seems very likely that the MRS caused high degrees of alveolar stress and strain in some patients.
In this study, we employ a high-fidelity computational simulator that reproduces the static and dynamic characteristics of several ARDS patients, to (a) compare the efficacy of the three RMs described above in improving key patient parameters describing oxygenation, carbon dioxide (CO2) retention and dynamic compliance and (b) investigate the effects of different PEEP settings in maintaining effective lung recruitment across a representative ARDS patient spectrum. Our central hypothesis is that computational simulation can be used to evaluate and understand the mode of operation of RMs for ARDS patients.
Methods
The computational simulator
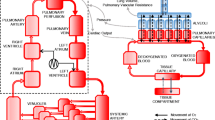
The simulation model considered in this study is an extended MATLAB™ implementation of several physiological models originally developed within the Nottingham Physiology Simulator [24-26]. The core models in the simulator have been designed to represent a dynamic in vivo cardio-vasculo-pulmonary state using a set of mass-conserving equations based on well-established physiological principles. The model simulates a lung divided into 100 alveolar compartments, with each compartment having a corresponding set of parameters accounting for stiffness, threshold opening pressures (TOPs) and extrinsic pressures as well as airway and vascular resistances. Recruitment is modeled as a time-dependent process [9,27] by the introduction of a ‘time-constant’ parameter (τc) for each collapsed alveolar compartment, denoting the time it takes for the collapsed alveolus to open after a threshold pressure has been reached. The mathematical principles and equations on which the simulator is based have been detailed in previous studies [25,26], which have also validated the simulator’s ability to accurately represent pulmonary disease states. Full details are provided in an additional file (see Additional file 1).
Computer simulation of RM protocols
Each protocol consists of a pre-RM stage, the RM stage and a post-RM stage. During the pre-RM stage, the in silico patients were subjected to identical end-expiratory pressures (10 cm H2O) and identical inspiratory pressure (15 cm H2O above PEEP). At the post RM stage, the inspiratory pressure is maintained at 15 cm H2O above PEEP while the PEEP is set to either 5 cm H2O or 10 cm H2O. Throughout the protocols, the fraction of inspired oxygen (FIO2), the hemoglobin level (Hb) and the cardiac output (CO) was maintained at the value suggested by the source data (which were used to configure each virtual patient), while the inspiratory to expiratory (IE) ratio and ventilation rate were chosen based on the available patient data (see below). Three RMs from the published literature were implemented in the simulator as detailed below and illustrated in Figure 1.
Pressure waveform supplied by the mechanical ventilator over the simulation time for all the RMs implemented in this study. (A) Maximal recruitment strategy with a final PEEP value of 10 cm H2O (MRS-10), (B) Maximal recruitment strategy with a final PEEP value of 5 cm H2O (MRS-5), (C) Sustained inflation with a final PEEP value of 10 cm H2O (SI-10), (D) Sustained inflation with a final PEEP value of 5 cm H2O (SI-5), (E) Prolonged recruitment maneuver with a final PEEP value of 10 cm H2O (PRM-10), (F) Prolonged recruitment maneuver with a final PEEP value of 5 cm H2O (PRM-5). From the plots, pressure at the end of expiration (that is the minimum pressure during a breath or in this case the PEEP setting) and peak inspiratory pressure (set by the ventilator) can be inferred. The MRS plots (A and B) indicate the recruitment and the PEEP titration phases. PEEP, positive end-expiratory pressure; RM, recruitment maneuver.
Maximal recruitment strategy (MRS)
The MRS [6] consists of two-minute steps of tidal ventilation in pressure-controlled mode, with a fixed driving pressure of 15 cm H2O (above PEEP). During the recruitment phase, PEEP was increased from 5 cm H2O to a maximum of 45 cm H2O in steps of 5 cm H2O, with each step lasting 2 minutes. During the PEEP titration phase, the PEEP is set to 25 cm H2O and then further reduced by 5 cm H2O in steps to the end-maneuver PEEP, with each step lasting 5 minutes. The end-maneuver PEEP was set to 5 cm H2O (MRS-5) or 10 cm H2O (MRS-10).
Sustained inflation (SI)
This SI [28] was simulated as a sustained pulmonary inflation maneuver, with a positive ventilator pressure of 40 cm H2O applied for 40 seconds. The end-maneuver PEEP was set to 5 cm H2O (SI-5) or 10 cm H2O (SI-10).
Prolonged recruitment maneuver (PRM)
The inspiratory pressure in the PRM [21] was progressively increased every 2 minutes in steps of 5 cm H2O from 15 cm H2O to 25 cm H2O, above a fixed PEEP of 15 cm H2O. The end-maneuver PEEP was set to 5 cm H2O (PRM-5) or 10 cm H2O (PRM-10).
The effectiveness of each RM was assessed via a common set of clinically relevant indicators: improvement in oxygenation (represented through the ratio of partial pressure of oxygen in arterial blood to the fraction of oxygen in inspired air, (PaO2/FIO2)), the change in peak alveolar pressures (Ppeak, representing the risk of barotrauma, calculated as the average of the maximum pressure in the most highly pressurized 20% of the 100 alveolar compartments, over the entire maneuver) the dynamic compliance, and the change in arterial carbon dioxide partial pressure (PaCO2).
Configuring the simulator to static and dynamic ARDS patient data
The model was configured to fit data from individual ARDS patients in two stages. In the first stage, the model was matched to static data reported by Nirmalan and colleagues [29], which listed arterial and mixed venous blood gas values and cardiac output measurements taken from patients treated for ARDS. The data obtained from Nirmalan [29] contain only static measurements and thus do not provide information concerning dynamic processes such as recruitment. Therefore, a second matching was done to determine the value of τc (representing the time it could take for collapsed alveoli to open after a threshold pressure is reached, (see Additional file 1)) for each compartment so as to best fit the data provided by Chiumello and colleagues [30], which reports blood gas measurements in ARDS patients over a 60-minute period as a result of step changes in PEEP. In both stages, advanced optimization algorithms were used to find physiologically realistic values of model parameters that best fit the available data – full details are provided in an additional file (see Additional file 2).
Results
Reproduction of ARDS patient data
The results of matching the model to static data from five patients from [29] are given in Table 1. The combined results for the five patients show an excellent linear correlation (Figure 2, with r = 0.997 (P <0.0001)) between the model-generated outputs and observations in the data. As shown in Figure 3, the outputs of the simulator also provide an extremely close fit to the dynamic data reported in [30].
Model outputs versus data recorded in [ 29 ] for five patients (Table 1 ). R, Pearson’s correlation coefficient. Units for PvO2, PvCO2 and PaO2 are in mmHg. PaO2, partial pressure of oxygen in arterial blood; PvCO2, partial pressure of carbon dioxide in venous blood; PvO2, partial pressure of oxygen in venous blood; Qs/Qt, shunt fraction.
Model PaO2fitted to data obtained from [36], resulting from changes in PEEP from 5 to 15 cm H2O and then back to 5 cm H2O. PaO2, partial pressure of oxygen in arterial blood; PEEP, positive end-expiratory pressure.
Comparative evaluation of RMs for a moderate ARDS state
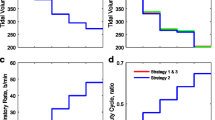
Figure 4 presents results obtained by applying the three RMs to the model configured to match Patient A in Table 1. According to the Berlin definition [31], patient A was classified as suffering from moderate ARDS (PaO2/FIO2 of 192.13 mmHg, see Table 1). As seen in Figure 4A, the application of the MRS causes the PaO2/FIO2 to increase by more than 400 mmHg. It remains raised throughout the recruitment stage and plateaus at its maximal value of 650 mmHg, continuing through to the PEEP titration phase. PaO2/FIO2 started to fall at the 40th minute interval, corresponding to the PEEP titration step to 10 cm H2O. Under MRS-5, the reduction of PEEP to 5 cm H2O resulted in a further fall of PaO2/FIO2 to near pre-RM levels of 200 mmHg. The SI maneuver resulted in a modest increase in the PaO2/FIO2 ratio (Figure 4B) from 200 mmHg to approximately 260 mmHg. A final PEEP value of 10 cm H2O was sufficient to maintain PaO2/FIO2 at 250 mmHg, while reducing the final PEEP value to 5 cm H2O produced a final PaO2/FIO2 of only 190 mmHg. Results of the application of the PRM (Figure 4C) show the PaO2/FIO2 ratio rising from 200 mmHg to a maximum value of 270 mmHg at the highest inspiratory pressure at the 15th minute interval. For PRM-10, the final PaO2/FIO2 remained at 250 mmHg whereas the PaO2/FIO2 dropped to below 200 mmHg for PRM-5. In all the RM protocols, a post-RM stage PEEP of 10 cm H2O results in a significantly higher PaO2/FIO2 than that obtained with the pre-RM stage PEEP of 10 cm H2O.
Model outputs for Patient A under the recruitment maneuvers given in Figure 1 . The continuous lines are outputs for PEEP = 10 cm H2O and dashed lines are outputs for PEEP = 5 cm H2O. The plot has nine panels: (A) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under MRS-10 and MRS-5, (B) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under SI-10 and SI-5, (C) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under PRM-10 and PRM-5, (D) the partial pressure of CO2 in arterial blood (mmHg) under MRS-10 and MRS-5, (E) the partial pressure of CO2 in arterial blood (mmHg) under SI-10 and SI-5, (F) the partial pressure of CO2 in arterial blood (mmHg) under PRM-10 and PRM-5, (G) dynamic compliance of the lung (ml mbar−1) under MRS-10 and MRS-5, (H) dynamic compliance of the lung (ml mbar−1) under SI-10 and SI-5, (I) dynamic compliance of the lung (ml mbar−1) under PRM-10 and PRM-5. CO2, carbon dioxide; MRS, maximum recruitment strategy; O2, oxygen; PEEP, positive end-expiratory pressure; PRM, prolonged recruitment maneuver; SI, sustained inflation.
Changes in PaCO2 are useful indicators of the pathological state of the lung, revealing the effectiveness of gas exchange, the presence of dead space, and acid-base balance of the blood. In Figure 4D, as the MRS begins, PaCO2 rises slightly, from 47 mmHg to 50 mmHg (until the 15th minute interval) where PaCO2 settles over the following 10 minutes (corresponding to the MRS reaching the peak ventilator pressure; see Figure 1A and B). This was followed by a reduction in PaCO2 until the 45th minute interval (as the PEEP titration stage ends) at which point the PaCO2 value achieved by MRS-5 deviates from that of MRS-10. The MRS-10 continues to make PaCO2 fall further whereas PaCO2 under MRS-5 returns to the level observed at the pre-RM stage. With SI, a slight rise in PaCO2 can be seen but the overall variation is minimal (Figure 4E) for both SI maneuvers (SI-5 and SI-10). Under the PRM (Figure 4F), from the baseline PaCO2 value of 47 mmHg, the PaCO2 rises and fluctuates around 52 mmHg until the 18th minute interval at which point it rises to a maximum value at 55 mmHg. The rest of the maneuver produced a slight drop in PaCO2 to a final value of 52 mmHg. No difference was observed between the PaCO2 values produced by PRM-10 and PRM-5 over the entire maneuver.
The MRS produced notable changes in the dynamic compliance of the lung (see Figure 4G). Beginning from an initial value of 30 ml mbar−1, as PEEP is increased, compliance fell to a minimum of 21 ml mbar−1. During PEEP titration, compliance gradually increased again to a peak of 48 ml mbar−1, the maximal value coinciding with the last step of the PEEP reduction. At this point, the higher final PEEP setting of MRS-10 resulted in the final dynamic compliance value settling at 43 ml mbar−1 while MRS-5 resulted in a lower final dynamic compliance value of 35 ml mbar−1. The SI maneuver was accompanied by a sharp increase in compliance; with SI-10 settling at a value of 32 ml mbar−1 and SI-5 settling at a slightly higher value of 35 ml mbar−1, as shown in Figure 4H. Finally, the PRM resulted in an overall drop in lung compliance while the maneuver is under progress, followed by an increase back to the baseline value upon cessation of the maneuver (Figure 4I). Under PRM-10, the final dynamic compliance value was recorded at 32 ml mbar−1 while under SI-5, the dynamic compliance settled at a higher value of 35 ml mbar−1.
Comparative evaluation of three RMs for a severe ARDS state
Figure 5 shows the results of the application of the RMs to Patient B (from Table 1), who was classified under the Berlin definition [31] as suffering from severe ARDS (baseline PaO2/FIO2 was less than 100 mmHg, see Table 1). The initial PaCO2 of 62 mmHg was also considerably higher than that of Patient A (47 mmHg), indicating severe hypercapnia. The increased severity of the initial ARDS state leads to reduced improvement in outcomes in each case; however, the relative efficacy of the different RMs was similar to that observed with Patient A. Also, as in the case of Patient A, reduction of the final PEEP value from 10 cm H2O to 5 cm H2O resulted in all improvements in recruitment being lost on completion of each maneuver.
Model outputs for Patient B under the recruitment maneuvers given in Figure 1 . The continuous lines are outputs for PEEP = 10 cm H2O and dashed lines are outputs for PEEP = 5 cm H2O. The plot has nine panels: (A) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under MRS-10 and MRS-5, (B) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under SI-10 and SI-5, (C) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under PRM-10 and PRM-5, (D) the partial pressure of CO2 in arterial blood (mmHg) under MRS-10 and MRS-5, (E) the partial pressure of CO2 in arterial blood (mmHg) under SI-10 and SI-5, (F) the partial pressure of CO2 in arterial blood (mmHg) under PRM-10 and PRM-5, (G) dynamic compliance of the lung (ml mbar−1) under MRS-10 and MRS-5, (H) dynamic compliance of the lung (ml mbar−1) under SI-10 and SI-5, (I) dynamic compliance of the lung (ml mbar−1) under PRM-10 and PRM-5. CO2, carbon dioxide; MRS, maximum recruitment strategy; O2, oxygen; PEEP, positive end-expiratory pressure; PRM, prolonged recruitment maneuver; SI, sustained inflation.
Comparative evaluation on three other patients
From Table 1, and using the Berlin definition, [31], Patient C would be considered as suffering from mild ARDS (baseline PaO2/FIO2 was equal 261 mmHg), Patient D as suffering from moderate ARDS (baseline PaO2/FIO2 was equal to 138 mmHg) and Patient E suffering from severe ARDS (baseline PaO2/FIO2 was equal to 65 mmHg). For patients C, D and E (Figures 6, 7 and 8 respectively) the MRS followed the pattern of the outcomes generated in Patients A and B. Unlike with Patients A and B however, the post-RM values of PaO2/FIO2 were maintained at their maximum values for all three patients for a final PEEP setting of 10 cm H2O in MRS-10 (Figures 6A, 7A, 8A). The SI maneuver produced a modest change in overall PaCO2 (Figures 6E, 7E, 8E) for all three patients.
Model outputs for Patient C under the recruitment maneuvers given in Figure 1 . The continuous lines are outputs for PEEP = 10 cm H2O and dashed lines are outputs for PEEP = 5 cm H2O. The plot has nine panels: (A) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under MRS-10 and MRS-5, (B) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under SI-10 and SI-5, (C) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under PRM-10 and PRM-5, (D) the partial pressure of CO2 in arterial blood (mmHg) under MRS-10 and MRS-5, (E) the partial pressure of CO2 in arterial blood (mmHg) under SI-10 and SI-5, (F) the partial pressure of CO2 in arterial blood (mmHg) under PRM-10 and PRM-5, (G) dynamic compliance of the lung (ml mbar−1) under MRS-10 and MRS-5, (H) dynamic compliance of the lung (ml mbar−1) under SI-10 and SI-5, (I) dynamic compliance of the lung (ml mbar−1) under PRM-10 and PRM-5. CO2, carbon dioxide; MRS, maximum recruitment strategy; O2, oxygen; PEEP, positive end-expiratory pressure; PRM, prolonged recruitment maneuver; SI, sustained inflation.
Model outputs for Patient D under the recruitment maneuvers given in Figure 1 . The continuous lines are outputs for PEEP = 10 cm H2O and dashed lines are outputs for PEEP = 5 cm H2O. The plot has nine panels: (A) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under MRS-10 and MRS-5, (B) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under SI-10 and SI-5, (C) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under PRM-10 and PRM-5, (D) the partial pressure of CO2 in arterial blood (mmHg) under MRS-10 and MRS-5, (E) the partial pressure of CO2 in arterial blood (mmHg) under SI-10 and SI-5, (F) the partial pressure of CO2 in arterial blood (mmHg) under PRM-10 and PRM-5, (G) dynamic compliance of the lung (ml mbar−1) under MRS-10 and MRS-5, (H) dynamic compliance of the lung (ml mbar−1) under SI-10 and SI-5, (I) dynamic compliance of the lung (ml mbar−1) under PRM-10 and PRM-5. CO2, carbon dioxide; MRS, maximum recruitment strategy; O2, oxygen; PEEP, positive end-expiratory pressure; PRM, prolonged recruitment maneuver; SI, sustained inflation.
Model outputs for Patient E under the recruitment maneuvers given in Figure 1 . The continuous lines are outputs for PEEP = 10 cm H2O and dashed lines are outputs for PEEP = 5 cm H2O. The plot has nine panels: (A) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under MRS-10 and MRS-5, (B) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under SI-10 and SI-5, (C) the partial pressure of O2 to fraction of inhaled O2 ratio (mmHg) under PRM-10 and PRM-5, (D) the partial pressure of CO2 in arterial blood (mmHg) under MRS-10 and MRS-5, (E) the partial pressure of CO2 in arterial blood (mmHg) under SI-10 and SI-5, (F) the partial pressure of CO2 in arterial blood (mmHg) under PRM-10 and PRM-5, (G) dynamic compliance of the lung (ml mbar−1) under MRS-10 and MRS-5, (H) dynamic compliance of the lung (ml mbar−1) under SI-10 and SI-5, (I) dynamic compliance of the lung (ml mbar−1) under PRM-10 and PRM-5. CO2, carbon dioxide; MRS, maximum recruitment strategy; O2, oxygen; PEEP, positive end-expiratory pressure; PRM, prolonged recruitment maneuver; SI, sustained inflation.
In Patient C (Figures 6B and C) for a post-RM PEEP setting of 10 cm H2O (SI-10 and PRM-10), no improvement was observed between pre-RM and post-RM values of PaO2/FIO2 (Figure 6B and C). With the post-RM PEEP of 5 cm H2O (SI-5 and PRM-5), the PaO2/FIO2 was reduced from its pre-RM value of 270 mmHg to a post-RM value of 240 mmHg. There was little noticeable change in initial and final values for the dynamic compliance during the SI and PRM maneuvers (Figures 6H and I).
Comparing the risk of lung injury
A major issue with all RMs is the high ventilator pressures delivered to the patient, which can contribute to ventilator-associated lung injury (VALI). Table 2 shows the peak alveolar pressure (Ppeak, calculated as the average of the maximum pressure in the most highly pressurised 20% of the 100 alveolar compartments over the entire maneuver) that were delivered to each patient during the three recruitment maneuvers with final PEEP values of 10 cm H2O. It is evident from Table 2 that although the MRS results in higher values of Ppeak than those produced by the other RMs, the resulting improvement in PaO2/FIO2 is considerably better than that achieved by the other two maneuvers in all five patients.
Computing minimum necessary PEEP values for the MRS
We next investigated whether the peak and final PEEP values originally proposed for the MRS in [6,22] are indeed the minimum values necessary to maintain effective lung recruitment in our virtual patients. It should be noted that a maximum PEEP of 45 cm H2O and a final PEEP of 25 cm H2O as reported in [6,22] were only recommended values and the authors proposed the use of CT scans to guide the selection of PEEP values. Figure 9A shows the percentage of alveolar compartments recruited by the MRS in each of the five patients for different peak values of PEEP (PEEPmax). Figure 9B shows the percentage of alveolar compartments that remained open after completion of the MRS for different final values of PEEP (PEEPend). As shown in Figure 9A, a value of PEEPmax of 35 cm H2O with a fixed driving pressure of 15 cm H2O (above PEEP) was sufficient to achieve recruitment in 95% of alveolar compartments for all five patients. This value is significantly lower than the maximum PEEP value of 45 cm H2O suggested in the original publications proposing the MRS [6,22]. For PEEPend, a value of 16 cm H2O was required to maintain 95% recruitment in all five patients – this is also significantly lower than the maximum value of 25 cm H2O specified in [22], although it is slightly higher than the value of 10 cm H2O suggested in [6]. Implementation of the MRS with the minimum necessary peak and final PEEP values suggested by our analysis produced the results shown in Figure 10.
The number of recruited compartments in each of the five patients with the maximum recruitment strategy for different values of (A) PEEPmax – the maximum value of PEEP at the end of the recruitment stage and (B) PEEPend – the final value of PEEP at the end of titration stage. PEEP, positive end-expiratory pressure.
The PaO2/FIO2 ratio, PaCO2 , and dynamic compliance plots of the five patients for the MRS implemented with a PEEPmaxvalue of 31 cm H2O and a PEEPendvalue of 16 cm H2 O. MRS, maximum recruitment strategy; PaCO2, partial pressure of arterial carbon dioxide; PaO2/FIO2, ratio of partial pressure of oxygen in arterial blood to fraction of oxygen in inspired air; PEEP, positive end-expiratory pressure; PEEPend, PEEP value at the end of simulation; PEEPmax, maximum PEEP during a recruitment maneuver.
Discussion
The marked improvement in PaO2/FIO2 seen in all five patients following the application of the MRS is striking, and might be explained as follows. During inspiration, a normal lung increases its volume uniformly, as almost all of its compartments have the same dynamic elastance and homogenous structure. This is not true in the case of diseased lungs, however. Gattinoni and colleagues [32] have shown that the ARDS lung is characterized by a small functional volume termed the ‘baby lung’, which stays open throughout the respiratory cycle. At end-expiration, an adequate PEEP can maintain some of the recruited lung regions open for the next respiratory cycle to take place. Therefore, selecting an appropriate recruitment strategy and modifying the PEEP applied at the end of an intensive ventilation period should increase and maintain the size of the baby lung and contribute to improving the gas exchange. As shown in Figure 9, in the simulator almost 100% of the alveolar compartments are recruited during a maximal recruitment maneuver, while 80% of the lung is recruited during a PRM or a SI maneuver. However, the last two maneuvers are followed by an immediate derecruitment, whereas the MRS is able to maintain alveolar recruitment for a significant period of time.
It should be noted that for each patient, apart from the variation in the ventilator pressure during the RM, no other parameters such as the ventilator settings of IE ratio, ventilation rate (VR), or FIO2 were changed. This explains the difference in outputs (Figure 9) between patients who each had most of their alveolar units recruited (Figure 8).
The results also display the presence of several interesting phenomena within the lung during the implementation of the various RM. For all RMs, a PEEP level of 10 cm H2O with identical driving pressure at the end of recruitment yielded an improved PaO2/FIO2 in comparison to that achieved with a PEEP level of 10 cm H2O before the RM was instigated. This implies that a higher level of oxygenation could be maintained with the same PEEP if an RM is utilized. In some cases (Patients A, B and E), a lower level of post-RM PEEP (5 cm H2O), was enough to maintain the PaO2/FIO2 at similar levels to that achieved with a pre-RM PEEP of 10 cm H2O.
During the PRM, large fluctuations were clearly noticeable in PaCO2 values in all patients. As expected, these coincided with the change in driving pressure that the PRM produced. A reduction in driving pressure increased the PaCO2 levels while an increase in driving pressure reduced PaCO2 levels. Furthermore, using MRS with a final PEEP of 10 cm H2O showed a post RM improvement in PaCO2 in all cases. It is highly likely that the above is due to the improved gas exchange following the recruitment of previously de-recruited units.
It is interesting that, in all cases, an increase in ventilator pressure caused a rise in PaCO2 initially. This is possibly due to the increasing pressure initially increasing pulmonary dead space, such that for the same minute ventilation, more ventilation was wasted, and consequently, less CO2 was eliminated.
The two patients with severe ARDS (Patients B and E) exhibited different responses to the same RM. For example, PaO2/FIO2 dropped significantly post-RM in Patient B for MRS-10, whereas in Patient E PaO2/FIO2 was maintained at a higher level at the end of the maneuver. This reflects the variations that can exist within the ARDS population and provides an example of different pathologies (for example varying distributions of TOPs in ARDS patient [33]), presenting with similar symptoms (in this case similar initial PaO2/FIO2 values). However, as seen in Figures 8 and 9, sufficient oxygen and recruitment could be attained in Patient E with lower inspiratory pressures than those required in Patient B. These results strongly motivate the development of patient-specific ventilation strategies, evaluated by individual PaO2 changes, rather than focusing solely on general algorithms.
The implemented model has a number of limitations. The model does not individually consider attributes such as superimposed pressure, the vertical gravitational affect; or surface tension changes on the alveolar and airway walls. Their effects have instead been lumped into the governing equation for the pressure volume relationship of individual alveolar units via the parameter Pext, which was determined individually for each alveolar unit within each individual patient during the model configuration stage (see Additional file 1). Effects of overdistension of alveoli have not been modeled and the model also assumes a fixed cardiac output. Therefore, attributes that may be associated with alveolar overdistension, namely, right ventricular impairment, reduced oxygen delivery and increased impact of venous shunting are presently not considered. This explains the smaller than expected rise in PaCO2 values [22] observed in our simulation during the recruitment phase of the MRS. Damage to the alveolar-capillary membrane, which can introduce and increase the bacterial and cytokine presence in the systemic circulation [34], and cause further inflammatory responses, is also not currently included in the model. Although the model can determine pulmonary function outcomes (corresponding to respiratory mechanics), information about clinical outcomes associated with RM, such as mortality, cannot be acquired. However, the model does allow for observations to changes in hemodynamic parameters (see Additional file 1 for relevant model equations and Additional file 3 for some examples), and also includes hysteresis (through the inclusion of parameters affecting the alveolar compliance directly, and through time-varying and pressure-dependant changes in the airway resistances (see Additional file 1)), hypoxic pulmonary vasoconstriction, ventilation perfusion mismatch and cyclical collapse-reopening.
The results presented here show the potential of computational simulation to offer an alternative to large-scale clinical trials which have, to date, failed to answer many key questions, such as:
-
What level of PEEP should be applied to maintain recruitment in newly opened alveoli?
-
What inspiratory pressures need to be maintained to recruit alveoli?
-
What are the values and distributions of critical opening times?
A significant limitation with conducting clinical trials is that it is very difficult to compare the results of different studies due to the different cohort of patients included in the trials and other interstudy variations. It should also be noted that there is no evidence of whether the development of atelectasis itself has an adverse affect on the patient. Permissive atelectasis with lower PEEP may be a less deleterious option than risking lung injury using higher PEEP and/or higher tidal volumes in some patients [35]. The optimal PEEP for many RMs is still to be determined and the most recent study in this area by Chiumello et al. [30] demonstrated the importance of considering RM timings with applying an RM [30,36].
Apart from the commonly administered RMs considered here, a number of other RMs have recently been proposed that should theoretically improve alveolar function but cannot be tested due to their experimental nature and the lack of patient data that would lend support for their introduction into practice [37-39]. The role of RMs also extends beyond ARDS patients; the administration of RMs has been shown to reduce markers of lung stress in patients following general anesthesia [40-43]. In both cases, computational simulation could play a key role in establishing the potential benefits of RMs and in the design of optimized patient- and disease-specific protocols.
An important question that needs to be addressed in future work in this area is the effect of RMs on the other organs [44]. Mortality linked to ALI and ARDS often involves multiple organ failure, as the disease is not limited to the lungs. RMs also alter the working physiology of surrounding organs, and their impact on the heart and circulation cannot currently be accurately measured [45,46]. Changes in intrathoracic and transpulmonary pressures have a secondary effect of decreasing venous return and cardiac preload, so patients also suffer the additional stress of a reduction in cardiac output during the procedure [47,48]. It is difficult to compare the risks associated with different RMs, although a stepwise RM has been shown to have a smaller effect on cardiac output than the more widely used SI [44,49]. In this study, we focused on the effect of recruitment maneuvers on the pulmonary system. However, we are developing hemodynamic computer simulations of the complete cardiac system that also integrate the pulmonary and systemic circulation in order to better understand these issues. Such research tools could offer an invaluable alternative or complement to time-consuming and expensive clinical trials, and could provide answers to many key unanswered questions about the clinical efficacy of RMs in health and disease.
Conclusions
Our results indicate that there is significant scope for reducing the high levels of PEEP specified in the MRS without compromising its effectiveness in maintaining adequate oxygenation. More generally, the study highlights the huge potential of computer simulation to assist in evaluating the clinical efficacy of RMs in health and disease, in understanding their modes of operation, and in supporting clinicians in the rational design of improved treatment strategies.
Key messages
-
We present the first application of computer simulation to evaluate the relative efficacy of different recruitment maneuvers with different levels of PEEP for ARDS patients.
-
Three recruitment maneuvers (SI, MRS followed by a titrated PEEP and PRM) were applied and evaluated on identical ‘virtual patients’.
-
The MRS with titrated PEEP exhibited the most prolonged improvement in oxygenation, but also exposed the alveolar compartments to the highest peak pressures.
-
Using the simulator, we were able to establish that there is significant scope for reducing the levels of PEEP specified in the MRS without compromising its effectiveness.
Abbreviations
- ALI:
-
acute lung injury
- ARDS:
-
acute respiratory distress syndrome
- CO:
-
cardiac output (l min−1)
- CO2 :
-
carbon dioxide
- CT:
-
computed tomography
- FIO2 :
-
fraction of inspired oxygen
- Hb:
-
hemoglobin content in blood (gm l−1)
- IE:
-
inspiratory to expiratory ratio
- MRS:
-
maximum recruitment strategy
- O2 :
-
oxygen
- P:
-
partial pressure (kPa) for example PO2, partial pressure of oxygen
- PaCO2 :
-
partial pressure of carbon dioxide in arterial blood
- PaO2 :
-
partial pressure of oxygen in arterial blood
- PaO2/FIO2 :
-
ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen
- PEEP:
-
positive end-expiratory pressure (cm H2O)
- PEEPend :
-
PEEP value at the end of simulation
- PEEPmax :
-
maximum PEEP during a recruitment maneuver
- Ppeak :
-
peak alveolar pressure
- PRM:
-
prolonged recruitment maneuver
- Pv:
-
ventilator pressure
- PvCO2 :
-
partial pressure of carbon dioxide in venous blood
- PvO2 :
-
partial pressure of oxygen in venous blood
- Qs/Qt:
-
shunt
- RM:
-
recruitment maneuver
- RQ:
-
respiratory quotient
- SI:
-
sustained inflation
- TOP:
-
threshold opening pressure; extra pressure within the collapsed alveolar compartment
- VALI:
-
ventilator-associated lung injury
- VO2 :
-
oxygen consumption (ml min−1)
- VR:
-
respiration rate set by the ventilator (breaths per minute, bpm)
References
Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–41.
Kushimoto S, Endo T, Yamanouchi S, Sakamoto T, Ishikura H, Kitazawa Y, et al. Relationship between extravascular lung water and severity categories of acute respiratory distress syndrome by the Berlin definition. Crit Care. 2013;17:R132.
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86.
Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–11.
Hedenstierna G, Rothen HU. Atelectasis formation during anesthesia: causes and measures to prevent it. J Clin Monit Comput. 2000;16:329–35.
de Matos GF, Stanzani F, Passos RH, Fontana MF, Albaladejo R, Caserta RE, et al. How large is the lung recruitability in early acute respiratory distress syndrome: a prospective case series of patients monitored by computed tomography. Crit Care. 2012;16:R4.
Investigators ART. Rationale, study design, and analysis plan of the Alveolar Recruitment for ARDS Trial (ART): study protocol for a randomized controlled trial. Trials. 2012;13:153.
Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, et al. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med. 2008;178:1156–63.
Hickling KG. The pressure-volume curve is greatly modified by recruitment: a mathematical model of ARDS lungs. Am J Respir Crit Care Med. 1998;158:194–202.
Massa CB, Allen GB, Bates JH. Modeling the dynamics of recruitment and derecruitment in mice with acute lung injury. J Appl Physiol. 2008;105:1813–21.
Ma B, Bates JH. Modeling the complex dynamics of derecruitment in the lung. Ann Biomed Eng. 2010;38:3466–77.
Sundaresan A, Chase JG, Shaw GM, Chiew YS, Desaive T. Model-based optimal PEEP in mechanically ventilated ARDS patients in the Intensive Care Unit. Biomed Eng Online. 2011;10:1–18.
Spieth PM, Gama de Abreu M. Lung recruitment in ARDS: we are still confused, but on a higher PEEP level. Crit Care. 2012;16:108.
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–45.
Oczenski W, Hormann C, Keller C, Lorenzl N, Kepka A, Schwarz S, et al. Recruitment maneuvers after a positive end-expiratory pressure trial do not induce sustained effects in early adult respiratory distress syndrome. Anesthesiology. 2004;101:620–5.
Brower RG, Morris A, MacIntyre N, Matthay MA, Hayden D, Thompson T, et al. Effects of recruitment maneuvers in patients with acute lung injury and acute respiratory distress syndrome ventilated with high positive end-expiratory pressure. Crit Care Med. 2003;31:2592–7.
Farias LL, Faffe DS, Xisto DG, Santana MC, Lassance R, Prota LF, et al. Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J Appl Physiol. 2005;98:53–61.
Meade MO, Cook DJ, Griffith LE, Hand LE, Lapinsky SE, Stewart TE, et al. A study of the physiologic responses to a lung recruitment maneuver in acute lung injury and acute respiratory distress syndrome. Respir Care. 2008;53:1441–9.
Musch G, Harris RS, Vidal Melo MF, O’Neill KR, Layfield JD, Winkler T, et al. Mechanism by which a sustained inflation can worsen oxygenation in acute lung injury. Anesthesiology. 2004;100:323–30.
Villagra A, Ochagavia A, Vatua S, Murias G, Del Mar FM, Lopez Aguilar J, et al. Recruitment maneuvers during lung protective ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:165–70.
Rzezinski AF, Oliveira GP, Santiago VR, Santos RS, Ornellas DS, Morales MM, et al. Prolonged recruitment manoeuvre improves lung function with less ultrastructural damage in experimental mild acute lung injury. Respir Physiol Neurobiol. 2009;169:271–81.
Borges JB, Okamoto VN, Matos GF, Caramez MP, Arantes PR, Barros F, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:268–78.
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–6.
Das A, Gao Z, Menon PP, Hardman JG, Bates DG. A systems engineering approach to validation of a pulmonary physiology simulator for clinical applications. J R Soc Interface. 2011;8:44–55.
Das A, Prathyush PM, Hardman JG, Bates DG. Optimization of mechanical ventilator settings for pulmonary disease states. IEEE Trans Biomed Eng. 2013;60:1599–607.
Wang W, Das A, Ali T, Cole O, Chikhani M, Haque M, et al. Can computer simulators accurately represent the pathophysiology of individual COPD patients? Intensive Care Med Exp. 2014;2:23.
Bates JH, Irvin CG. Time dependence of recruitment and derecruitment in the lung: a theoretical model. J Appl Physiol. 2002;93:705–13.
Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS. Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med. 1999;25:1297–301.
Nirmalan M, Willard T, Columb MO, Nightingale P. Effect of changes in arterial-mixed venous oxygen content difference (C(a-v)O2) on indices of pulmonary oxygen transfer in a model ARDS lung. Br J Anaesth. 2001;86:477–85.
Chiumello D, Coppola S, Froio S, Mietto C, Brazzi L, Carlesso E, et al. Time to reach a new steady state after changes of positive end expiratory pressure. Intensive Care Med. 2013;39:1377–85.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31:776–84.
Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, et al. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med. 2001;164:131–40.
Halbertsma FJ, Vaneker M, Pickkers P, Neeleman C, Scheffer GJ, van der Hoeven JG. A single recruitment maneuver in ventilated critically ill children can translocate pulmonary cytokines into the circulation. J Crit Care. 2010;25:10–5.
Steinberg KP, Kacmarek RM. Respiratory controversies in the critical care setting. Should tidal volume be 6 mL/kg predicted body weight in virtually all patients with acute respiratory failure? Respir Care. 2007;52:556–67.
Brower RG. Time to reach a new equilibrium after changes in PEEP in acute respiratory distress syndrome patients. Intensive Care Med. 2013;39:2053–5.
Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med. 2001;164:122–30.
Lim CM, Koh Y, Park W, Chin JY, Shim TS, Lee SD, et al. Mechanistic scheme and effect of “extended sigh” as a recruitment maneuver in patients with acute respiratory distress syndrome: a preliminary study. Crit Care Med. 2001;29:1255–60.
Riva DR, Contador RS, Baez-Garcia CS, Xisto DG, Cagido VR, Martini SV, et al. Recruitment maneuver: RAMP versus CPAP pressure profile in a model of acute lung injury. Respir Physiol Neurobiol. 2009;169:62–8.
Tusman G, Bohm SH, Melkun F, Staltari D, Quinzio C, Nador C, et al. Alveolar recruitment strategy increases arterial oxygenation during one-lung ventilation. Ann Thorac Surg. 2002;73:1204–9.
Magnusson L, Tenling A, Lemoine R, Hogman M, Tyden H, Hedenstierna G. The safety of one, or repeated, vital capacity maneuvers during general anesthesia. Anesth Analg. 2000;91:702–7.
Magnusson L, Spahn DR. New concepts of atelectasis during general anaesthesia. Br J Anaesth. 2003;91:61–72.
Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Prevention of atelectasis during general anaesthesia. Lancet. 1995;345:1387–91.
Guerin C, Debord S, Leray V, Delannoy B, Bayle F, Bourdin G, et al. Efficacy and safety of recruitment maneuvers in acute respiratory distress syndrome. Ann Intensive Care. 2011;1:9.
Monge García M, Cano AG, Romero MG, Monrové J. Respiratory and hemodynamic changes during lung recruitment maneuvering through progressive increases and decreases in PEEP level. Med Intensiva. 2012;36:77–88.
Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, et al. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology. 2002;96:795–802.
Nielsen J, Ostergaard M, Kjaergaard J, Tingleff J, Berthelsen PG, Nygard E, et al. Lung recruitment maneuver depresses central hemodynamics in patients following cardiac surgery. Intensive Care Med. 2005;31:1189–94.
Pinsky MR. The hemodynamic consequences of mechanical ventilation: an evolving story. Intensive Care Med. 1997;23:493–503.
Odenstedt H, Lindgren S, Olegard C, Erlandsson K, Lethvall S, Aneman A, et al. Slow moderate pressure recruitment maneuver minimizes negative circulatory and lung mechanic side effects: evaluation of recruitment maneuvers using electric impedance tomography. Intensive Care Med. 2005;31:1706–14.
Acknowledgements
This work was supported by the UK Engineering and Physical Sciences Research Council (EP/F057016/2, EP/F057059/1, EP/1036680/2), and the UK Medical Research Council (G1002017, MR/K019783).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AD, DGB and JGH conceived, designed and coordinated the study. AD, WW, DGB and JGH developed the computational simulator and implemented the computer simulations. AD, OC, MC, TA, MH and JGH contributed to the acquisition, analysis, and interpretation of data and protocols. OC, MC, TA and JGH contributed to clinical evaluation of the results of the study. All authors contributed to drafting, revising and finalizing the manuscript. All authors read and approved the final manuscript.
Additional files
Additional file 1:
Simulation model description and equations. The file describes in detail the simulation model employed in the paper.
Additional file 2:
Configuring the simulator to patient data using global optimization and the tabulated configuration results. The file presents the optimization strategy used in matching the model to the ARDS patient data and the resultant parameter values for the different models tabulated for each patient.
Additional file 3:
Additional figures and experiments. The file reports the effects on PaO2/FIO2 of changes in hemoglobin levels, cardiac output, and FIO2 for the MRS-10 RM applied to patient A and presents some further model validation results.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Das, A., Cole, O., Chikhani, M. et al. Evaluation of lung recruitment maneuvers in acute respiratory distress syndrome using computer simulation. Crit Care 19, 8 (2015). https://doi.org/10.1186/s13054-014-0723-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-014-0723-6