Abstract
Background
The variants of nucleoporins are extremely rare in hereditary steroid-resistant nephrotic syndrome (SRNS). Most of the patients carrying such variants progress to end stage kidney disease (ESKD) in their childhood. More clinical and genetic data from these patients are needed to characterize their genotype–phenotype relationships and elucidate the role of nucleoporins in SRNS.
Methods
Four patients of SRNS carrying biallelic variants in the NUP93, NUP107 and NUP160 genes were presented. The clinical and molecular genetic characteristics of these patients were summarized, and relevant literature was reviewed.
Results
All four patients in this study were female and initially presented with SRNS. The median age at the onset of the disease was 5.08 years, ranging from 1 to 10.5 years. Among the four patients, three progressed to ESKD at a median age of 7 years, ranging from 1.5 to 10.5 years, while one patient reached stage 3 chronic kidney disease (CKD3). Kidney biopsies revealed focal segmental glomerulosclerosis in three patients. Biallelic variants were detected in NUP93 in one patient, NUP107 in two patients, as well as NUP160 in one patient respectively. Among these variants, five yielded single amino acid substitutions, one led to nonsense mutation causing premature termination of NUP107 translation, one caused a single nucleotide deletion resulting in frameshift and truncation of NUP107. Furthermore, one splicing donor mutation was observed in NUP160. None of these variants had been reported previously.
Conclusion
This report indicates that biallelic variants in NUP93, NUP107 and NUP160 can cause severe early-onset SRNS, which rapidly progresses to ESKD. Moreover, these findings expand the spectrum of phenotypes and genotypes and highlight the importance of next-generation sequencing in elucidating the molecular basis of SRNS and allowing rational treatment for affected individuals.
Similar content being viewed by others
Introduction
Nephrotic syndrome (NS) is common in children. Most of them respond well to glucocorticoid, while a minority are steroid-resistant [1,2,3]. Steroid-resistant nephrotic syndrome (SRNS) primarily presents as focal segmental glomerulosclerosis (FSGS), which is associated with an unfavorable renal prognosis [4, 5]. Despite extensive research efforts, the etiology and pathogenesis of SRNS remain incompletely understood. Recent studies have identified an increasing number of genes associated with the development of SRNS, totally accounting for 29.5% of SRNS as reported by Hildebrandt [6]. About 66% of SRNS occurring within the first year of their life are caused by monogenic variants [7]. Thus far, more than 50 genes have been identified for SRNS worldwide [8]. Most of these gene products are located in slit diaphragm, cytoskeleton, mitochondria, lysosome and endocytic compartment of podocytes [8, 9].
Currently, variants in several nucleoporins (NUPs) have been identified as the underlying causes of SRNS. The nuclear pore complex includes a variety of NUPs that are distributed across the nuclear envelope and play a critical role in macro-molecular transportation between nucleus and cytoplasm [10, 11]. However, the reported cases of such variants are limited. Therefore, additional clinical and genetic data are required to characterize genotype–phenotype relationships and elucidate the role of NUPs in SRNS. This study aims to summarize the clinical and molecular genetic characteristics of four cases of SRNS caused by variants in NUP93, NUP107 and NUP160 genes with the intention of providing new insights into this rare disease.
Patients and methods
Case presentation
Case 1 was a 1-year-old female patient who initially presented with edema on the lower limbs and eyelids. She developed normally and was well-nourished. Upon physical examination, the patient presented with hypertension with a blood pressure of 115/68mmHg (≥95th percentile + 12 mmHg), while no other evident abnormalities were observed. The urine test showed proteinuria 3+ and hematuria 2+. The serum creatinine was 0.58mg/dl (eGFR 57ml/min/1.73m2). Subsequent renal biopsy revealed FSGS. Despite receiving treatment with a combination of prednisone and cyclosporin A, the patient remained nephrotic after approximately six months of treatment and eventually progressed to end-stage kidney disease (ESKD). Whole-exome sequencing (WES) identified biallelic variants in the NUP93 gene.
Case 2 was a 5.3-year-old female patient who was hospitalized due to edema on lower limbs, with blood pressure of 141/108mmHg (≥95th percentile + 12 mmHg). Physical examination revealed no other significant abnormalities. Her growth and development were normal without any malformations. Urine examination showed hematuria 2+ and proteinuria 3+, while the serum creatinine level was 0.4mg/dl (eGFR 110ml/min/1.73m2). Despite four weeks of treatment with prednisone, the patient showed no response. So renal biopsy and whole-exome sequencing (WES) were recommended. The renal biopsy revealed global sclerosis in 11 glomeruli and segmental glomerulosclerosis in 4 among total 30 glomeruli. WES identified biallelic variants in the NUP107 gene.
Case 3 involved a female child aged 10.5 years who presented with chest tightness and nausea. Physical examination showed hypertension with blood pressure ranging from 151/108 mmHg to 200/149 mmHg (≥95th percentile + 12 mmHg). There were no edema or growth and developmental delays. The plasma renin level was within the normal range, and the whole aortic computer tomography angiography (CTA) showed no remarkable findings. Echocardiography showed left ventricular hypertrophy with left ventricular ejection fractions (LVEF) at 38.7%. Ultrasound examination revealed no abnormalities in the kidneys, uterus, or ovaries. Urine analysis revealed a significant proteinuria level of 4+ without hematuria. The level of serum creatinine was 6.26 mg/dl (eGFR 9.6ml/min/1.73m2). Subsequently, the patient underwent peritoneal dialysis, and whole-exome sequencing (WES) identified biallelic variants in the NUP107 gene.
Case 4 was a 3.5-year-old girl presenting with eyelid edema and hypertension. Urinalysis revealed proteinuria 4+ and hematuria 2+. The patient showed mild intellectual disability, as evidenced by a full-scale score of 48 on the Wechsler Preschool and Primary Scale of Intelligence (WPPSI), indicating an intellectual delay. A comprehensive neuropsychiatric assessment indicated delays in both gross and fine motor skills, adaptive abilities, language development and social behavior. Cognitive assessment demonstrated abnormal cognitive play and social communication behaviors. The Autism Behavior Checklist (ABC) confirmed the presence of autistic behaviors, with the Childhood Autism Rating Scale (CARS) indicating mild to moderate autism spectrum disorder. The ultrasound examination revealed the presence of a cord-like uterine, structure measuring 13 mm in length and 3 mm in anteroposterior diameter. The baseline level of serum creatinine was 0.34mg/dl (eGFR 114ml/min/1.73m2). However, there was an increase level in serum creatinine to 0.98mg/dl (eGFR 42ml/min/1.73m2) during the follow-up, suggesting the development of stage 3 chronic kidney disease (CKD3). Renal biopsy revealed FSGS. The patient was initially treated with prednisone and tacrolimus, which were discontinued due to no response. Thereafter WES identified biallelic variants in the NUP160 gene.
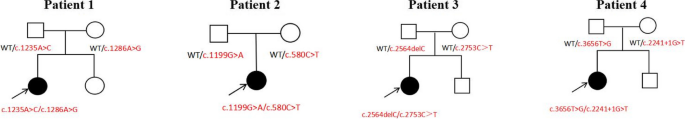
All of these cases had no family history of the disease. The specific family diagram is illustrated in Fig. 1.
Detection and analysis of nucleoporin gene variants
After obtaining the informed consent of the patients’ parents, blood samples were collected from both the patients and their parents. DNA was extracted from peripheral white blood cells by using the MagPure Buffy Coat DNA Midi KF Kit according to manufacturer’s standard protocol. Genomic DNA was broken into 100–500 bp fragments by BGI’s enzyme kit (Segmentase, BGI), and 280–320 bp fragments were collected by magnetic bead. PCR amplification was performed using universal primers complementary to the adapter sequence to form a sequencing library. All amplified libraries were hybridized with exome capture probes (Agilent, USA) and sequenced. The clean reads derived from targeted sequencing and filtering were then aligned to the human genome reference (hg19) by using the BWA. Single-nucleotide variants (SNVs) and INDELs were detected with Sentieon (the same algorithm with GATK) analysis. The pathogenic variants were screened by ClinVar, OMIM, and HGMD databases. Functional prediction of missense mutations was conducted using PolyPhen-2, SIFT, and MutationTaster. All variants and potential pathogenic variants were validated via conventional Sanger sequencing methods.
Results
Clinical and renal pathological features and long-term outcome
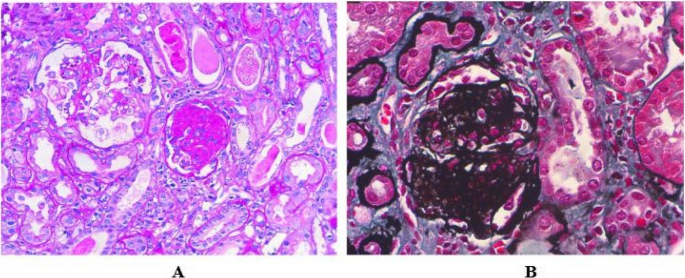
The clinical and laboratory data of four patients are shown in Table 1. All patients initially presented with massive proteinuria with or without hematuria. Patient 2 and 4 had normal serum creatinine levels and eGFR at the onset, while patient 1 and 3 had elevated levels of serum creatinine and impaired renal function at beginning. Moreover, patient 3 presented with heart failure as an extra-renal manifestation, and patient 4 showed mild intellectual disability and uterine dysplasia. Renal biopsy revealed focal and segmental glomerular sclerosis (Fig. 2).
Patient 1 rapidly progressed to ESKD within six months of onset and regrettably passed away at 1.5 years of age. Patient 2 developed renal failure at 9 years old and subsequently underwent renal transplantation. Patient 3 was diagnosed with ESKD at the initial presentation and was on maintenance peritoneal dialysis. And patient 4 was diagnosed with CKD3 at 5 years old during the follow-up period (Table 1).
Identification of pathological variants in the NUP genes
Biallelic variants in the NUP genes were detected by WES in all patients. Specifically, patient 1 had biallelic missense mutations c.1235A>C (p.Tyr412Ser) and c.1286A>G (p.Tyr429Cys) in the NUP93 gene. Patient 2 showed biallelic variants c.1199G>A (p.Gly400Glu) and c.580C>T (p.Arg194*) in the NUP107 gene, and patient 3 also had biallelic variants c.2564delC (p.Pro855fsTer*23) and c.2753C>T (p.Pro918Leu) in NUP107, resulting in amino acid substitutions and proteins truncation. Patient 4 was identified with a missense mutation c.3656T>G (p.Leu1219Trp) and a splicing donor mutation c.2241+1G>T in the NUP160 gene. Ployphen2, MutationTaster analysis indicated that all the missense mutations were harmful (Table 2).
Clinical and molecular genetic characterization of reported patients carrying biallelic variants of NUP genes
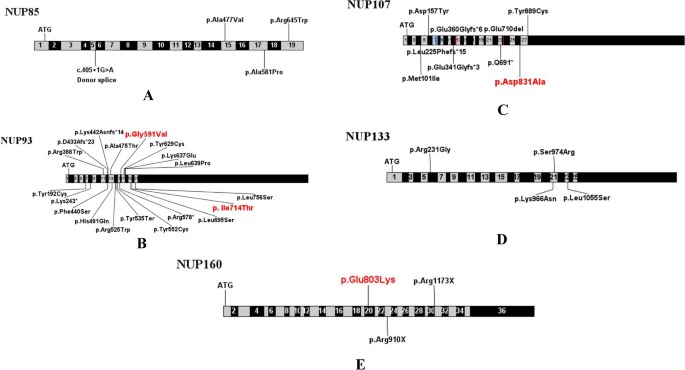
To date, a total of 60 cases of NUP-associated SRNS have been reported worldwide (Table 3). Among these reported cases, variants of NUP93 gene were observed in 24 individuals (40%), while NUP107 variants in 20 individuals (33.3%). Additionally, there were also eight cases (13.3%) with NUP133 mutations, four cases (6.7%) with NUP85 mutations, one case (1.7%) with NUP205 mutation and three cases (5%) with NUP160 mutations. The majority of these reported cases initially presented with SRNS and eventually progressed to ESKD. The detailed information of these variants were presented in Table 3 and Fig. 3, involving 22 variants in NUP93, 11 in NUP107, 6 in NUP133, 4 in NUP85, 1 in NUP205, and 3 in NUP160. Missense mutations were the majority of all NUP mutations.
Discussion
NUPs are situated in the nuclear membrane, and approximately 30 kinds of NUPs assemble to form the nuclear pore complex (NPC), serving as a special and unique transport channel across the nuclear membrane [29]. The NPC comprises a core scaffold, a nuclear basket, transmembrane nucleoporins and a central selective channel [11]. NUP93 is located in the inner ring of the core scaffold, while NUP107 and NUP160 are located in the outer ring also known as the “Y” complex [30]. NUP93, NUP107 and NUP160 interact with other NUPs and participate in the assembly of NPCs, which are crucial for trans-nuclear membrane transportation. Any alterations in the NUPs or defects in transport channels can hinder transmembrane transport, resulting in the abnormal accumulation of materials in nucleus or cytoplasm. Consequently, variants in NUPs are associated with a variety of diseases [31]. Previous studies have linked NUP93, NUP107 and NUP160 to cancer, congenital heart disease, neurological diseases and gonadal dysgenesis [32,33,34,35,36]. Recently several SRNS cases caused by variants in NUPs have been reported and attracted the attention of pediatric nephrologists.
Previous studies had indicated that variants in NUP93 and NUP107 were the most frequent mutated NUP genes in individuals with SRNS. Patients with variants in these genes rapidly progressed to ESKD at a young age. Conversely, cases of SRNS with NUP160 variants presented relatively later and progressed to ESKD at an older age than those with NUP93 and NUP107 variants. Furthermore, patients with variants in NUP93, NUP107, and NUP133 were more likely to present with extra-renal manifestations.
Previously reported NUP variants included missense, nonsense, frameshift, small deletion and splicing mutations, among which missense mutations were found to be the most prevalent. Notably, variant hotspots were found in NUP93 and NUP107 genes. Specifically, the c.1772G>T (p.G591V) variant in NUP93 was identified as a pathogenic European founder variant and the c.1537+ 1G>A (deletion of exon 13) was found to be another pathogenic variant in Germans [13]. The c.2492A > C (D831A) variant in the NUP107 gene was considered to be unique to East Asians [23]. Furthermore, the variant c.2407G>A (p.Glu803Lys) in the NUP160 may be a hotspot variant in Asian according to previous reports.
The specific mechanisms underlying steroid-resistant nephrotic syndrome (SRNS) caused by NUPs remain unclear. A previous study demonstrated the presence of glomerular dysplasia and abnormal podocyte processes in zebrafish models with NUP107 knockdown [22]. Knockdown of NUP160 gene resulted in podocyte proliferation incapability, increased apoptosis, autophagy and cell migration, and altered expression and localization of nephrin, podocin, CD2AP and α-actinin-4 [37]. Furthermore, Braun [25] discovered upregulation of cdc42 in podocytes with knockout of NUP85, NUP107, and NUP133 genes. In addition, knockdown of NUP93 gene in podocytes disrupted BMP7-dependent SMAD signaling, potentially implicating it in the pathogenesis of SRNS [16].
Next-generation sequencing (NGS) is a powerful and high-throughput genetic test helping to identify the causative variants of genetic diseases. This technique offers valuable insights and evidence for the purposes of diagnosis, treatment, and genetic counseling [38,39,40]. Targeted panel sequencing is faster and cheaper than WES, making it a preferred choice for patients whose clinical data are highly consistent with a specific genetic defect or a known group of genes [41]. However, the limitation of targeted panel is that only genes within the panel are sequenced, which may result in missing genes located beyond the panel. As the cost of sequencing becomes cheaper and cheaper, WES has been widely accepted especially for patients with ambiguous phenotypes that make it difficult to apply targeted panel sequencing [42].
Cystic kidney diseases and renal tubulopathies subjected to WES result in relatively high rates of positive findings [43]. Generally speaking, an earlier onset of a disease is associated with a higher possibility of genetic etiology. Identifying the causes of sporadic non-syndromic SRNS is challenging. Overall, WES should be considered for SRNS patients with multisystem involvement as well as with unexplained clinical manifestations. In this study, all four patients lacked specific manifestation and two of them showed multisystemic involvement. Therefore, gene variant screening was the only way to identify genetic causes. Fortunately WES uncovered definitive pathogenic biallelic variants of NUP genes, thereby the ineffective treatment of prednisone and immunosuppressants was immediately discontinued. Our report demonstrates the necessity and diagnostic utility of genetic analysis in sporadic cases of SRNS and highlights the role of NGS in understanding the molecular mechanisms of SRNS and facilitating rational and individualized treatment for patients.
This paper is believed to be the first to report on a series of Chinese cases of SRNS associated with variants of NUP genes. Furthermore, all these variants in NUP93, NUP107 and NUP160 have not been previously reported. Since this study is solely a case report, the limited number of cases prevented us from drawing conclusive phenotypic and genotypic correlations. In addition, how NUP variants cause SRNS remains unclear and requires further comprehensive molecular research.
Conclusion
In summary, this study reports four cases of sporadic SRNS caused by novel variants in the NUP93, NUP107, and NUP160 genes in Chinese children. The findings extend the spectrum of phenotypes and genotypes, and also highlight the importance of NGS in elucidating the molecular mechanisms of SRNS and allowing personalized treatment for affected individuals.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SRNS:
-
Steroid-resistant nephrotic syndrome
- ESKD:
-
End stage kidney disease
- CKD:
-
Chronic kidney disease
- CKD3:
-
Stage 3 chronic kidney disease
- NUP:
-
Nucleoporin
- WES:
-
Whole-exome sequencing
- NPC:
-
Nuclear pore complex
- NGS:
-
Next-generation sequencing
References
Trautmann A, Vivarelli M, Samuel S, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35(8):1529–61.
Downie M L, Gallibois C, Parekh R S, et al. Nephrotic syndrome in infants and children: pathophysiology and management[Z]. England: Taylor & Francis, 2017: 37, 248-258.
Tullus K, Webb H, Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc Health. 2018;2(12):880–90.
Lee JM, Kronbichler A, Shin JI, et al. Current understandings in treating children with steroid-resistant nephrotic syndrome. Pediatr Nephrol (Berlin, West). 2021;36(4):747–61.
Shin JI, Kronbichler A, Oh J, et al. Nephrotic syndrome: genetics, mechanism, and therapies. Biomed Res Int. 2018;2018:6215942–6.
Sadowski CE, Lovric S, Ashraf S, et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26(6):1279–89.
Hinkes BG, Mucha B, Vlangos CN, et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics. 2007;119(4):e907–19.
Preston R, Stuart HM, Lennon R. Genetic testing in steroid-resistant nephrotic syndrome: why, who, when and how? Pediatr Nephrol (Berlin, West). 2019;34(2):195–210.
Trautmann A, Lipska-Ziętkiewicz BS, Schaefer F. Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the podonet registry. Front Pediatr. 2018;6:200.
Lin DH, Hoelz A. The structure of the nuclear pore complex (an update). Ann Rev Biochem. 2019;88(1):725–83.
Hampoelz B, Andres-Pons A, Kastritis P, et al. Structure and assembly of the nuclear pore complex. Ann Rev Biophys. 2019;48(1):515–36.
Bierzynska A, Bull K, Miellet S, et al. Exploring the relevance of NUP93 variants in steroid-resistant nephrotic syndrome using next generation sequencing and a fly kidney model. Pediatr Nephrol (Berlin, West). 2022;37(11):2643–56.
Bezdíčka M, Štolbová Š, Seeman T, et al. Genetic diagnosis of steroid-resistant nephrotic syndrome in a longitudinal collection of Czech and Slovak patients: a high proportion of causative variants in NUP93. Pediatr Nephrol (Berlin, West). 2018;33(8):1347–63.
Rossanti R, Shono A, Miura K, et al. Molecular assay for an intronic variant in NUP93 that causes steroid resistant nephrotic syndrome. J Hum Genet. 2019;64(7):673–9.
Sandokji I, Marquez J, Ji W, et al. Identification of novel mutations and phenotype in the steroid resistant nephrotic syndrome gene NUP93: a case report. BMC Nephrol. 2019;20(1):271.
Braun DA, Sadowski CE, Kohl S, et al. Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat Genet. 2016;48(4):457–65.
Zhao B, Chen J, Liao Y, et al. Steroid-resistant nephrotic syndrome in infants caused by a novel compound heterozygous mutation of the NUP93: A CARE case report. Medicine (Baltimore). 2021;100(6):e24627.
Cason RK, Williams A, Chryst-Stangl M, et al. Collapsing focal segmental glomerulosclerosis in siblings with compound heterozygous variants in NUP93 expand the spectrum of kidney phenotypes associated with nucleoporin gene mutations. Front Pediatr. 2022;10:915174.
Acharya R, Upadhyay K. End-stage renal disease in a child with focal segmental glomerulosclerosis associated with a homozygous NUP93 variant. Clin Case Rep. 2021;9(11):e05111.
Hashimoto T, Harita Y, Takizawa K, et al. In Vivo expression of NUP93 and its alteration by NUP93 mutations causing focal segmental glomerulosclerosis. Kidney Int Rep. 2019;4(9):1312–22.
Al Riyami M S, Al Alawi I, Al Gaithi B, et al. Genetic analysis and outcomes of Omani children with steroid-resistant nephrotic syndrome. Mol Genet Genomic Med. 2023;11(9):e2201.
Miyake N, Tsukaguchi H, Koshimizu E, et al. Biallelic mutations in nuclear pore complex subunit NUP107 cause early-childhood-onset steroid-resistant nephrotic syndrome. Am J Hum Genet. 2015;97(4):555–66.
Park E, Ahn YH, Kang HG, et al. NUP107 mutations in children with steroid-resistant nephrotic syndrome. Nephrol Dial Transplan. 2017;32(6):1013–7.
Rosti RO, Sotak BN, Bielas SL, et al. Homozygous mutation in NUP107 leads to microcephaly with steroid-resistant nephrotic condition similar to Galloway-Mowat syndrome. J Med Genet. 2017;54(6):399–403.
Braun DA, Lovric S, Schapiro D, et al. Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome. J Clin Investig. 2018;128(10):4313–28.
Zhao F, Zhu JY, Richman A, et al. Mutations in NUP160 are implicated in steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2019;30(5):840–53.
Fujita A, Tsukaguchi H, Koshimizu E, et al. Homozygous splicing mutation in NUP133 causes Galloway-Mowat syndrome. Ann Neurol. 2018;84(6):814–28.
Wang Q, Gu R, Li FW, et al. Steroid-resistant nephrotic syndrome caused by nuclear pore gene NUP133 variation. Clin Genet. 2023;104(2):272–4.
Schuller AP, Wojtynek M, Mankus D, et al. The cellular environment shapes the nuclear pore complex architecture. Nature. 2021;598(7882):667–71.
Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol. 2017;18(2):73–89.
Jamali T, Jamali Y, Mehrbod M, et al. Chapter six - Nuclear Pore Complex: Biochemistry and Biophysics of Nucleocytoplasmic Transport in Health and Disease[M]//Jeon K W. International Review of Cell and Molecular Biology. Academic Press, 2011:233-286.
Ouyang X, Hao X, Liu S, et al. Expression of Nup93 is associated with the proliferation, migration and invasion capacity of cervical cancer cells. Acta Biochimica Et Biophysica Sinica. 2019;51(12):1276–85.
Pan L, Song XW, Song JC, et al. Downregulation of NUP93 aggravates hypoxia-induced death of cardiomyocytes in vitro through abnormal regulation of gene transcription. Acta Pharmacol Sin. 2023;44(5):969–83.
Nong JS, Zhou X, Liu JQ, et al. Nucleoporin 107 is a prognostic biomarker in hepatocellular carcinoma associated with immune infiltration. Cancer Med. 2023;12(9):10990–1009.
Ren Y, Diao F, Katari S, et al. Functional study of a novel missense single-nucleotide variant of NUP107 in two daughters of Mexican origin with premature ovarian insufficiency. Mol Genet Genom Med. 2018;6(2):276–81.
Tarazon E, Rivera M, Rosello-Lleti E, et al. Heart failure induces significant changes in nuclear pore complex of human cardiomyocytes. Plos One. 2012;7(11):e48957.
Wang P, Zhao F, Nie X, et al. Knockdown of NUP160 inhibits cell proliferation, induces apoptosis, autophagy and cell migration, and alters the expression and localization of podocyte associated molecules in mouse podocytes. Gene. 2018;664:12–21.
Serra G, Antona V, D’Alessandro MM, Maggio MC, Verde V, Corsello G. Novel SCNN1A gene splicing-site mutation causing autosomal recessive pseudohypoaldosteronism type 1 (PHA1) in two Italian patients belonging to the same small town. Ital J Pediatr. 2021;47(1):138.
Piro E, Schierz IAM, Antona V, et al. Neonatal hyperinsulinemic hypoglycemia: case report of kabuki syndrome due to a novel KMT2D splicing-site mutation. Ital J Pediatr. 2020;46(1):136.
Serra G, Corsello G, Antona V, et al. Autosomal recessive polycystic kidney disease: case report of a newborn with rare PKHD1 mutation, rapid renal enlargement and early fatal outcome. Ital J Pediatr. 2020;46(1):154.
Platt CD, Zaman F, Bainter W, et al. Efficacy and economics of targeted panel versus whole-exome sequencing in 878 patients with suspected primary immunodeficiency. J Allergy Clin Immunol. 2021;147(2):723–6.
Lee JY, Oh SH, Keum C, Lee BL, Chung WY. Clinical application of prospective whole-exome sequencing in the diagnosis of genetic disease: Experience of a regional disease center in South Korea. Ann Hum Genet. Published online October 5, 2023.
Vilboux T, Doherty DA, Glass IA, et al. Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med. 2017;19(8):875–82.
Acknowledgments
The authors thank all patients and their families for participation in this study.
Funding
The work for this study was supported by the National Key Scientific Research and Development Program of China (No.2022YFC2705193) and the Key Scientific Research and Development Program of Hubei Province (No.2022BCA047).
Author information
Authors and Affiliations
Contributions
HYXL and ZJH conceived the study. HYXL collected and analyzed the data and drafted the initial manuscript. SHY, YY, ZLQ, YZW, WY and YFJ helped to collect and supplement the data and participated in the care of these patients. QLR and ZY interpreted the results of WES and kidney biopsies. ZJH was the research grant recipient, revised the manuscript and supervised all the process. All authors had read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent documents for this study were obtained from patients’ parents. The study was approved by the the Medical Ethics Committee of Tongji Medical College and was conducted in accordance with HIPAA regulations and with the tenets of the Declaration of Helsinki.
Consent for publication
Consent for publication were obtained from the institution and patients’ parents.
Competing interests
The authors declare no competing interests in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, Y., Sha, H., Yang, Y. et al. Mutations in the NUP93, NUP107 and NUP160 genes cause steroid-resistant nephrotic syndrome in Chinese children. Ital J Pediatr 50, 81 (2024). https://doi.org/10.1186/s13052-024-01656-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-024-01656-3